Abstract
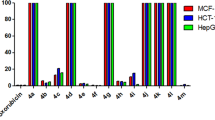
Multi-component reactions for the preparation of 4H-chromene derivatives under microwave irradiation from different aromatic aldehydes with a mixture of malononitrile and phenol derivatives were established. The cytotoxic activity of the target compounds was evaluated against four cancer cell lines MCF-7, HCT-116, HepG-2 and A549 in comparison with vinblastine and colchicine as reference drugs. Generally, several compounds showed good cell growth inhibitory activity as compared to standard drugs. The structure–activity relationship studies reported that the substitution at 4- and 6-positions in 4H-chromene nucleus with the specific halogen atom increases the ability of the molecule against the different cell lines. The structures of the synthesized compounds were established on the basis of spectral data, IR, 1H NMR, 13C NMR and MS data.

Similar content being viewed by others
References
Akbarzadeh T, Rafinejad A, Malekian Mollaghasem J, Safavi M, Fallah-Tafti A, Pordeli M, Kabudanian Ardestani S, Shafiee A, Foroumadi A (2012) 2-Amino-3-cyano-4-(5-arylisoxazol-3-yl)-4H-chromenes: synthesis and in vitro cytotoxic activity. Arch Pharm 345:386–392
Bhat M, Siddiqui N, Khan S (2008) Synthesis of novel 3-(4-acetyl-5H/methyl-5-substituted phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones as potential anticonvulsant agents. Acta Pol Pharm 65:235–239
Birch KA, Heath WF, Hermeling RN, Johnston CM, Stramm L, Dell C, Smith C, Williamson JR, Reifel-Miller A (1996) LY290181, an inhibitor of diabetes induced vascular dysfunction, blocks protein kinase C-stimulated transcriptional activation through inhibition of transcription factor binding to a phorbol response element. Diabetes 45:642–650
Cheng JF, Ishikawa A, Ono Y, Arrhenius T, Nadzan A (2003) Novel chromene derivatives as TNF-α inhibitors. Bioorg Med Chem Lett 13:3647–3650
Chetan BS, Nimesh MS, Manish PP, Ranjan GP (2012) Microwave assisted synthesis of novel 4H-chromene derivatives bearing phenoxypyrazole and their antimicrobial activity assess. J Serb Chem Soc 77:1–17
El-Agrody AM, Khattab ESAEH, Fouda AM (2014) Synthesis, structure–activity relationship (SAR) studies on some 4-Aryl-4H-chromenes and relationship between lipophilicity and antitumor activity. Lett Drug Des Discov 11:1167–1176
Hosseini-Zare MS, Mahdavi M, Saeedi M, Asadi M, Javanshir S, Shafiee A, Foroumadi A (2012) Synthesis of 2,3-diaryl-5H-imidazo[2,1-a]isoindol-5-ones via the one-pot reaction of 1,2-diketones, 2-formylbenzoic acids, and ammonium acetate. Tetrahedron Lett 53:3448–3451
Kathrotiya HG, Patel MP (2012) Microwave-assisted synthesis of 3′-indolyl substituted 4H-chromenes catalyzed by DMAP and their antimicrobial activity. Med Chem Res 21:3406–3416
Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, Xu L, Crogan-Grundy C, Denis R, Barriault N, Vaillancourt L, Charron S, Dodd J, Attardo G, Labrecque D, Lamothe S, Gourdeau H, Tseng B, Drewe J, Cai SX (2005) Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure–activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg Med Chem Lett 15:4745–4751
Kheirollahi A, Pordeli M, Safavi M, Mashkouri S, Naimi-Jamal MR, Ardestani SK (2014) Cytotoxic and apoptotic affects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharmacol 387:1199–1208
Magedov IV, Manpadi M, Evdokimov NM, Elias EM, Rozhkova E, Ogasawara MA, Bettale JD, Przheval’skii NM, Rogelj S, Kornienko A (2007) Antiproliferative and apoptosis inducing properties of pyrano[3,2-c]pyridones accessible by a one-step multicomponent synthesis. Bioorg Med Chem Lett 17:3872–3876
Makarem S, Mohammadi AA, Fakhari AR (2008) A multi-component electro-organic synthesis of 2-amino-4H-chromenes. Tetrahedron Lett 49:7194–7196
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Musa MA, Badisa VLD, Latinwo LM, Waryoba C, Ugochukwu N (2010) In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res 30:4613–4617
Nareshkumar J, Jiayi X, Ramesh MK, Fuyong D, Guo JZ, Emmanuel P (2009) Identification and structure–activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J Med Chem 52:7544–7569
Nefzi A, Ostresh JM, Houghten RA (1997) The current status of heterocyclic combinatorial libraries. Chem Rev 97:449–472
Nimesh RK, Dhaval DH, Prashant TM, Saurabh KP (2011) Synthesis and evaluation of in vitro antitubercular activity and antimicrobial activity of some novel 4H-chromeno[2,3-d]pyrimi-dine via 2-amino-4-phenyl-4H-chromene-3-carbonitriles. Med Chem Res 20:854–864
Patil SA, Patil R, Pfeffer LM, Miller DD (2013) Chromenes: potential new chemotherapeutic agents for cancer. Future Med Chem 5:1647–1660
Rafinejad A, Fallah-Tafti A, Tiwari R, Shirazi AN, Mandal D, Shafiee A, Parang K, Foroumadi A, Akbarzadeh T (2012) 4-Aryl-4H-naphthopyrans derivatives: one-pot synthesis, evaluation of Src kinase inhibitory and anti-proliferative activities. DARU J Pharm Sci 20:100–106
Rahman AU, Choudhary MI, Thomsen WJ (2001) Bioassay technique for drug development. Harwood Academic Publishers; ISBN 0-203-34349-2 (Adobe e-Reader Format), ISBN 90-5823-051-1 (Print Edition)
Sabry NM, Mohamed HM, Khattab ESAEH, Motlaq SS, El-Agrody AM (2011) Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur J Med Chem 46:765–772
Saeedi M, Mahdavi M, Foroumadi A, Shafiee A (2013) Synthesis of novel fused 4,5-dihydro-1,2,3-triazolo[1,5-a][1,4]benzodiazepine derivatives via four-component Ugi–Smiles-type reaction. Tetrahedron 69:3506–3510
Saffari Z, Aryapour H, Akbarzadeh A, Foroumadi A, Jafari N, Zarabi MF, Farhangi A (2014) In vitro antitumor evaluation of 4H-chromene-3-carbonitrile derivatives as a new series of apoptotic inducers. Tumor Biol 35:5845–5855
Singh OM, Devi NS, Thokchom DS, Sharma GJ (2010) Novel 3-alkanoyl/aroyl/-heteroaroyl-2H-chromene-2-thiones: synthesis and evaluation of their antioxidant activities. Eur J Med Chem 45:2250–2257
Snedecor GM, Cochran WG (1982) Statistical methods, 7th edn. Lowa state University Press, Ames, pp 325–330
Szulawska-Mroczek A, Szumilak M, Szczesio M, Olczak A, Nazarski RB, Lewgowd W, Czyz M, Stanczak A (2013) Synthesis and biological evaluation of new bischromone derivatives with antiproliferative activity. Arch Pharm Chem 346:34–43
Thomas N, Zachariah SM (2013) In Silico drug design and analysis of 4-Phenyl-4H-chromene derivatives as anticancer and anti-inflammatory agents. Int J Pharm Sci Rev Res 22:50–54
Thompson LA (2000) Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr Opin Chem Biol 4:324–337
Vukovic N, Sukdolak S, Solujic S, Niciforovic N (2010) Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: synthesis and in vitro assessments. Food Chem 120:1011–1018
Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z (2000) Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 97:7124–7129
Wiener C, Schroeder CH, West BD, Link KP (1962) Studies on the 4-hydroxycoumarins. XVIII. 3-[α-(acetamidomethyl)benzyl]-4-hydroxycoumarin and related products. J Org Chem 27:3086–3088
Zhang D, Ma Y, Liu Y, Liu ZP (2014) Synthesis of sulfonylhydrazone- and acylhydrazone-substituted 8-ethoxy-3-nitro-2H-chromenes as potent antiproliferative and apoptosis inducing agents. Arch Pharm Chem 347:576–588
Acknowledgments
The author deeply thanks the Regional Center for Mycology and Biotechnology (RCMP), Al-Azhar University, Cairo, Egypt, for carrying out the antitumor study and also, Mr. Ali Y. A. Alshahrani for making the 1H NMR and 13C NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouda, A.M. Synthesis of several 4H-chromene derivatives of expected antitumor activity. Med Chem Res 25, 1229–1238 (2016). https://doi.org/10.1007/s00044-016-1565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1565-3