Abstract
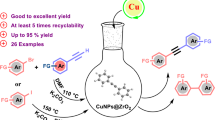
The copper particles, supported on Al2O3 were for the first time used as heterogeneous, highly active, easily available, and recyclable copper catalyst of direct carboxylation of various terminal alkynes with CO2 (2 bar) in the presence of cesium carbonate, allowing for the synthesis of alkyl 2-alkynoates. The catalyst contains large-scale CuNPs covered by oxide layer and less prone to erosion, resulting in its high selectivity and efficiency unusual for the particles of this size.
Graphical Abstract












Similar content being viewed by others
References
Aresta M (2010) Carbon dioxide as chemical feedstock. Wiley-VCH, Weinheim, doi:10.1002/9783527629916
Liu Q, Wu L, Jackstell R, Beller M (2015) Using carbon dioxide as a building block in organic synthesis. Nat Commun 6:5933–5947. doi:10.1038/ncomms6933
Williams CM, Johnson JB, Rovis T (2008) Nickel-catalyzed reductive carboxylation of styrenes using CO2. J Am Chem Soc 130:14936–14937. doi:10.1021/ja8062925
Greenhalgh MD, Thomas SP (2012) Iron-catalyzed highly regioselective synthesis of α-aryl carboxylic acids from styrene derivatives and CO2. J Am Chem Soc 134:11900–11903. doi:10.1021/ja3045053
Ostapowicz TG, Schmitz M, Krystof M, Klankermayer J, Leitner W (2013) Carbon dioxide as a C1 building block for the formation of carboxylic acids by formal catalytic hydrocarboxylation. Angew Chem Int Ed 52:12119–12123. doi:10.1002/anie.201304529
Tsuda T, Morikawa S, Sumiya R, Saegusa T (1988) Nickel(0)-catalyzed cycloaddition of diynes and carbon dioxide to bicyclic α-pyrones. J Org Chem 53:3140–3145. doi:10.1021/jo00249a003
Tekavec TN, Arif AM, Louie J (2004) Regioselectivity in nickel(0) catalyzed cycloadditions of carbon dioxide with diynes. Tetrahedron 60:7431–7437. doi:10.1016/j.tet.2004.06.025
Fujihara T, Xu T, Semba K, Terao J, Tsuji Y (2011) Copper-catalyzed hydrocarboxylation of alkynes using carbon dioxide and hydrosilanes. Angew Chem Int Ed 50:523–527. doi:10.1002/anie.201006292
Takimoto M, Mori M (2001) Cross-coupling reaction of oxo-π-allylnickel complex generated from 1,3-diene under an atmosphere of carbon dioxide. J Am Chem Soc 123:2895–2896. doi:10.1021/ja004004f
Takimoto M, Mori M (2002) Novel catalytic CO2 incorporation reaction: nickel-catalyzed regio- and stereoselective ring-closing carboxylation of bis-1,3-dienes. J Am Chem Soc 124:10008–10009. doi:10.1021/ja026620c
Takaya J, Iwasawa N (2008) Hydrocarboxylation of allenes with CO2 catalyzed by silyl pincer-type palladium complex. J Am Chem Soc 130:15254–15255. doi:10.1021/ja806677w
Osakada K, Sato R, Yamamoto T (1994) Nickel-complex-promoted carboxylation of haloarenes involving insertion of CO2 into NiII-C bonds. Organometallics 13:4645–4647. doi:10.1021/om00023a078
Correa A, Martin R (2009) Palladium-catalyzed direct carboxylation of aryl bromides with carbon dioxide. J Am Chem Soc 131:15974–15975. doi:10.1021/ja905264a
Gaillard S, Cazin CSJ, Nolan SP (2012) N-heterocyclic carbene gold(I) and copper(I) complexes in C-H bond activation. Acc Chem Res 45:778–787. doi:10.1021/ar200188f
Zang L, Cheng J, Ohishi T, Hou Z (2010) Copper-catalyzed direct carboxylation of C–H bonds with carbon dioxide. Angew Chem Int Ed 49:8670–8673. doi:10.1002/anie.201003995
Correa A, Martin R (2009) Metal-catalyzed carboxylation of organometallic reagents with carbon dioxide. Angew Chem Int Ed 48:6201–6204. doi:10.1002/anie.200900667
Federsel C, Jackstell R, Beller M (2010) State-of-the-art catalysts for hydrogenation of carbon dioxide. Angew Chem Int Ed 49:6254–6257. doi:10.1002/anie.201000533
Tsuji Y, Fujihara T (2012) Carbon dioxide as a carbon source in organic transformation: carbon–carbon bond forming reactions by transition-metal catalysts. Chem Commun 48:9956–9964. doi:10.1039/C2CC33848C
Manjolinho F, Arndt M, Gooßen K, Gooßen LJ (2012) Catalytic C–H carboxylation of terminal alkynes with carbon dioxide. ACS Catal 2:2014–2021. doi:10.1021/cs300448v
Yu D, Tan MX, Zhang Y (2012) Carboxylation of terminal alkynes with carbon dioxide catalyzed by poly(N-heterocyclic carbene)-supported silver nanoparticles. Adv Synth Catal 354:969–974. doi:10.1002/adsc.201100934
Li S, Sun J, Zhang Z, Xie R, Fang X, Zhou M (2016) Carboxylation of terminal alkynes with CO2 catalyzed by novel silver N-heterocyclic carbene complexes. Dalton Trans 45:10577–10584. doi:10.1039/C6DT01746K
Yuan Y, Chen C, Zeng C, Mousavi B, Chaemchuen S, Verpoort F (2017) Carboxylation of terminal alkynes with carbon dioxide catalyzed by an in situ Ag2O/N-heterocyclic carbene precursor system. ChemCatChem. doi:10.1002/cctc.201601379. Accepted manuscript online: 5 Dec 2016
Tsuda T, Ueda K, Saegusa T (1974) Carbon dioxide insertion into organocopper and organosilver compounds. JCS. Chem Commun 10:380–381. doi:10.1039/C39740000380
Tsuda T, Chujo Y, Saegusa T (1975) Reversible carbon dioxide fixation by organocopper complexes. Chem Commun 24:963–964. doi:10.1039/C39750000963
Fukue Y, Oi S, Inoue Y (1994) Direct synthesis of alkyl 2-alkynoates from alk-l-ynes, CO2, and bromoalkanes catalysed by copper(I) or silver(I) salt. Chem Commun 18:2091. doi:10.1039/C39940002091
Yu B, Diao Z-F, Guo C-X, Zhong C-L, He L-N, Zhao Y-N, Song Q-W, Liu A-H, Wang J-Q (2013) Carboxylation of terminal alkynes at ambient CO2 pressure in ethylene carbonate. Green Chem 15:2401–2407. doi:10.1039/C3GC40896E
Li F-W, Suo Q-L, Hong H-L, Zhu N, Wang Y-Q, Han L-M (2014) DBU and copper(I) mediated carboxylation of terminal alkynes using supercritical CO2 as a reactant and solvent. Tetrahedron Lett 55:3878–3880. doi:10.1016/j.tetlet.2014.05.024
Gooßen LJ, Rodríguez N, Manjolinho F, Lange PP (2010) Synthesis of propiolic acids via copper-catalyzed insertion of carbon dioxide into the C–H bond of terminal alkynes. Adv Synth Catal 352:2913–2917. doi:10.1002/adsc.201000564
Inamoto K, Asano N, Kobayashi K, Yonemoto M, Kondo Y (2012) A copper-based catalytic system for carboxylation of terminal alkynes: synthesis of alkyl 2-alkynoates. Org Biomol Chem 10:1514–1516. doi:10.1039/C2OB06884B
Yu D, Zhang Y (2010) Copper- and copper-N-heterocyclic carbene-catalyzed C–H activating carboxylation of terminal alkynes with CO2 at ambient conditions. PNAS 107:20184–20189. doi:10.1073/pnas.1010962107
Xie J-N, Yu B, Zhou Z-H, Fu H-C, Wanga N, He L-N (2015) Copper(I)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure. Tetrahedron Lett 56:7059–7062. doi:10.1016/j.tetlet.2015.11.028
Yu B, Li W, He L-N (2015) Copper(I)@carbon-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure. ACS Catal 5:3940–3944. doi:10.1021/acscatal.5b00764
Tao F, Spivey J, Ostafin A (2014) Metal nanoparticles for catalysis: advances and applications. Royal Society of Chemistry, London. doi:10.1039/9781782621034
Amalia AJ, Rana RK (2009) Stabilisation of Pd(0) on surface functionalised Fe3O4 nanoparticles: magnetically recoverable and stable recyclable catalyst for hydrogenation and Suzuki–Miyaura reactions. Green Chem 11:1781–1786. doi:10.1039/B916261P
Choudary BM, Madhi S, Chowdari NS, Kantam ML, Sreedhar B (2002) Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J Am Chem Soc 124:14127–14136. doi:10.1021/ja026975w
Okumura K, Tomiyama T, Okuda S, Yoshida H, Niwa M (2010) Origin of the excellent catalytic activity of Pd loaded on ultra-stable Y zeolites in Suzuki–Miyaura reactions. J Catal 273:156–166. doi:10.1016/j.jcat.2010.05.009
Dhara K, Sarkar K., Srimani D, Saha SK, Chattopadhyaye P, Bhaumik A (2010) A new functionalized mesoporous matrix supported Pd(II)-Schiff base complex: an efficient catalyst for the Suzuki–Miyaura coupling reaction. Dalton Trans 39:6395–6402. doi:10.1039/C003142A
Woo H, Mohan B, Heo E, Park JC, Song H, Park KH (2013) CuO hollow nanosphere-catalyzed cross-coupling of aryl iodides with thiols. Nanoscale Res Lett 8:390. doi:10.1186/1556-276X-8-390
Rout L, Sen TK, Punniyamurthy T (2007) Efficient CuO-nanoparticle-catalyzed C-S cross-coupling of thiols with iodobenzene. Angew Chem Int Ed 46:5583–5586. doi:10.1002/anie.200701282
Kidwai M, Bhardwaj S, Poddar R (2010) C-Arylation reactions catalyzed by CuO-nanoparticles under ligand free conditions. Beilstein. J Org Chem 6:35. doi:10.3762/bjoc.6.35
Azeredo JB, Godoi M, Schwab RS, Botteselle GV, Braga AL (2013) Synthesis of thiol esters using nano CuO/ionic liquid as an eco-friendly reductive system under microwave irradiation. Eur J Org Chem 2013:5188–5194. doi:10.1002/ejoc.201300295
Ahmady AZ, Keshavarz M, Kardani M, Mohtasham N (2015) CuO nanoparticles as an efficient catalyst for the synthesis of flavanones. Orient J Chem 31:1841–1846. doi:10.13005/ojc/310369
Sun S, Zhang X, Sun Y, Zhang J, Yang S, Song X, Yang Z (2013) A facile strategy for the synthesis of hierarchical CuO nanourchins and their application as non-enzymatic glucose sensors. RSC Adv 3:13712–13719. doi:10.1039/C3RA41098F
Alonso F, Moglie Y, Radivoy G, Yus M (2011) Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org Biomol Chem 9:6385–6395. doi:10.1039/C1OB05735A
Alonso F, Moglie Y, Radivoy G (2015) Copper nanoparticles in click chemistry. Acc Chem Res 48:2516–2528. doi:10.1021/acs.accounts.5b00293
Diaz-Droguetta DE, Espinoza R, Fuenzalida VM (2011) Appl Surf Sci 257:4597–4602. doi:10.1016/j.apsusc.2010.12.082
Sun S (2015) Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 7:10850–10882. doi:10.1039/C5NR02178B
Chen W, Fan Z, Lai Z (2013) Synthesis of core–shell heterostructured Cu/Cu2O nanowires monitored by in situ XRD as efficient visible-light photocatalysts. J Mater Chem A 1:13862–13868. doi:10.1039/C3TA13413J
Ai Z, Zhang L, Lee S, Ho W (2009) Interfacial hydrothermal synthesis of Cu@Cu2O core-shell microspheres with enhanced visible-light-driven photocatalytic activity. J Phys Chem C 113:20896–20902. doi:10.1021/jp9083647
Bendavid LI, Carter EA (2013) CO2 adsorption on Cu2O(111): A DFT + U and DFT-D study. J Phys Chem C 117:26048–26059. doi:10.1021/jp407468t
Mishra AK, Roldan A, De Leeuw NH (2016) A density functional theory study of the adsorption behaviour of CO2 on Cu2O surfaces. J Chem Phys 145:044709. doi:10.1063/1.4958804
Selivanova AV, Tyurin VS, Beletskaya IP (2014) Palladium nanoparticles supported on poly(N-vinylimidazole-co-N-vinylcaprolactam) as an effective recyclable catalyst for the Suzuki reaction. ChemPlusChem 79:1278–1283. doi:10.1002/cplu.201402111
Sigeev AS, Peregudov AS, Cheprakov AV, Beletskaya IP (2015) The Palladium slow-release pre-catalysts and nanoparticles in the “phosphine-free” Mizoroki–Heck and Suzuki–Miyaura reactions. Adv Synth Catal 357:417–429. doi:10.1002/adsc.201400562
Bondarenko GN, Beletskaya IP (2015) Activated carbon as an efficient support for gold nanoparticles that catalyze the hydrogenation of nitro compounds with molecular hydrogen. Mendeleev Commun 25:443–445. doi:10.1016/j.mencom.2015.11.015
Fripiat JJ, Bosmans H, Rouxet PG (1967) Proton mobility in solids. I. Hydrogenic vibration modes and proton delocalization in boehmite. J Phys Chem 71:1097–1111. doi:10.1021/j100863a048
Yu V, Zhang Y (2011) The direct carboxylation of terminal alkynes with carbon dioxide. Green Chem 13:1275–1279. doi:10.1039/C0GC00819B
Zhao D, Gao C, Su X, He Y, You J, Xue Y (2010) Copper-catalyzed decarboxylative cross-coupling of alkynyl carboxylic acids with aryl halides. Chem Commun 46:9049–9051. doi:10.1039/C0CC03772A
Roth GJ, Liepold B, Müller SG, Bestmann HJ (2004) Further Improvements of the synthesis of alkynes from aldehydes. Synthesis 2004:59–62. doi:10.1055/s-2003-44346
Kachala VV, Khemchyan LL, Kashin AS AS, Orlov NV, Grachev AA, Zalesskiy SS, Ananikov VP (2013) Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ Chem Rev 82:648–685. doi:10.1070/RC2013v082n07ABEH004413
Kashin AS, Ananikov VP (2011) A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ Chem Bull Int Ed 60:2602–2607. doi:10.1007/s11172-011-0399-x
Acknowledgements
This work was supported by Russian Science Foundation (Grant No. 14-33-00001). Electron microscopy, XPS and X-ray characterization were performed in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bondarenko, G.N., Dvurechenskaya, E.G., Magommedov, E.S. et al. Copper(0) Nanoparticles Supported on Al2O3 as Catalyst for Carboxylation of Terminal Alkynes. Catal Lett 147, 2570–2580 (2017). https://doi.org/10.1007/s10562-017-2127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2127-0