Abstract
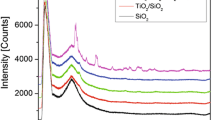
The preparation of vanadium-phosphate catalysts by Atomic Layer Deposition (ALD) was studied for application to selective oxidation of n-butane to maleic anhydride (MA). Modification of bulk V2O5 by ALD with trimethylphosphate significantly increased the selectivity to MA but the catalyst performance was still much worse than that observed for bulk vanadium phosphate (VPO). Silica-supported VPO was then prepared by ALD of P and V and compared to a VPO/SiO2 catalyst prepared by impregnation. Raman spectra showed that the ALD-prepared materials were more homogeneous, and temperature-programmed desorption of 2-propanol demonstrated that the VPO phase covered the silica more uniformly when deposited by ALD. However, the bulk VPO catalyst remained the most selective for MA. Possible reasons for why the supported catalysts showed lower performance are discussed and suggestions made for improving these catalysts.
Graphical Abstract









Similar content being viewed by others
References
Bartholomew CH, Farrauto RJ (2005) Catalytic Oxidations of Inorganic and Organic Compounds. In: Fundamentals of Industrial Catalytic Processes. pp 560–634
Contractor RM (1999) Dupont’s CFB technology for maleic anhydride. Chem Eng Sci. 54:5627–5632. https://doi.org/10.1016/S0009-2509(99)00295-X
Patience GS (2010) Bockrath RE Butane oxidation process development in a circulating fluidized bed. Appl Catal A Gen 376:4–12. https://doi.org/10.1016/j.apcata.2009.10.023
Zhou G, Shah PR, Montini T et al (2007) Oxidation enthalpies for reduction of ceria surfaces. Surf Sci 601:2512–2519. https://doi.org/10.1016/j.susc.2007.04.238
Harding WD, Birkeland KE, Kung HH (1994) Selective oxidation of butane on phosphorus-modified silica supported vanadia catalysts. Catal Letters 28:1–7. https://doi.org/10.1007/BF00812463
Birkeland KE, Babitz SM, Bethke GK et al (1997) Supported VPO Catalysts for Selective Oxidation of Butane. II. Characterization of VPO/SiO2 Catalysts. J Phys Chem B 101:6895–6902. https://doi.org/10.1021/jp962571c
Overbeek RA, Pekelharing ARCJ, van Dillen AJ, Geus JW (1996) Preparation, characterization and testing of newly developed silica-supported V-P-O catalysts. Appl Catal A Gen 135:231–248. https://doi.org/10.1016/0926-860X(95)00243-X
Martinez-Lara M, Moreno-Real L, Pozas-Tormo R et al (1992) Catalytic activity of vanadyl phosphate supported on TiO2 (anatase) and SiO2 (silica). Can J Chem 70:5–13. https://doi.org/10.1139/v92-002
Kuo PS, Yang BL (1989) AlPO4 as a support material for VPO catalysts. J Catal 117:301–310. https://doi.org/10.1016/0021-9517(89)90341-2
Do N-T, Baerns M (1998) Effect of support material on the catalytic performance of V2O5/P2O5 catalysts for the selective oxidation of but-1-ene and furan to maleic anhydride and its consecutive nonselective oxidation: I. Results of Catalytic Testing. Appl Catal 45:1–7. https://doi.org/10.1016/S0166-9834(00)82388-X
Strempel VE, Löffler D, Kröhnert J et al (2015) Enhancing of catalytic properties of vanadia via surface doping with phosphorus using atomic layer deposition. J Vac Sci Technol A 34:01A135. https://doi.org/10.1116/1.4936390
Onn TM, Zhang S, Arroyo-Ramirez L et al (2017) High-surface-area ceria prepared by ALD on Al2O3 support. Appl Catal B Environ 201:430–437. https://doi.org/10.1016/j.apcatb.2016.08.054
Wang C, Mao X, Lee JD et al (2018) A characterization study of reactive sites in ALD-synthesized WOx/ZrO2 catalysts. Catalysts 8:292. https://doi.org/10.3390/catal8070292
Wang C-Y, Kwon O, Gorte RJ, Vohs JM (2022) Synthesis of high-surface area tungstated zirconia by atomic layer deposition on mesoporous silica. Microporous Mesoporous Mater 335:111821. https://doi.org/10.1016/j.micromeso.2022.111821
Nguyen PT, Hoffman RD, Sleight AW (1995) Structure of (VO)2P2O7. Mater Res Bull 30:1055–1063. https://doi.org/10.1016/0025-5408(95)00116-6
Bakhmutsky K, Wieder NL, Baldassare T et al (2011) A thermodynamic study of the redox properties of supported Co particles. Appl Catal A Gen 397:266–271. https://doi.org/10.1016/j.apcata.2011.03.013
Wang D, Barteau MA (2001) Kinetics of butane oxidation by a vanadyl pyrophosphate catalyst. J Catal 197:17–25. https://doi.org/10.1006/jcat.2000.3061
Johnson JW, Johnston DC, Jacobson AJ, Brody JF (1984) Preparation and characterization of vanadyl hydrogen phosphate hemihydrate and its topotactic transformation to vanadyl pyrophosphate. J Am Chem Soc 106:8123–8128. https://doi.org/10.1021/ja00338a020
Cao T, Kwon O, Lin C et al (2021) Two-dimensional perovskite crystals formed by atomic layer deposition of CaTiO3 on γ-Al2 O3. Nanomaterials 11:2207. https://doi.org/10.3390/nano11092207
Musschoot J, Deduytsche D, Van Meirhaeghe RL, Detavernier C (2009) ALD of vanadium oxide. ECS Trans 25:29. https://doi.org/10.1149/1.3205040
Deo G, Hardcastle FD, Richards M et al (1990) Raman spectroscopy of vanadium oxide supported on alumina. In: Novel materials in heterogeneous catalysis. American Chemical Society, pp. 29–317
Wachs IE, Jehng J-M, Deo G et al (1996) In situ Raman spectroscopy studies of bulk and surface metal oxide phases during oxidation reactions. Catal Today 32:47–55. https://doi.org/10.1016/S0920-5861(96)00091-0
Zhang X, Yang D, Liu W, Rui X (2020) VOPO4⋅2H2O: Large-scale synthesis and zinc-ion storage application. Front Energy Res. https://doi.org/10.3389/fenrg.2020.00211
Wang J, Tan S, Xiong F et al (2020) VOPO4·2H2O as a new cathode material for rechargeable Ca-ion batteries. Chem Commun 56:3805–3808. https://doi.org/10.1039/D0CC00772B
Cao T, Kwon O, Vohs JM, Gorte RJ (2022) LaFeO3 films on SiO2 for supported-Pt catalysts. Int J Green Energy 19:380–388. https://doi.org/10.1080/15435075.2021.1946815
Luo J, Yu J, Gorte RJ et al (2014) The effect of oxide acidity on HMF etherification. Catal Sci Technol 4:3074–3081. https://doi.org/10.1039/C4CY00563E
Huang R, Chang J, Choi H et al (2022) Furfural upgrading by aldol condensation with ketones over solid-base catalysts. Catal Letters. https://doi.org/10.1007/s10562-022-03960-1
Shen K, Fan M, Kwon O et al (2023) Reversible perovskite-fluorite phase transition in alumina-supported CeFeOx films. J Mater Chem A 11:4183–4193. https://doi.org/10.1039/D2TA06215A
Shen K, Fan M, Rai RK et al (2023) Structure and redox properties of CeMnO3 thin films. J Solid State Chem 323:124055. https://doi.org/10.1016/j.jssc.2023.124055
Fan M, Ji Y, Lawal A et al (2022) Site and structural requirements for the dehydra-decyclization of cyclic ethers on ZrO2. Catalysts 12:902. https://doi.org/10.3390/catal12080902
Acknowledgements
Jian Chang acknowledges support from the Vagelos Institute for Energy Science Technology at the University of Pennsylvania. Additional support was provided by the Catalysis Center for Energy Innovation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award number DE-SC0001004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, J., Gorte, R.J. & Vohs, J.M. Supported VPO Catalysts for Maleic Anhydride by Atomic Layer Deposition. Catal Lett 154, 1072–1080 (2024). https://doi.org/10.1007/s10562-023-04373-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04373-4