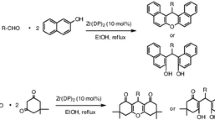
Abstract
A facile and convenient method has been developed for the one-pot three-component synthesis of 2-amino-3-cyano substituted tetrahydrobenzo[b]pyran derivatives from the reactions of aromatic aldehydes, malononitrile and dimedone or 1,3-cyclohexanedione in the presence of a catalytic amount of sodium dodecyl sulphate as an efficient surfactant type catalyst in water at room temperature. Synthesis of 2-amino-3-cyano substituted spiropyrans was also achieved under the same reaction conditions starting from ninhydrin/isatins, malononitrile and dimedone or 1,3-cyclohexanedione. All the reactions were completed within 2.5 h and the desired products afforded in good to excellent yields. Gram scale production of the desired compound was also achieved. Use of water as green solvent, commercially available low cost surfactant type catalyst, high atom economy, excellent yields, energy efficiency, no column chromatographic purifications, reusability of the solvent media, multiple carbon–carbon and carbon-heteroatom bond formations are some of the major advantages of this newly developed protocol.
Graphical Abstract








Similar content being viewed by others
References
Kaur M, Kaur M, Bandopadhyay T, Sharma A, Priya A, Singh A, Banerjee B (2022) Phy Sci Rev. https://doi.org/10.1515/psr-2022-0003
Ma J, Hecht SM (2004) Chem Comm 10:1190–1191
Ray S, Majumder HK, Chakravarty AK, Mukhopadhyay S, Gil RR, Cordell GA (1996) J Nat Prod 59:27–29
Makino M, Fujimoto Y (1999) Phytochemistry 50:273–277
Delost MD, Smith DT, Anderson BJ, Njardarson JT (2018) J Med Chem 61:10996–11020
Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, Xu L, Crogan-Grundy C, Denis R, Barriault N, Vaillancourt L, Charron S, Dodd J, Attardo G, Labrecque D, Lamothe S, Gourdeau H, Tseng B, Drewe J, Cai SX (2005) Bioorg Med Chem Lett 15:4745–4751
Mahmoodi M, Aliabadi A, Emami S, Safavi M, Rajabalian S, Mohagheghi MA, Khoshzaban A, Samzadeh-Kermani A, Lamei N, Shafiee A, Foroumadi A (2010) Arch Pharm Chem Life Sci 343:411–416
Kemnitzer W, Drewe J, Jiang S, Zhang H, Zhao J, Crogan-Grundy C, Xu L, Lamothe S, Gourdeau H, Denis R, Tseng B, Kasibhatla S, Cai SX (2007) J Med Chem 50:2858
Fouda AM (2016) Med Chem Res 25:1229–1238
Amr AGE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam AEFG (2006) Bioorg Med Chem 14:5481–5488
Smith CW, Bailey JM, Billingham MEJ, Chandrasekhar S, Dell CP, Harvey AK, Hicks CA, Kingston AE, Wishart GN (1995) Bioorg Med Chem Lett 5:2783–2788
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) Eur J Med Chem 44:3805–3809
Abdelrazek FM, Metz P, Farrag EK (2004) Arch Pharm Pharm Med Chem 337:482–485
Paliwal PK, Jetti SR, Jain S (2013) Med Chem Res 22:2984–2990
Vala ND, Jardosh HH, Patel MP (2016) Chin Chem Lett 27:168
Kidwai M, Jain A, Nemaysh V, Kumar R (2013) Med Chem Res 22:2717–2723
Brahmachari G, Banerjee B (2013) ACS Sustain Chem Eng 2:411–422
Hasaninejad A, Shekouhy M, Golzar N, Zare A, Doroodmand MM (2011) Appl Catal A Gen 402:11–22
Banerjee S, Horn A, Khatri H, Sereda G (2011) Tetrahedron Lett 52:1878–1881
Wang LM, Shao JH, Tian H, Wang YH, Liu B (2006) J Fluor Chem 127:97–100
Ghobadpoor A, Eskandari MM, Zare A, Karami M (2021) Iran J Catal 11:69–75
Magar CV, Solanke KS, Mane SB, Choudhary SS, Pawar RP (2007) Synth Commun 37:4353–4357
Mohammadi AA, Asghariganjeh MR, Hadadzahmatkesh A (2017) Arab J Chem 10:S2213–S2216
Pourpanah SS, Habibi-Khorassani SM, Shahraki M (2015) Chinese J Catal 36:757–763
Mahdieh H, Farhad S, Mostafa G (2019) J Nanosci Nanotechnol 19:3447–3458
Oskooie HA, Heravi MM, Karimi N, Zadeh ME (2011) Synth Commun 41:436–440
Sabitha G, Arundhathi K, Sudhakar K, Sastry BS, Yadav JS (2009) Synth Comm 39:433–442
Ataie F, Davoodnia A, Khojastehnezhad A (2019) Polycyclic Aromat Compd 414:781–794
Biglari M, Shirini F, Mahmoodi NO, Zabihzadeh M, Mashhadinezhad MA (2020) J Mol Struct 1205:127652. https://doi.org/10.1016/j.molstruc.2019.127652
Ranu BC, Banerjee S, Roy S (2008) Indian J Chem 47:1108–1112
Devi I, Bhuyan PJ (2004) Tetrahedron Lett 45:8625–8627
Azarifar D, Khatami SM, Zolfigol MA, Nejat-Yami R (2013) J Iran Chem Soc 11:1223–1230
Mobinikhaledi A, Fard MAB (2010) Acta Chim Slov 57:931–935
Balalaie S, Sheikh-Ahmadi M, Bararjanian M (2007) Catal Commun 8:1724–1728
Shi D, Mou J, Zhuang Q, Wang X (2004) J Chem Res 12:821–823
Hu H, Qiu F, Ying A, Yang J, Meng H (2014) J Mol Sci 15:6897–6909
Jin TS, Wang AQ, Wang X, Zhang JS, Li TS (2004) Synlett 5:0871–0873
Gao S, Tsai CH, Tseng C, Yao CF (2008) Tetrahedron 64:9143–9149
Patra A, Mahapatra T (2010) J Chem Res 34:689–693
Balalaie S, Bararjanian M, Sheikh-Ahmadi M, Hekmat S, Salehi P (2007) Synth Commun 37:1097–1108
Banerjee B (2018) Curr Org Chem 22:208–233
Kaur G, Sharma A, Banerjee B (2018) J Serb Chem Soc 83:1071–1097
Banerjee B, Bhardwaj V, Kaur A, Kaur G, Singh A (2020) Curr Org Chem 23:3191–3205
Basafa S, Davoodnia A, Beyramabadi SA, Pordel M (2021) Chem Methodol 5:59–69
Selmi A, Zarei A, Tachoua W, Puschmann H, Teymourinia H, Ramazani A (2022) Chem Methodol 6:463–474
Banik BK, Banerjee B (2022) In Organocatalysis: A Green Tool for Sustainable Developments. De Gruyter, Berlin, Boston. https://doi.org/10.1515/9783110732542
Banerjee B, Kaur G, Priya A (2022) Green sustainable process for chemical and environmental engineering and science. Elsevier, Amsterdam, pp 337–350
Manabe K, Mori Y, Wakabayashi T, Nagayama S, Kobayashi S (2000) J Am Chem Soc 122:7202–7207
Kaur G, Thakur S, Kaundal P, Chandel K, Banerjee B (2018) ChemistrySelect 3:12918–12936
Manabe K, Iimura S, Sun XM, Kobayashi S (2002) J Am Chem Soc 124:11971–11978
Lang S (2002) Curr Opin in Colloid Interface Sci 7:12–20
Satpute SK, Banat IM, Dhakephalkar PK, Banpurkar AG, Chopade BA (2010) Biotechnol Adv 28:436–450
Kumar A, Rao MS, Rao VK (2010) Aust J Chem 63:1538–1540
Ghosh P, Mandal A (2011) Catal Commun 12:744–747
Bansal R, Soni PK, Sharma J, Bhardwaj SK, Halve AK (2017) Curr Chem Lett 6:135–142
Ghosh P, Mandal A (2013) Green Chem Lett Rev 6:45–54
Mehrabi H, Abusaidi H (2010) J Iran Chem Soc 7:890–894
Hemmati S, Safarimehr P, Safaei M, Hekmati M (2016) J Heterocycl Chem 54:1640–1644
Veisi H, Azadbakht R, Ezadifar M, Hemmati S (2013) J Heterocycl Chem 50:E241–E246
Sahu PK, Sahu PK, Agarwal DD (2014) RSC Adv 4:40414–40420
Sahu PK (2016) RSC Adv 6:67651–67661
Shi DQ, Shi JW, Yao H (2009) Synth Commun 39:664–675
Kong D, Wang Q, Zhu Z, Wang X, Shi Z, Lin Q, Wu M (2017) Tetrahedron Lett 58:2644–2647
Wang W, Wang SX, Qin XY, Li JT (2005) Synth Commun 35:1263–1269
Bhutia ZT, Panjikar PC, Iyer S, Chatterjee A, Banerjee M (2020) ACS Omega 5:13333–13343
Tornquist BL, Bueno GP, Willig JCM, Oliveira IM, Stefani HA, Rafique J, Saba S, Iglesias BA, Botteselle GV, Manarin F (2018) ChemistrySelect 3:6358–6363
Guidotti BB, Silva TS, Correia JTM, Coelho F (2020) Org Biomol Chem 18:7330–7335
Costantino U, Fringuelli F, Orrù M, Nocchetti M, Piermatti O, Pizzo F (2009) Eur J Org Chem 2009:1214–1220
McKay CS, Kennedy DC, Pezacki JP (2009) Tetrahedron Lett 50:1893–1896
Kamali TA, Bakherad M, Nasrollahzadeh M, Farhangi S, Habibi D (2009) Tetrahedron Lett 50:5459–5462
Background review for sodium laurilsulfate used as an excipient, European Medicines Agency, (2015) EMA/CHMP/351898/2014. Available at: https://www.ema.europa.eu/en/documents/report/background-review-sodium-laurilsulfate-used-excipient-context-revision-guideline-excipients-label_en.pdf
Kaur G, Devi P, Thakur S, Kumar A, Chandel R, Banerjee B (2018) ChemistrySelect 3:9892–9910
Brahmachari G, Banerjee B (2012) Asian J Org Chem 1:251–258
Banerjee B, Brahmachari G (2014) J Chem Res 38:745–750
Brahmachari G, Laskar S, Banerjee B (2014) J Heterocycl Chem 51:303–308
Kaur G, Shamim M, Bhardwaj V, Gupta VK, Banerjee B (2020) Synth Commun 50:1545–1560
Taherkhani H, Ramazani A, Sajjadifar S, Aghahossieini H, Rezaei A (2021) ACS Omega 6:25608–25622
Rezayati S, Kalantari F, Ramazani A, Sajjadifar S, Aghahosseini H, Rezaei A (2022) Inorg Chem 61:992–1010
Baghernejad B, Harzevili MR (2021) Chem Methodol 5:90–95
Hassanzadeh-Afruzi F, Asgharnasl S, Mehraeen S, Amiri-Khamakani Z, Maleki A (2021) Sci Rep 11:19852. https://doi.org/10.1038/s41598-021-99120-3
Kamalzare M, Ahghari MR, Bayat M, Maleki A (2021) Sci Rep 11:20021. https://doi.org/10.1038/s41598-021-99121-2
Maleki A, Jafari AA, Yousefi S (2017) Carbohydr Polym 175:409–416
Maleki A, Azadegan S (2017) Inorg Nano-Met Chem 47:917–924
Maleki A, Azadegan S (2017) J Inorg Organomet Polym 27:714–719
Maleki A, Ghassemi M, Firouzi-Haji R (2018) Pure Appl Chem 90:387–394
Maleki A, Movahed H, Ravaghi P (2017) Carbohydr Polym 156:259–267
Maleki A, Aghaei M, Ghamari N (2016) Appl Organometal Chem 30:939
Hajizadeh Z, Maleki A (2018) Mol Catal 460:87–93
Maleki A, Azizia M, Emdadi Z (2018) Green Chem Lett Rev 114:573–582
Maleki A, Varzi Z, Hassanzadeh-Afruzi F (2019) Polyhedron 171:193–202
Maleki A, Hajizadeh Z (2019) Silicon 11:2789–2798
Kaur G, Bala K, Devi S, Banerjee B (2018) Curr Green Chem 5:150–167
Brahmachari G, Banerjee B (2016) Curr Organocatal 3:93–124
Singh A, Kaur G, Kaur A, Gupta VK, Banerjee B (2020) Curr Green Chem 7:128–140
Kaur G, Singh A, Bala K, Devi M, Kumari A, Devi S, Devi R, Gupta VK, Banerjee B (2019) Curr Org Chem 23:1778–1788
Banerjee B, Priya A, Sharma A, Kaur G, Kaur M (2022) Phy Sci Rev 7:539–565
Banik BK, Banerjee B, Kaur G, Saroch S, Kumar R (2020) Molecules 25:5918. https://doi.org/10.3390/molecules25245918
Sharma A, Singh A, Priya A, Kaur M, Gupta VK, Jaitak V, Banerjee B (2022) Synth Commun 52:1614–1627
Banerjee B, Singh A, Kaur G (2022) Phy Sci Rev 7:301–323
Kaur G, Moudgil R, Shamim M, Gupta VK, Banerjee B (2021) Synth Commun 51:1100–1120
Kaur G, Kumar R, Saroch S, Gupta VK, Banerjee B (2021) Curr Organocatal 8:147–159
Kaur G, Singh D, Singh A, Banerjee B (2021) Synth Commun 51:1045–1057
Kaur M, Priya A, Sharma A, Singh A, Banerjee B (2022) Synth Commun 52:1635–1656
Saluja P, Aggarwal K, Khurana JM (2013) Synth Commun 43:3239–3246
Jin SS, Ding MH, Guo HY (2013) Heterocycl Commun 19:139–143
Zhu SL, Ji SJ, Zhang Y (2007) Tetrahedron 63:9365–9372
Zhao LQ, Zhou B, Li YQ (2011) Heteroat Chem 22:673–677
Acknowledgements
Dr. B. Banerjee is thankful to the Akal University, Talwandi Sabo, Punjab, India and the Kalgidhar Trust, Baru Sahib, Himachal Pradesh, India for the support. Authors are grateful to AMRC, IIT Mandi, Himachal Pradesh, India for the spectral measurements such as FT-IR, 1H and 13C NMR, HRMS and single X-ray crystal data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the memory of our heavenly colleague, Dr. Arvinder Singh, Associate Professor, Department of Botany, Akal University, Talwandi Sabo, Bathinda.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, B., Priya, A., Kaur, M. et al. Sodium Dodecyl Sulphate Catalyzed One-Pot Three-Component Synthesis of Structurally Diverse 2-Amino-3-cyano Substituted Tetrahydrobenzo[b]pyrans and Spiropyrans in Water at Room Temperature. Catal Lett 153, 3547–3560 (2023). https://doi.org/10.1007/s10562-022-04256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04256-0