Abstract
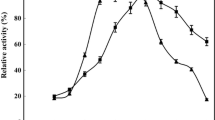
Recently, enzyme immobilization via self-assembly of magnetic metal–organic frameworks (mMOFs) has attracted attention for researchers. In this study, a Bacillus protease KHB3 was embedded into a magnetic metal organic framework. Temperature activity results shown that the free protease displayed the optimal activity at 40 °C, while the immobilized protease showed the highest activity at 60 °C. Besides, a 20% improvement in the activity of the immobilized form was found at 80 °C against the free protease. Furthermore, the Vmax of immobilized enzyme was 1.75 fold more than the free enzyme. Results exhibited that the immobilized protease showed 1.54 and 1.55 folds more activity than the control toward fibrin and gelatin, respectively. The immobilized protease reserved about twofold higher activity than the free protease after 21 days of storage. Results revealed that the immobilized enzyme showed 38% clot lysis, while the free protease showed 26% at a similar condition. The hydrolysis degree of the free protease and immobilized protease was obtained about 17 and 45% after 8 h incubation at 50 °C, respectively. Overall, our results show the high potential of these enzymes in the clot lysis capacity and construction of high-value compounds from protein hydrolysis of shrimp protein waste.
Graphic Abstract










Similar content being viewed by others
References
Wu S, Snajdrova R, Moore JC, Baldenius K, Bornscheuer UT (2021) Biocatalysis: enzymatic synthesis for industrial applications. Angew Chem Int Ed 60:88–119
Thompson MP, Thompson MP, Peñafiel I, Cosgrove SC, Turner NJ (2019) Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals. Org Process Res Dev 23:9–18
Datta S, Christena LR, Rajaram YRS (2013) Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3:1–9
Gimenez-Marques M, Hidalgo T, Serre C, Horcajada P (2016) Nanostructured metal organic frameworks and their bio-related applications. Coord Chem Rev 307:342–360
Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S, Lollar C, Wang X, Zhou HC (2017) Enzyme–MOF (metal–organic framework) composites. Chem Soc Rev 46:3386–3401
Nadar SS, Rathod VK (2018) Magnetic-metal organic framework (magnetic-MOF): a novel platform for enzyme immobilization and nanozyme applications. Int J Biol Macromol 120:2293–2302
Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P (2017) Metal–organic frameworks at the biointerface: synthetic strategies and applications. Acc Chem Res 50:1423–1432
Gkaniatsou E, Sicard C, Ricoux R, Mahy JP, Steunou N, Serre C (2017) Metal–organic frameworks: a novel host platform for enzymatic catalysis and detection. Mater Horiz 4:55–63
Nadar SS, Rathod VK (2019) One pot synthesis of α-amylase metal organic framework (MOF)-sponge via dip-coating technique. Int J Biol Macromol 138:1035–1043
Patil PD, Yadav GD (2018) Rapid in situ encapsulation of laccase into metal-organic framework support (ZIF-8) under biocompatible conditions. ChemistrySelect 3:4669–4675
Samui A, Chowdhuri AR, Mahto TK, Sahu SK (2016) Fabrication of a magnetic nanoparticle embedded NH2-MIL-88B MOF hybrid for highly efficient covalent immobilization of lipase. RSC Adv 6:66385–66393
Nadar SS, Rathod VK (2017) Facile synthesis of glucoamylase embedded metal-organic frameworks (glucoamylase-MOF) with enhanced stability. Int J Biol Macromol 95:511–519
Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, Doonan CJ, Falcaro P (2015) Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun 6:7240–7248
Junior OB, Bedran-Russo A, Flor JBS, Borges AFS, Ximenes VF, Frem RCG, Lisboa-Filho PN (2019) Encapsulation of collagenase within biomimetically mineralized metal–organic frameworks: designing biocomposites to prevent collagen degradation. New J Chem 43:1017
Pei X, Wu Y, Wang J, Chen Z, Liu W, Sub W, Liu F (2020) Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal–organic framework for efficient biocatalysis. Nanoscale 12:967
Rafieia R, Tangestaninejad S, Horcajad P, Moghadam M, Mirkhania V, Mohammadpoor-Baltork I, Kardanpour R, Zadehahmadi F (2018) Efficient biodiesel production using a lipase@ZIF-67 nanobioreactor. Chem Eng J 334:1233–1241
Zhao M, Zhang XM, Hui DC (2015) Rational synthesis of novel recyclable Fe3O4@MOF nanocomposites for enzymatic digestion. Chem Commun 51:8116–8119
Hou C, Wang Y, Ding Q, Jiang L, Li M, Zhu W, Pan D, Zhu H, Liu M (2015) Facile synthesis of enzyme-embedded magnetic metal–organic frameworks as a reusable mimic multi-enzyme system: mimetic peroxidase properties and colorimetric sensor. Nanoscale 7:18770
Lin C, Xu K, Zheng R, Zheng Y (2019) Immobilization of amidase into a magnetic hierarchically porous metal–organic framework for efficient biocatalysis. Chem Commun 55:5697
Wu E, Li Y, Huang Q, Yang Z, Wei A, Hu Q (2019) Laccase immobilization on amino-functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere 233:327–335
Cao SL, Xu H, Lai LH, Gu WM, Xu P, Xiong J, Yin H, Li XH, Ma YZ, Zhou J, Zong MH, Lou WY (2017) Magnetic ZIF-8/cellulose/Fe3O4 nanocomposite: preparation, characterization, and enzyme immobilization. Bioresour Bioprocess 4:56–64
Sharma M, Gat Y, Arya S, Kumar V, Panghal A, Kumar A (2019) A review on microbial alkaline protease: an essential tool for various industrial approaches. Ind Biotechnol 15(2):69–78
Peng Y, Yang X, Zhang Y (2005) Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol 69:126–132
Krishnamurthy A, Belur PD (2018) A novel fibrinolytic serine metalloprotease from the marine Serratia marcescens subsp. sakuensis: purification and characterization. Int J Biol Macromol 112:110–118
Peng Y, Huang Q, Zhang RH, Zhang YZ (2003) Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional chinese soybean food. Comp Biochem Physiol B Biochem Mol Biol 134:45–52
Chandramohan M, Chang YY, Beatrice PHK, Ponnaiah P, Narendrakumar G, Samrot AV (2019) Production, characterization and optimization of fibrinolytic protease from Bacillus pseudomycoides strain MA02 isolated from poultry slaughter house soils. Biocatal Agric Biotechnol 22:101371–101378
Wang CT, Ji BP, Li B, Nout R, Li PL, Ji H, Chen LF (2006) Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from chinese traditional Douchi. J Ind Microbiol Biotechnol 33:750–758
Kim HC, Choi BS, Sapkota K, Kim S, Lee HJ, Yoo JC, Kim SJ (2011) Purification and characterization of a novel, highly potent fibrinolytic enzyme from Paecilomyces tenuipes. Process Biochem 46:1545–1553
Avhad DN, Vanjari SS, Rathod VK (2013) A novel fibrinolytic enzyme from Bacillus sphaericus MTCC 3672: optimization and purification studies. Int J Curr Microbiol Appl Sci 1:1–13
Taneja K, Bajaj BK, Kumar S, Dilbaghi N (2017) Production, purification and characterization of fibrinolytic enzyme from Serratia sp. KG-2–1 using optimized media. 3 Biotech 7:184–199
Yang H, Liu Y, Ning Y, Wang C, Zhang X, Weng P, Wu Z (2020) Characterization of an intracellular alkaline serine protease from Bacillus velezensis SW5 with fibrinolytic activity. Curr Microbiol 77:1610–1621
Sahoo A, Mahanty B, Daverey A, Dutt K (2020) Nattokinase production from Bacillus subtilis using cheese whey: effect of nitrogen supplementation and dynamic modelling. J Water Process Eng 38:101533
Wang C, Du M, Zheng D, Kong F, Zu G, Feng Y (2009) Purification and characterization of Nattokinase from Bacillus subtilis natto B-12. J Agric Food Chem 57:9722–9729
Simkhada JR, Maner P, Cho SS, Ydoo JC (2010) A novel fibrinolytic protease from Streptomyces sp. CS684. Process Biochem 45:88–93
Khursade PS, Galande SH, Krishna P, Prakasham S, Prakashama RS (2019) Stenotrophomonas maltophilia Gd2: a potential and novel isolate for fibrinolytic enzyme production. Saudi J Biol Sci 26(7):1567–1575
Kumar SS, Haridas M, Abdulhameed S (2020) A novel fibrinolytic enzyme from marine Pseudomonas aeruginosa KU1 and its rapid in vivo thrombolysis with little haemolysis. Int J Biol Macromol 162:470–479
Badoei-dalfard A, Karami Z (2013) Screening and isolation of an organic solvent tolerant-protease from Bacillus sp. JER02: activity optimization by response surface methodology. J Mol Catal B Enzyme 89:15–23
Badoei-dalfard A, Karami Z, Ravan H (2015) Purification and characterization of a thermo- and organic solvent-tolerant alkaline protease from Bacillus sp. JER02. Prep Biochem Biotechnol 45:128–143
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685
Hashemabadi M, Badoei-Dalfard A (2019) Fabrication of magnetic CLEA-protease nanocomposite: high progression in biotechnology and protein waste management. Catal Lett 149(7):1753–1764
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kotb E (2015) Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK 07 isolated from Kishk, a traditional Egyptian fermented food. Appl Biochem Microbiol 51:34–43
Kim W, Choi K, Kim Y, Park H, Choi J, Lee Y, Oh H, Kwon I, Lee S (1996) Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11–4 screened from Chungkook-Jang. Appl Environ Microbiol 62:2482–2488
Manni L, Ghorbel-Bellaaj O, Jellouli K, Younes I, Nasri M (2010) Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from Shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl Biochem Biotechnol 162:345–357
Badoei-dalfard A, Khankari S, Karami Z (2020) One-pot synthesis and biochemical characterization of protease metal organic framework (protease@MOF) and its application on the hydrolysis of fish protein-waste. Colloids Surf B 196:111318
Choi JH, Kim JE, Kim S, Yoon J, Park DH, Shin HJ, Leeb HJ, Cho SS (2017) Purification and partial characterization of a low molecular fibrinolytic serine metalloprotease C142 from the culture supernatant of Bacillus subtilis C142. Int J Biol Macromol 104:724–731
Mahajan PM, Nayak S, Lele SS (2012) Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: media optimization, purification and characterization. J Biosci Bioeng 113(3):307–314
Kim SH, Choi NS (2000) Purification and characterization of subtilisin DJ-4secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Biosci Biotechnol Biochem 64:1722–1725
Wu X, Yang C, Ge J (2017) Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour Bioprocess 4:24–28
Nadar SS, Rathod VK (2018) Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzyme Microb Technol 108:11–20
Zhu Q, Zhuang W, Chen Y, Wang Z, Hernandez BV, Wu J, Yang P, Liu D, Zhu C, Ying H, Zhu Z (2018) Nano-biocatalysts of Cyt c@ZIF-8/GO composites with high recyclability via a de novo approach. ACS Appl Mater Interfaces 10(18):16066–16076
Shakir H, Mahmood R, Irfan M, Deeba F, Javed I, Qazi J (2019) Protease production from Bacillus safensis in submerged fermentation using response surface methodology. Rev Mex Ing Quim 18(1):375–385
Wang L, Guan S, Bai J, Jiang Y, Song Y, Zheng X, Gao J (2020) Enzyme immobilized in BioMOFs: facile synthesis and improved catalytic performance. Int J Biol Macromol 144:19–28
Li Z, Ding Y, Li S, Jiang Y, Liu Z, Ge J (2016) Highly active, stable and self-antimicrobial enzyme catalysts prepared by biomimetic mineralization of copper hydroxysulfate. Nanoscale 8:17440–17445
Reddy MR, Reddy KS, Chouhan YR, Bee H, Reddy G (2017) Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresour Technol 243:254–263
Sanghvi G, Patel H, Vaishnav D, Oza T, Dave G, Kunjadia P, Sheth N (2016) A novel alkaline keratinase from Bacillus subtilis DP1 with potential utility in cosmetic formulation. Int J Biol Macromol 87:256–262
Khankari S, Badoei-Dalfard A, Karami Z (2021) Cross-linked enzyme aggregates of fibrinolytic protease BC1 immobilized on magnetic chitosan nanoparticles (CLEAs-Fib-mChi): synthesis, purification, and characterization. Appl Biochem Biotechnol 193(6):2004–2027
Rahman RA, Zaliha RN, Geok LP, Basri M, Salleh AB (2006) An organic solvent-stable alkaline protease from Pseudomonas aeruginosa strain K: enzyme purification and characterization. Enzyme Microb Technol 39:1484–1491
Li Q, Yi L, Marek P, Iverson BL (2013) Commercial proteases: present and future. FEBS Lett 587(8):1155–1163
Sinha R, Khare S (2015) Immobilization of halophilic Bacillus sp. EMB9 protease on functionalized silica nanoparticles and application in whey protein hydrolysis. Bioprocess Biosyst Eng 38:739–748
Wu RB, Wu CL, Liu D, Yang XH, Huang JF, Zhang J, Liao B, He HL (2018) Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem 248:346–352
Acknowledgements
The authors express their gratitude to the Research Council of the Shahid Bahonar University of Kerman, Kerman (Iran), for financial support during this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karami, Z., Tamri, H. & Badoei-dalfard, A. Immobilization of Protease KHB3 onto Magnetic Metal–Organic Frameworks and Investigation of Its Biotechnological Applications. Catal Lett 152, 2256–2269 (2022). https://doi.org/10.1007/s10562-021-03808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03808-0