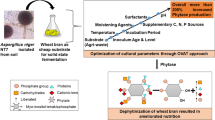
Abstract
In the present study, statistically optimized production of thermotolerant Aspergillus fumigatus phytase was carried out in batch fermentation (1-L). An enhancement in phytase production was noted up to 3.3-folds as compared to unoptimized and using One Variable at a Time strategy. Phytase was purified up to 26-folds with a single band of ~ 62 kDa. The optimum activity of purified phytase was recorded at pH 5.5 and 65 °C. The kinetic parameters, Km and Vmax, were observed to be, 0.75 mM, and 345 µmol/min/mg-protein,respectively. The activity was constrained by silver and mercuric ions and slightly increased by calcium ions. The phytase showed high dephytinization up to 78.7% of wheat flour (10 g) at a small reaction volume (30 mL). On up-scaling to 3-L reaction, a slight decrease in dephytinization to 72.5% was recorded. This is the first report where hyperactive purified phytase from thermotolerant A. fumigatus has been demonstrated to degrade wheat flour-based phytic acid up to a 3-L scale at the bioreactor level. This finding can be explored at a commercial scale for multi-dimensional future biotechnological applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phytases are of great potential in the manufacturing and processing of the feed and food industry. Global market insights assume the growth of the feed enzyme industry to cross over 2 billion USD by 2024 [1]. Among major feed enzymes, phytase (EC 3.1.3.8) has a market share of 83.6% in terms of revenue [2]. Phytase is the enzyme that breaks phospho-monoester bonds in phytates and is primarily applied to reduce phytate content in the feed as well as in other foodstuffs. Several phytase-producing microbes have been reported with their potential applications in diverse areas [3]. The presence of phytase in human food will provide the essential metal ions and myoinositols by releasing from its substrate the phytic acid which further functions as therapeutic for several ailments e.g. Alzehmier’s disease, Crohn’s disease and irritable bowel syndrome. Further with the rise in nutritional and health issues, there is strong demand for the functional food with increase up to 96 billion USD by 2020 [4]. Among various cereal grains, wheat flour is the most commonly used grain in India and other Asian countries on daily basis. Whole wheat flour consists approximately four mg/g of anti-nutrient phytic acid [5]. The most abundant form of organic phosphorous is the phytic acid (myo – inositol—hexakisphosphate) which is present in other plant products like oil seeds, legumes and nuts [6, 7]. Thus, treatment of these cereals and legumes using phytase enzyme will open up the nutritional treasure locked inside these plant products. Livestock production has reported about 60–70% of its expenditure on feeding swine and poultry. Large amounts of this feed provided to monogastric animals are wasted as they are unable to digest due to a lack of required enzymes [2]. Phosphorous is one of the major nutrients in the plant-based diet of monogastric animals, present in the form of phytin [8]. Its pessimistic effect on mineral uptake is a great concern regarding its existence in the aliment. In the gut of monogastric animals, phytic acid shows a chelation effect on minerals such as calcium (Ca), copper (Cu), magnesium (Mg), iron (Fe), manganese (Mn), and zinc (Zn) [9, 10].
. The marvelous capacity of various fungi to produce extracellular enzymes during utilizing substrates for their own growth makes them good source for industrial enzyme production [11]. Several microbes are reported for producing phytases, however, most of the work has been done using filamentous fungi for example Aspergillus niger, Sporotrichum Thermophile, Thermoascus Aurantiacus, A. niger CFR 335, A. niger st-6 [12]. Generally, fungal phytases are known for their production in solid state fermentation but due to their rigidity for scale up and complexity in extraction steps further makes it less feasible. Moreover, fungal microbes like A. ficuum, A. niger are few moulds that are studied for phytase production in submerged fermentation [7]. Most of the phytase-producing fungal strains reported so far, generally, grow at low temperatures (≤ 30ºC) with a long incubation period. Biocatalysts need to maintain three-dimensional conformations during exposure to high temperatures at which enzymes may lose their biocatalytic activity. Several studies have suggested phytase denaturation at high temperatures [13]. However, there is a constant need for thermostable or thermotolerant strains which are optimized for the industrial production of enzymes. The latest statistical experiment design offers a well-organized approach that identifies the interactions between different factors affecting the production to generate a certain optimum response [14].
Research on phytase primarily is progressed around its role in phytic acid degradation for nutritional improvement in the area of human nutrition [14, 15]. In the present study, a bioprocess was developed for hyperactive phytase production from a wild strain of thermotolerant Aspergillus fumigatus in a statistically optimized medium through response surface methodology. The phytase was purified to homogeneity, and process parameters optimization was performed to determine high biocatalytic activity. The purified phytase application was successfully demonstrated for efficient dephytinization of wheat flour at 3-L of fermentation. These findings suggested that thermotolerant hyperactive phytase from A. fumigatus can be potentially used as multi-facet biocatalyst for future biotechnological applications.
2 Materials and Methods
2.1 Chemicals and Reagents
All the media constituents were acquired from Hi-Media (Mumbai, India). The substrate (Ca-phytate) used during the in vitro assay was purchased from Hi-Media (Mumbai, India). The solvents were used of High-performance liquid chromatography (HPLC) grade (Merck, Germany). All chemicals or reagents were used of high purity-grade from commercial sources.
2.2 Microorganism and Growth Conditions
Phytase-producing fungus A. fumigatus was previously isolated from the rhizospheric soil of maize fields [16]. The fungus was selected due to its hyperactivity against the substrate calcium phytate for phytase production. Reported optimized medium using One Variable at a Time (OVAT) strategy was employed for extracellular phytase production in submerged medium (pH 5.5) with following composition (w/v): maltose (3.62%), NH4NO3 (0.50%), mineral solution [1.5 mL (v/v) of mineral stock solution consist of KCl (600 mM), MgSO4.7H2O (200 mM), FeSO4.7H2O (3 mM), CaCl2.2H2O (300 mM), and MnSO4(6 mM)], calcium-phytate (70 μM, v/v of stock solution 3.5 mM) as an inducer, and inoculum (125 × 104 spores per 100 mL, v/v) at 40 °C for incubation up to 96 h. The dry cell weight (dcw) analysis was performed after drying biomass at 70 °C for incubation of 48 h [17].
2.3 Phytase Assay
The molybdenum blue method was used to measure phytase activity [18]. Brief, a 1-mL reaction consists of the substrate (250 µM), and the enzyme in a sodium-acetate (0.25 mM, pH 5.5) buffer was incubated for 15 min (at 55 °C) followed by 10% trichloroacetic acid (TCA) (250 µL) addition to stop the reaction. For the assay of released phosphate 1 ml of reagent (FeSO4 0.7 H2O (7.32%); Ammonium molybdate 4H2O(1.0%); H2SO4 (3.2 v/v) was added and mixed properly in reaction supernatant. Product formation was estimated spectrophotometrically at 750 nm using sodium-dihydrogen phosphate [16]. One unit of phytase is defined as the amount of enzyme that liberates one μmole inorganic phosphate/ml/min under the assay conditions.
2.4 Statistical Optimization for Phytase Production
Several components (media, carbon source, nitrogen source, etc.) screened previously for phytase production [16], were used to construct a factorial design to study simultaneous interactions among all the components for their individual and cumulative effect on growth and phytase activity. Using Plackett–Burman design (Design-Expert software 9.0.3, Stat-Ease, Minneapolis, MN, USA), effect of eight independent factors i.e. maltose (3–4%), NH4NO3 (0.1–1.0%), Ca-phytate (0.1–2.0 mL/100 mL), mineral solution (0.5–5.0 mL/100 mL), pH (4.5–6.5), temperature (30–50 °C), CaCl2.2H2O (0.01–0.2%) and inoculum (140–400 × 104 spores) on phytase production by A. fumigatus was screened. With different combinations of low and high levels of variable-based different experiments were performed to create a Pareto chart to identify the positive factors. Using pareto chart, four factors influenced phytase production in submerged fermentation (SmF) i.e. inducer Ca-phytate, mineral solution, CaCl2.2H2O, and inoculum size based on which central composite design (CCD) experiment was designed. The second-order polynomial coefficients were analyzed by Design-Expert software 9.0.3 (Stat-Ease, Minneapolis, MN, USA) [19]. A total set of 30 experiments were performed. Phytase production using a statistical model was validated under a predicted set of conditions by performing experiments at shake flask level (50 mL).
2.5 Bench-Scale Production and Phytase Purification
Bench-scale production of phytase (1-L, BioFlow C-32 New Brunswick Scientific, USA) was carried out in statistically optimized medium (pH, 5.5) with final composition (w/v): maltose (3.62%), NH4NO3 (0.50%), CaCl2.2H2O (0.105%), mineral solution (2.75 mL, v/v), Ca-phytate (1.05%) and inoculum (39 × 105 spores) at 40 °C and agitation rate of 150 rpm for incubation of 72 h. To purify the phytase, the culture filtrate was subjected to salt precipitation using different saturation of ammonium sulphate (10–90%). The protein contents were evaluated through the Bradford method using bovine serum albumin [20]. The precipitates were extensively dialyzed against sodium-acetate (0.25 M, pH 5.5) buffer. The dialyzed proteins sample (~ 1.0–1.5 mg) was loaded on a manually packed Sephadex G-100 column [21]. The sodium-acetate (0.25 M; pH 5.5) was used as an elution buffer, and thereafter the obtained fractions were monitored spectrophotometrically. All the purification stages were processed at 4 °C. The polyacrylamide gel electrophoresis (PAGE) investigation with and without sodium-dodecyl sulfate (SDS) was evaluated to measure molecular weight through gel electrophoresis system (Genetix Biotech Asia Pvt. Ltd, India) using BLUeyepre-stained [22].
2.6 Biochemical Characterization and Stability of Purified Phytase
Initially, the phytase activity was measured in various buffers glycine–HCl (pH, 2.5–3.5), citrate (pH, 3.0–6.0), and sodium-acetate (pH, 4.0–5.5). The influence of buffer concentration (0.10–0.50 M) on the activity was evaluated at pH 5.5 using a standard assay. Similarly, activity at different temperatures (35–70 °C) was assayed in sodium-acetate buffer (0.30 M, pH 5.5). The kinetic parameters (Km and Vmax) were measured using Ca-phytate (50–500 µM), and their corresponding values were determined by Lineweaver–Burk plot at room temperature under assay conditions [23]. The effect of various metal ions additives and inhibitors (FeCl3, HgCl2, ZnSO4.7H2O, CoCl2, MgSO4.6H2O, CuSO4.5H2O, NaCl, Pb (NO3)2, AgNO3, BaCl2.2H2O, NaN3, CaCl2.2H2O, MnCl2.2H2O, urea, ethylenediaminetetraacetic acid (EDTA), dithiothreitol (DTT), phenylmethylsulphonyl fluoride (PMSF), and polyethylene glycol (PEG) on the phytase activity were tested at the concentration of 1 mM under assay conditions. The influence of several non-polar and polar organic solvents (ethanol, methanol, propanol, acetone, benzene, and 1,4-dioxan) on activity were measured by their addition of 10% (v/v) to the reaction mixture. The thermostability profile of phytase was noted at various temperatures (35–70 °C) up to 2 h of incubation under assay conditions.
2.7 Phytic Acid Degradation in Wheat Flour
To determine the applicability of purified phytase, dephytinization of wheat flour was tested. The reaction was primarily performed at a lab scale (30 mL) to determine the extent of degradation before scale-up. The substrate wheat flour (10 g) was acted upon by purified phytase (10 U, 65 °C, and 150 rpm). After 30 min, the supernatant was estimated for inorganic phosphate released during the degradation process. Separate enzyme controls were run simultaneously. To evaluate the process sustainability, the experiment was scaled up to 3-L reaction volume (7-L, Brunswick Scientific, USA) using 1 kg of wheat flour and 1000 U of the enzyme under similar conditions.
2.8 Instrumental Analysis
All spectrophotometric analysis was performed UV–VIS spectrophotometer (Lambda 12, Perkin Elmer, Massachusetts, USA). The Fourier transform infrared (FTIR) spectroscopy analysis was evaluated by Avatar 370 spectrometer, Thermo Nicolet, MA, USA [24]. Phytic acids analysis was performed by HPLC (Waters, Bangaluru, India) equipped with a C-18 (150 × 4.6 mm, 5 µm) Reverse phase Oyster ODS3 column (Merck, Germany) [25]. The orthophosphoric acid (25 mM) with methanol (1%, v/v) was used as the mobile phase (1 mL/min) and peaks were recorded at 190 nm. All the experiments were performed in triplicates unless otherwise stated and means values ± S.D. were presented.
3 Results and Discussion
3.1 Statistical Optimization of Phytase Production Medium
The optimization of medium components and process parameters was necessary to improve phytase production. The maximum phytase activity (1.64 U/mL) was observed after 72 h of cultivation with 10.1 mg/mL-dcw of fungal biomass in the medium (Fig. S1). The limitation of not accounting interactions among different variables during optimization by OVAT strategy could be overcome by using statistical designs. Therefore, the medium components (maltose, ammonium nitrate, calcium chloride, Ca-phytate, trace elements) and the other physiological parameters (pH, temperature, inoculum) screened and optimized previously were referred for further optimization for maximal phytase production [16].
3.1.1 Plackett–Burman Design
The set of 12-experiments was evaluated, and 0 to 2.13 U/mL changes in activity were noted. To note the significance of the tested variables on phytase production, the results of the Plackett–Burman design were employed to prepare a Pareto chart (Fig. S2). Out of eight selected independent variables, four variables i.e., Ca-phytate, mineral solution, CaCl2.2H2O, and inoculums have enhanced phytase production. The first-order polynomial equation was derived from ANOVA analysis to describe the influence of different variables on phytase production.
R1 = + 0.11—0.023*A—6.917E—003*B + 0.026*C + 0.058*D—0.016*F + 0.028*G + 0.041*H
R1 is Phytase activity (U/mL) and A, B, C, D, E, F, G, and H are the coded values of various-variables, i.e. A-maltose, B-ammonium nitrate, C-Ca-phytate, D-mineral solution, F-temperature, G-CaCl2.2H2O, and H-inoculum. There are several studies that showed the effect of maltose, inoculum age, peptone, initial pH, inoculum size, incubation temperature and incubation time on phytase production [4, 6, 11, 26,27,28]. The statistical optimization was positively contributed towards phytase production by Pediococcus acidilactici SMVDUDB2 and S. cerevisiae using the Plackett–Burman design [4, 27].
3.1.2 CCD Measurements
On the Plackett–Burman design-based results, the effect of variables Ca-phytate, mineral solution, CaCl2.2H2O, and inoculum on the production of phytase were examined. The set of 30-experiments were performed as suggested by the design expert software (Table 1). The model was validated by observing experimental values quite close to the predicted values by a design expert. The maximum enzyme production (2.13 U/mL) was observed with run order number 22 with observed positive contributors i.e. 2.75 mL mineral solution as supplementary trace elements with 1.05 mL Ca-phytate as substrate inducer along with 0.105 g CaCl2.2H2O with an initial inoculum of 390 spores/mL, which were further incubated at 40 °C for 3 days to carry out SmF. After variance analysis, the regression equation was derived as follows:
R1 = + 1.94 + 0.18*A + 0.080*B −0.018*C −0.020*D + 0.18*AB + 8.375E-003*AC −0.040*AD −9.000E-003*BC + 0.028*BD −0.019*CD −0.44*A2 −0.37*B2 −0.31C2 −0.34*D2
A, B, C, D are coded values for a different variable, where A-mineral solution, B-Ca-phytate, C- CaCl2.2H2O, and D-inoculum. The CCD showed a significant role to optimize all the positively contributing factors. Therefore, it can be potentially used for the optimization of key parameters in the bioprocesses, including phytase production [4, 29].
3.1.3 Analysis of Variance (ANOVA)
ANOVA for the CCD is represented in Table S1. The significance of the model is predicted by its P-value (< 0.0001) and thus it also determines its efficacy for optimization. The coefficient of determination (R2) was 0.9668, which shows 96.98% agility of the model. The quadratic process order was observed best and was used for subsequent measurements due to a small standard deviation (0.16), high R-squared (0.96), and low press (1.89). The quadratic regression model evaluation suggested that a correlation coefficient (0.96) close to 1 denotes a significant correlation between predicted and observed responses. However, several studies report significant production models with a range of coefficient of determination (R2) values for phytase production e.g. high R2 value (0.9807) for phytase production from A. niger NT7 to a moderate R2 value of 0.7778 for phytase production from Saccharomyces cerevisiae has been reported [27, 29]. A coefficient of variance (CV 21.1) lower value represented high reliability of performed experiments. The three-dimensional plots were created for regression analysis of CCD of pairwise combinations of four factors for the production of phytase (Fig. 1). The 3D surface (with its peak and shape) interprets the optimum concentration required for phytase production considering two interacting variables. Figures 1A-C suggest a positive interaction of mineral solution as a variable with other three positive contributors i.e. Ca-phytate, CaCl2.2H2O, and inoculum, respectively, with intermediate to sharp peaks reflecting the optimum concentration in each graph for maximal phytase production. The optimum levels of concentration determined by the model were represented near the centre of dome-shaped response surface plots in each graph. The phytase activity decreases as the graph deviates from the optimum levels of concentration which corresponds to low production of phytase at peripheral concentration levels. The decrease in activity is represented by the gradient red to green in each plot. The other positive variable calcium phytate when plotted with CaCl2.2H2O (Fig. 1D) and inoculum (Fig. 1E) to maximize phytase production shows the optimum levels during their interaction. Inoculum and CaCl2.2H2O were plotted (Fig. 1F) to show their interaction while keeping the optimal concentration of the other two variables constant.
Three-dimensional surface plots generated by CCD to observe the effect of four positive variables on Phytase activity (U/mL) is plotted on the y-axis against two independent variables at a time on the x-axis and z-axis respectively: A Mineral solution and Calcium chloride dihydrate B Ca-phytate and Mineral solution C Mineral solution and inoculum D Ca-phytate and Calcium chloride dihydrate E Inoculum and Ca-phytate F Calcium chloride dihydrate and inoculum
3.1.4 Model Validation
The CCD proposed a predicted phytase activity (1.94 U/mL) as a primary response to the predicted optimal concentration of four positive variables. The maximum phytase production (2.13 U/mL) was obtained by performing the experiments which showed actual values closely related to the predicted values measured through ANOVA.. The perturbation plot displayed the optimum-value for variables mineral solution 2.75 mL of the stock solution, CaCl2.2H2O 0.105%, inoculums 390 spores per 100 mL, Ca-phytate 1.05 mL of the stock solution (Fig. S3). The validity of the model was proved by point determination. It was found that actual values of CCD runs were closely related to the predicted values (Fig. S4). Here, the production of phytase was statistically optimized to 1.3-folds. Statistical optimization for determining interactions among media components and other physiochemical parameters is a holistic approach opted for fungal phytase production [27, 28, 30]. Both submerged and solid-state fermentation has been extensively reported using statistical optimization for phytase production. In SmF, A. niger phytase production was enhanced to 1.5-fold in a recent report using statistical optimization [31]. Kanti and Sudiana [32] reported phytase production from A. niger, Neurospora sitophila and Rhizophus oryzae under submerged fermentation, with activity varied from 6.5 to 11.1 U/mL. Phytase production from Aspergillus ficuum under SmF conditions was reported with 50% higher activity (3.45 U/mL) than its previous results [33]. In the case of solid-state fermentation, several substrates have been reported to produce phytase. A 2.5-fold enhancement has been reported from A. niger NT7 by utilizing the statistical approach [15].
3.2 Batch Fermentation in Submerged Bioreactor and Downstream Processing
Both fed-batch and batch fermentations have been largely used for producing biomass and enzymes [28, 34]. The present study demonstrates phytase production at 40 °C (2.14 U/mL) from A. fumigatus in a statistically optimized submerged medium (1-L). Enzyme production at the bench-scale was not affected during scale-up in batch fermentation [27]. Production of phytase at 28 °C (2.27 U/mL) from C. tropicalis was reported in a 10-L batch reactor at pH-6.5 [7]. Similarly, phytase production from S. cerevisiae in 10L fermentor has also been reported by the same group [27]. In case of recombinant Pichia anomala phytase, phytase production was done at 3L scale at 30 °C [5]. The enzyme produced in batch fermentation was recovered to approximately 25.5%. The crude enzyme (528 U) was subjected to ammonium sulfate precipitation (Fig. 2A). A. fumigatus was purified to homogeneity to approximately 26-folds on Sephadex G-100 column. The essence of phytase purification from A. fumigatus is given in the Table 2. Extracellular phytase from A. niger 7A-1 was reported with 5.39% protein recovery [35]. Phytase from Aspergillus aculeatus was recovered in a low amount of 5.1% [23], whereas overall 15% protein was recovered from A. fumigatus isolated from African giant snail (Achatina fulica) [36]. The specific activity of phytase from A. fumigatus was 287 U/mg, which corresponds to its hyperactivity against the substrate Ca-phytate. However, variability in specific activity of A. fumigatus phytase (0.23 U/mg) isolated from A. fulica was observed [36]. Molecular weight was determined using an 8% cross-linked polyacrylamide gel. The analyses of the gel run under denaturing conditions revealed a single band of ~ 62 kDa (Fig. 2B). However native PAGE had shown a band size of ~ 146 kDa (Fig. S5). These results of SDS-PAGE and Native-PAGE suggested that there can be a strong possibility of glycosylation in purified extracellular phytase secreted by A. fumigatus [37]. Therefore, FTIR analysis was performed to determine the presence of carbohydrate moieties in the native structure of purified phytase (Fig. 2C). Two peaks corresponding to characteristic absorption by beta-sheets of polypeptide and by sugar moieties of glycoprotein at 1637.1 and 1103.4 cm−1 were observed, respectively [38].
Purification and FTIR studies of A. fumigatus phytase: A Ammonium sulphate saturation on phytase precipitation from cell-free extract (Bar graph with red Y-axis corresponds to the phytase activity; Line graph with blue Y-axis represents the protein concentration), B SDS-PAGE analysis of purified phytase (Lane 1: Molecular Weight determination markers (kDa); and Lane 2: purified phytase), and C FTIR spectra of purified phytase for determination of carbohydrate moieties
3.3 Characteristics of Purified Phytase
3.3.1 Optimization of Kinetic Parameters
The hyperactive phytase showed an abroad pH activity profile from 2.5 to 6.0 in 0.25 M of buffers with an optimum value of 5.5 at 55 °C (Fig. 3A). An activity was improved 14% at optimum sodium-acetate buffer (pH, 5.5) concentration of 0.30 M (Fig. 3B). Similarly, the residual phytase activity was observed 43% higher at the optimum temperature of 65 °C [29] (Fig. 3C). Optimum temperature of the purified enzyme from A. niger was 56 °C [35]. To determine the metabolic fate of substrate, it is important to know the kinetics of purified enzymes. The kinetic parameters (Km and Vmax) of A. fumigatus phytase were determined by using different concentrations of substrate (Ca-phytate) varied from 50 to 500 µM at 55 °C. A reciprocal plot between 1/V and 1/[S] was used to calculate the substrate affinity (Km) and maximum velocity (Vmax) values which were noted 0.75 mM and 345 µmol/min/mg-protein, respectively (Fig. 3D). Thus, the value of Km and Vmax for A. fumigatus phytase corresponds to high affinity towards the substrate Ca-phytate, and Vmax is attained when all the phytase active sites are occupied for product formation. However, phytase from A. aculeatus APF1 showed variability with a low affinity towards the s>ubstrate with Km and Vmax values of 3.21 mM and 3.78 U/mg protein, respectively [23]. A high catalytic efficiency (Kcat/Km) of 476 min−1 mM−1 was observed for purified phytase. Overall, A. fumigatus phytase showed significantly higher Kcat/Km up to 800-fold as compared to other strains A. aculeatus APF1 [23], A. oryzae SBS50 [12], A. niger 7A-1 [35], and A. niger 11T53A9 (Table 3) [39].
3.3.2 Effect of Inhibitors Additives, Metal Ions, and Organic Solvents
Different metal ions, inhibitors, and solvents affect the residual activity of an enzyme to a different extent (Fig. 4A). Here, phytase from A. fumigatus was strongly inhibited by Hg2+, Ag2+, Pb2+, Cu2+, and Zn2+. Heavy metals such as Hg2+, Ag+, Pb2+ have powerful affinities for -SH groups [35]. For several enzymes, these metals are non-competitive inhibitors; however, Mg2+ decreased the purified phytase activity to a lesser extent (10%). Mg2+ was also reported to have an inhibitory effect on the A. niger NT7 phytase, on the other hand, phytase activity was slightly enhanced by Ca2+ ions which shows similarity with A. fumigatus phytase [15]. Several reports are suggesting the role of calcium ions in increasing stability by enhancing heat tolerance of phytase from other microbes [40, 41]. The presence of Ca2+ ion increased the thermostability of phytase with 100% residual activity when pre-incubated at 20–50 °C for 30 min of incubation [40]. PMSF which is a serine protease inhibitor inhibits 10% phytase activity in the present study. Phytase activity was strongly decreased by Fe2+, Mn2+, and Cu2+. In contrast, Co2+, Zn2+, Mg2+ had shown a positive effect in the case of alkaline phosphatase [42]. Zinc oxide nanocomposites have reportedly increased the thermostability and shelf-life of phytase [41]. Phytase activity was affected by various organic solvents. Some of the solvents showed little or no effect on phytase catalytic activity on the other hand some increased (hexane-110%) or completely stopped (phenol) its activity (Fig. 4B). In a similar manner phytase from A. niger had completely reserved its catalytic activity (112%) in hexane [43]. These results demonstrated that hydrophobic residues may not have a crucial role in the catalytic properties of the phytase enzyme.
3.3.3 Thermostability Profile
For the industrial application of most enzymes viz. phytase, thermostability is thought to be an important and functional criterion. A gradual decrease in the residual activity of purified phytase from the wild strain of A. fumigatus was noted with an increase in the temperature from 35 to 70 ºC (Fig. 4C). The phytase retained approximately 90% (after 20 min) at 70 ºC and more than 50% residual activity at 2 h of incubation. However, in the case of third generation phytase (Quantum, AB Enzymes, Germany) approximately 90% of activity at 70 ºC (after 30 min), was retained while 20% of activity was dropped in the case of wild-type [18]. The glycosylation of purified phytase may be the reason for its high-temperature stability [44].
3.4 Dephytinization of Wheat Flour
Wheat is one of the most common staple food/grains which is used immensely in both the food and feed industry in all its form. So, this is important to increase its nutritional value in all aspects. But, due to the pessimist effect of phytic acid in mineral absorption, the present examination was done to observe the extent of its degradation in wheat flour using A. fumigatus phytase. At a shaking flask level (30 mL), the purified phytase catalyzes the phytic acid (78.7%) present in the wheat flour (10 g) along with the associated liberation of inorganic phosphate (9.1 mg/g) within 30 min of reaction incubation (Table 4). Further, the reaction was scaled up to 100-folds (3-L), and a slightly lower degradation was noted to 72.5% in wheat flour. These results were validated by a significant decrease in peak area in the test (26.7%) as compares to control (100%) in HPLC measurements for phytic acid degradation in wheat flour (Fig. S6). This study is demonstrated using a significantly higher amount of wheat flour (1 kg) as compared to previous studies between 1.0 to 10 g [5, 45, 46]. Overall, phosphate release (8.94 mg/g) from wheat flour by A. fumigatus phytase was noted up to 13.8-folds higher as compared to fungal phytases from Humicola nigrescens [45], P. anomala [5], and Rhizopus oligosporus [46].
4 Conclusion
This study reports beneficial uses of statistical tools to enhance phytase production from thermotolerant A. fumigatus up to 3.3-fold as compared to unoptimized and OVAT production conditions under SmF. The purified phytase exhibited high specific activity for ca-phytate as substrate (~ 287 U/mg). The bioprocess parameters optimization for purified phytase showed better kinetics properties and higher stability at different temperatures and in various organic solvents. The hyperactive phytase was efficiently used for dephytinization of wheat flour at a 3-L scale. Overall, the extracellular phytases from thermotolerant A. fumigatus was founded a promising biocatalyst over Arpergillus strains. Further, hyperactivity of phytase for dephytinization of phytic acid can be harnessed for its application as a biocatalyst in the development of various agricultural and functional biotechnological processes in the future.
References
Meghwanshi GK, Kaur N, Verma S et al (2020) Enzymes for pharmaceutical and therapeutic applications. Biotechnol Appl Biochem 67:586–601. https://doi.org/10.1002/bab.1919
Vasudevan UM, Jaiswal AK, Krishna S et al (2019) Thermostable phytase in feed and fuel industries. Bioresour Technol 278:400–407. https://doi.org/10.1016/j.biortech.2019.01.065
Singh B, Boukhris I, Pragya X et al (2020) Contribution of microbial phytases to the improvement of plant growth and nutrition: a review. Pedosphere 30:295–313. https://doi.org/10.1016/S1002-0160(20)60010-8
Bhagat D, Raina N, Kumar A et al (2020) Probiotic properties of a phytase producing Pediococcus acidilactici strain SMVDUDB2 isolated from traditional fermented cheese product. Kalarei Sci Rep 10:1926. https://doi.org/10.1038/s41598-020-58676-2
Joshi S, Satyanarayana T (2015) Bioprocess for efficient production of recombinant Pichia anomala phytase and its applicability in dephytinizing chick feed and whole wheat flat Indian breads. J Ind Microbiol Biotechnol 42:1389–1400. https://doi.org/10.1007/s10295-015-1670-1
Shah PC, Kumar VR, Dastager SG et al (2017) Phytase production by Aspergillus niger NCIM 563 for a novel application to degrade organophosphorus pesticides. AMB Expr 7:66. https://doi.org/10.1186/s13568-017-0370-9
Puppala KR, Naik T, Shaik A et al (2018) Evaluation of Candida tropicalis (NCIM 3321) extracellular phytase having plant growth promoting potential and process development. Biocatal Agric Biotechnol 13:225–235. https://doi.org/10.1016/j.bcab.2017.12.013
Lamid M, Al-Arif A, Asmarani O et al (2018) Characterization of phytase enzymes as feed additive for poultry and feed. IOP Conf Ser Earth Environ Sci 137:012009. https://doi.org/10.1088/1755-1315/137/1/012009
Cao L, Wang W, Yang C et al (2007) Application of microbial phytase in fish feed. Enzyme Microb Technol 40:497–507. https://doi.org/10.1016/j.enzmictec.2007.01.007
Liu Z, Wang H, Wang X-E et al (2008) Effect of wheat pearling on flour phytase activity, phytic acid, iron, and zinc content. LWT- Food Sci Technol 41:521–527. https://doi.org/10.1016/j.lwt.2007.04.001
Jatuwong K, Kumla J, Suwannarach N et al (2020) Bioprocessing of agricultural residues as substrates and optimal conditions for phytase production of chestnut mushroom, Pholiota adiposa, in solid state fermentation. J Fungi 6:384. https://doi.org/10.3390/jof6040384
Sapna X, Singh B (2017) Purification and characterization of a protease-resistant phytase of Aspergillus oryzae SBS50 whose properties make it exceptionally useful as a feed supplement. Int J Biol Macromol 103:458–466. https://doi.org/10.1016/j.ijbiomac.2017.05.077
Bilal M, Wang Z, Cui J et al (2020) Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers – A drive towards greener and eco-friendlier biocatalytic systems. Sci Total Environ 722:137903. https://doi.org/10.1016/j.scitotenv.2020.137903
Sharma A, Satyanarayana T (2011) Optimization of medium components and cultural variables for enhanced production of acidic high maltose-forming and Ca2+-independent α-amylase by Bacillus acidicola. J Biosci Bioeng 111:550–553. https://doi.org/10.1016/j.jbiosc.2011.01.004
Kumari N, Bansal S (2021) Statistical modeling and optimization of microbial phytase production towards utilization as a feed supplement. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01672-x
Thakur N, Kumar P, Chand D (2017) Enhanced production of phytase from thermotolerant Aspergillus fumigatus isolated from rhizospheric zone of maize fields. J Innov Pharmaceut Biol Sci 4:114–120
Patel SKS, Kalia VC, Joo JB et al (2020) Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol 297:122433. https://doi.org/10.1016/j.biortech.2019.122433
Ushasree UM, Krishna S, Jalaja V et al (2017) Microbial phytase: impact of advances in genetic engineering in revolutionizing its properties and applications. Bioresour Technol 245:1790–1799. https://doi.org/10.1016/j.biortech.2017.05.060
Awad GEA, Helal MMI, Danial EN et al (2014) Optimization of phytase production by Penicillium purpurogenum GE1 under solid state fermentation by using Box-Behnken design. Saudi J Biol Sci 21:81–88. https://doi.org/10.1016/j.sjbs.2013.06.004
Patel SKS, Choi H, Lee J-K (2019) Multi-metal based inorganic–protein hybrid system for enzyme immobilization. ACS Sustainable Chem Eng 7:13633–13638. https://doi.org/10.1021/acssuschemeng.9b02583
Ornela PHD, Guimarães LHS (2020) Purification and characterization of an alkalistablephytase produced by Rhizopus microsporus var. microsporus in submerged fermentation. J Appl Biol 8:16–25. https://doi.org/10.7324/JABB.2020.80403
Pandey D, Patel SKS, Singh R et al (2019) Solvent-tolerant acyltransferase from Bacillus sp. APB-6: purification and characterization. Indian J Microbiol 59:500–507. https://doi.org/10.1007/s12088-019-00836-8
Saxena A, Verma M, Singh B et al (2020) Characteristics of an acidic phytase from Aspergillus aculeatus APF1 for dephytinization of biofortified wheat genotypes. Appl Biochem Biotechnol 191:679–694. https://doi.org/10.1007/s12010-019-03205-9
Patel SKS, Gupta RK, Kondaveeti S et al (2020) Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol 315:123791. https://doi.org/10.1016/j.biortech.2020.123791
Devi N, Patel SKS, Kumar P et al (2021) Bioprocess scale-up for acetohydroxamic acid production by hyperactive acyltransferase of immobilized Rhodococcus pyridinivorans. Catal Lett. https://doi.org/10.1007/s10562-021-03696-4
Jain J, Singh B (2017) Phytase production and development of an ideal dephytinization process for amelioration of food nutrition using microbial phytases. Appl Biochem Biotechnol 181(4):1485–1495. https://doi.org/10.1007/s12010-016-2297-z
Puppala KR, Kumar VR, Khire J et al (2019) Dephytinizing and probiotic potentials of Saccharomyces cerevisiae (NCIM 3662) strain for amelioration of nutritional quality of functional foods. Probiotics Antimicrob Proteins 11:604–617. https://doi.org/10.1007/s12602-018-9394-y
Makolomakwa M, Puri AK et al (2017) Thermo-acid-stable phytase-mediated enhancement of bioethanol production using Colocasia esculenta. Bioresour Technol 235:396–404. https://doi.org/10.1016/j.biortech.2017.03.157
Badoei-Dalfard A, Parhamfar M, Karami Z (2019) Characterization of a thermostable, acidic-phytase from Bacillus tequilensis Dm018; medium optimization by response surface methodology. Catal Lett 149(11):2961–2972. https://doi.org/10.1007/s10562-019-02881-w
Jatuwong K, Suwannarach N, Kumla J et al (2020) Bioprocess for production, characteristics, and biotechnological applications of fungal phytases. Front Microbiol 11:188. https://doi.org/10.3389/fmicb.2020.00188
Mahmood S, Shahid MG, Nadeem M et al (2021) Screening of phytate degrading fungi and optimization of culture conditions for phytase synthesis using agro-industrial by-products. Pak J Bot 53:763–770. https://doi.org/10.30848/PJB2021-2(12)
Kanti A, Sudiana IM (2018) Production of phytase, amylase and cellulase by Aspergillus, Rhizophus and Neurospora on mixed rice straw powder and soybean curd residue. IOP Conf Ser Earth Environ Sci 166:012010. https://doi.org/10.1088/1755-1315/166/1/012010
Coban HB, Demirci A (2015) Improved submerged Aspergillus ficuum phytase production in bench-top bioreactors by optimization of fermentation medium. Acta Aliment 44:549–560. https://doi.org/10.1556/066.2015.44.0027
Zhang C, Ma Y, Miao H et al (2020) Transcriptomic analysis of Pichia pastoris (Komagataella phaffii) GS115 during heterologous protein production using a high-cell-density fed-batch cultivation strategy. Front Microbiol 11:463. https://doi.org/10.3389/fmicb.2020.00463
Neira-Vielma AA, Aguilar CN, Ilyina A et al (2018) Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnol Rep 17:49–54. https://doi.org/10.1016/j.btre.2017.12.004
Sanni DM, Lawal OT, Enujiugha VN (2019) Purification and characterization of phytase from Aspergillus fumigatus isolated from African giant snail (Achatina fulica). Biocatal Agric Biotechnol 17:225–232. https://doi.org/10.1016/j.bcab.2018.11.017
Ajith S, Ghosh J, Shet D et al (2019) Partial purification and characterization of phytase from Aspergillus foetidus MTCC 11682. AMB Expr 9:3. https://doi.org/10.1186/s13568-018-0725-x
Derenne A, Derfoufi K-M, Cowper B et al (2020) FTIR spectroscopy as an analytical tool to compare glycosylation in therapeutic monoclonal antibodies. Anal Chim Acta 1112:62–71. https://doi.org/10.1016/j.aca.2020.03.038
Greiner R, Silva LGD, Couri S (2009) Purification and characterisation of an extracellular phytase from Aspergillus niger 11T53A9. Braz J Microbiol 40:795–807. https://doi.org/10.1590/S1517-838220090004000010
Roy PM, Datta S, Ghosh S (2017) A novel extracellular low-temperature active phytase from Bacillus aryabhattai RS1 with potential application in plant growth. Biotechnol Prog 33:633–641. https://doi.org/10.1002/btpr.2452
Rebello S, Anoopkumar AN, Puthur S et al (2018) Zinc oxide phytase nanocomposites as contributory tools to improved thermostability and shelflife. Bioresour Technol Rep 3:1–6. https://doi.org/10.1016/j.biteb.2018.05.007
Sreedevi S, Reddy BN (2013) Purification and biochemical characterization of phytase from newly isolated Bacillus subtilis C43. Adv BioTech 12:8. https://www.advancedbiotech.in/621.pdf
Vats P, Banerjee UC (2005) Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. J Ind Microbiol Biotechnol 32:141–147. https://doi.org/10.1007/s10295-005-0214-5
Nezhad NG, Rahman RNZRA, Normi YM et al (2020) Integrative structural and computational biology of phytases for the animal feed industry. Catalysts 10:844. https://doi.org/10.3390/catal10080844
Bala A, Jain J, Kumari A et al (2014) Production of an extracellular phytase from a thermophilic mould Humicola nigrescens in solid state fermentation and its application in dephytinization. Biocatal Agricult Biotechnol 3:259–264. https://doi.org/10.1016/j.bcab.2014.07.002
Suresh S, Radha KV (2015) Effect of a mixed substrate on phytase production by Rhizopus oligosporus MTCC 556 using solid state fermentation and determination of dephytinization activities in food grains. Food Sci Biotechnol 24:551–559. https://doi.org/10.1007/s10068-015-0072-5
Acknowledgements
The authors gratefully acknowledge Himachal Pradesh University, Summer Hill, Shimla, India for providing the financial assistance as a senior research fellowship (Biotech No. 1498) to N.T. and necessary facilities for the completion of this research work.
Funding
Himachal Pradesh University,Summer Hill,Shimla,India,(Biotech No. 1498)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thakur, N., Patel, S.K.S., Kumar, P. et al. Bioprocess for Hyperactive Thermotolerant Aspergillus fumigatus Phytase and its Application in Dephytinization of Wheat Flour. Catal Lett 152, 3220–3232 (2022). https://doi.org/10.1007/s10562-021-03886-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03886-0