Abstract
This review reports the first discoveries in homogeneous catalysis in Rennes. Ruthenium(II) catalysts are shown to promote the activation of terminal alkynes and the synthesis of vinylcarbamates on addition of CO2 and amines. The ruthenium–vinylidene are the key catalytic species. Applications to the addition of carboxylic acids to alkynes have opened the access to various enolesters and dienylesters via controlled catalyzed Markovnikov or anti-Markonikov additions. The catalytic synthesis of α-methylene cyclic carbonates and oxazolidinones from propargylic alcohols and CO2 is presented and shown to lead to allene derivatives via palladium catalysis. Cp*RuX(COD) catalyst promote the head to tail oxidative couplings of alkene with alkyne, and the synthesis of functional dienes via 1,4 addition of H-nucleophiles to the biscarbene-ruthenium intermediate arising from head to head couplings of alkynes, whereas Cp*RuL2 + catalysts favour activation of alkynes via vinylidene intermediate.
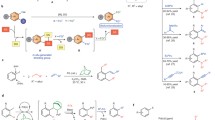
Graphical Abstract
This historical review presents the first steps in homogeneous catalysis in Rennes starting in 1985 with ruthenium(II) catalysts promoting the activation of terminal alkynes via ruthenium–vinylidene or oxidative couplings for the synthesis of CO2 derivatives: vinylcarbamates, carbonates or oxazolidinones, enolesters and functional dienes.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Homogeneous catalysis in Rennes started almost 30 years ago when we explored with an industrial company some ways to use CO2 as a phosgene substitute. Our first steps were guided by the aim to discover selective combinations of simple accessible substrates, avoiding protecting groups and toxic reagents. This led us to discover an atom economy catalytic synthesis of vinylcarbamates and oxoalkylcarbamates from terminal alkynes, CO2 and secondary amines with ruthenium catalysts. This catalytic synthesis was applied to the selective Markovnikov or anti-Markovnikov addition of carboxylic acids to alkynes affording enol esters and 1,3-dienol esters. These new catalytic transformations of alkynes questioned the involvement of vinylidene–ruthenium in catalysis which was proved, developed in Rennes and led to a new chemistry of the stoichiometric activation of terminal alkynes and preparation of vinylidene-, carbene-, allenylidene- cumulenylidene- and indenylidene-ruthenium complexes and to the chemistry of carbon- rich organometallic complexes.
The direct incorporation of CO2 as a functional group was developed via the organocatalytic activation with phosphine of propargyl alcohol derivatives to produce a variety of unsaturated cyclic carbonates which became the straightforward precursors of oxazolidinones, allenols, unsaturated ketones or heterocycles.
In parallel to the activation of alkynes to generate active vinylidene intermediates our Rennes team explored the selective transformation of alkynes by oxidative coupling with electron-rich ruthenium(II) catalysts, derivatives of Cp*RuXL2 complexes, (Cp* = C5Me5), which was revealed first to be an excellent route to various functional dienes.
The above innovations in catalytic syntheses involving alkynes, by rising questions about mechanisms greatly contributed to the improvement of ruthenium organometallic chemistry.
At the time CO2 chemistry is again the target of investigations to take profit of this stable, simple and abundant small molecule, and that selective catalytic transformations of alkynes is still contributing to both organic synthesis and molecular materials science, it might be of interest to tell this story on the first 10 years of catalysis in Rennes starting in 1985.
2 Ruthenium-Catalyzed Synthesis of Vinyl-carbamates
In cooperation with the French company SNPE, we were interested in finding solutions to replace toxic phosgene derivatives in some industrial processes. One target was the production of vinylcarbamate, a varnish precursor, previously produced from R2NCOCl and a metal-enolate derivative. As a secondary amine and carbon dioxide are in equilibrium with ammonium carbamate we explored the activation of alkynes with potential electrophilic ruthenium catalysts to favour nucleophilic addition of carbamates to readily available alkynes. This work was initiated by a post-doctor fellow from Tsukuba, Yoshiyuki Sasaki [1], and a Rennes PhD student, Roger Mahé [2].
Our first attempt with Ru3(CO)12 as a catalyst for activation of phenylacetylene led to moderate yields of Z and E-PhCH=CH–O2CNEt2 [1]. The use of more electrophilic ruthenium(II) catalysts drastically improved the reaction of secondary amine with alkyne under 50 bar of CO2. RuCl2(py)2(nbd), (py = pyridine, nbd = norbonadiene) and RuCl2(PMe3)(C6Me6) catalysts led to the formation of a variety of vinylcarbamates (Scheme 1) [2]. The Z isomer was always the major isomer and the use of RuCl3.H2O also led to the Z > E isomers of carbamates PhCH=CH–O2CNR2.
The only anti-Markovnikov addition products were observed. In addition the reaction was restricted to terminal alkynes. We could already propose [2] that this catalytic reaction proceeded via the in situ formation of a ruthenium(II)-vinylidene species [Ru(II)=C=CHR] for which the coordinated carbon was expected to be electrophilic. A metal-vinylidene species in catalysis was proposed for the first time suggesting that ruthenium(II)-vinylidenes are more active toward nucleophiles than ruthenium(II)-η2-alkyne intermediates.
This view was realistic as some metal-vinylidene complexes were already known. The first well-recognized vinylidene complex MCl(C5H5)(L)2(C=C(CN)2) (M=Mo, W) was obtained by R. B. King by chloride migration of an α-chloroalkenyl ligand [3]. Cationic (M=C=CHR+ vinylidene complexes were previously made by protonation of metal-alkynyl derivatives [4–7]. An early established method of preparation of metal vinylidene consisted also in the addition of an electrophilic group at the β-carbon of alkynyl-metal complexes [8–10]. The most straightforward method appeared to be the direct activation via 1,2-hydrogen migration of η2-coordinated terminal alkynes [11]. Only a few examples were shown later to proceed via oxidative addition of alkyne C–H bond to metal center followed by 1,3-hydride migration [12]. Very soon metal-vinylidene reviews appeared [13–15] pointing out the methods of synthesis and giving evidence for the electrophilic character of the carbon linked to the metal which favoured nucleophilic addition on that carbon atom.
Le Bozec [16] demonstrated that the catalyst precursors RuCl2(PR3)(arene), used for catalytic vinylcarbamate synthesis, under very mild conditions, activate terminal alkynes via a vinylidene–ruthenium(II) intermediates to generate a variety of ruthenium–carbene complexes, simply on addition of alcohols [16]. Consequently the proposed mechanism as soon as 1986, based on vinylidene–ruthenium intermediate (Scheme 1) [2] was realistic and only the last step, the protonation of the Ru site or of the Ru–C bond could not be observed since.
This catalytic synthesis of vinylcarbamates was boosted when Christian Bruneau, a CNRS fellow, joined our group in the fall 1986. The reaction could be applied to a variety of secondary amines and terminal alkynes using catalysts of type RuCl2(PR3)(arene) and preferentially Ru(methallyl)2(diphosphine). It was shown that the simple vinylcarbamate could be prepared from acetylene itself [17, 18]. The simple catalyst RuCl3·3H2O could perform the vinylcarbamate synthesis from acetylene in up to 46 % yield in acetonitrile under 15 atm of CO2 [17]. [RuCl2(NBD)]n, which in situ generates RuCl2(HNR2)2(NBD) in the presence of amine was shown by C. Bruneau and S. Lécolier (SNPE) to be the most efficient catalyst precursor for this transformation of acetylene into the useful vinylcarbamates (20 atm CO2, 80 °C) [18] (Eq. 1)

C. Bruneau and J. Höfer showed that the catalytic addition of ammonium carbamate to isopropenylacetylene led to the regioselective synthesis of Z > E O-1-(1,3-dienyl)carbamates (Eq. 2) [19]. For this reaction the best ruthenium(II) catalysts were of the type Ru(methallyl)2(Ph2P(CH2)nPPh2). The complete evaluation of the previously cited ruthenium(II) catalysts for catalytic vinylcarbamate synthesis appeared in 1989 [20]

When the above reaction of secondary amines and CO2 was applied to propargylic alcohols, it led to the selective formation of O-β-oxopropylcarbamates. In the presence of [RuCl2(NBD)]n catalyst precursor 30–40 % yields of carbamates were obtained (Eq. 3) [21, 22].

As the triple bond is transformed into an acetyl group, the reaction could proceed via initial carbamate addition at carbon C2 of the coordinated alkyne. However it will be shown later that when the reaction was performed in the presence of NEt3, the unsaturated α-methylene cyclic carbonate was formed in small amount, thus the oxoalkyl carbamates can also arise from secondary amine addition to the cyclic carbonate (Eq. 3) (see Sect. 4.)
3 Ruthenium-Catalyzed Synthesis of Enol Esters from Terminal Alkynes
The regioselective synthesis of vinylcarbamates led us to evaluate the ruthenium(II) activation of alkynes towards carboxylic acids addition in an attempt to produce enol and dienol esters useful acylating reagents, such as vinylacetate or in peptide synthesis, or polymer precursors. According to the nature of both catalysts and substrates, both regioselective Markovnikov and anti-Markovnikov additions could be obtained.
The regioselective Markovnikov addition of carboxylic acids to terminal alkynes was first performed by C. Ruppin with RuCl3·3H2O/2 PR3 but preferentially with RuCl2(PR3)(arene) [23]. The reaction was applied to the addition of N-protected aminoacids to butylacetylene and propyne [24] without racemization at 80 °C and the products were used in peptide synthesis by Z. Kabouche and C. Bruneau (Eq. 4) [24, 25]. These reaction conditions with RuCl2(PR3)(arene) catalysts were much milder than those with Ru3(CO)12, [Ru(O2CH)(CO)2]n [26] or Ru(cyclooctadienyl)2/PBu3 [27]. The mechanism studies concluded that the reaction proceeded via external addition of carboxylate to the η2-coordinated triple bond.

The addition of carboxylic acids to isopropenylacetylene, performed by K. Philippot, selectively afforded 2-acyloxy-1,3-dienes using the RuCl2(PMe3)(p-cymene) catalyst. The reaction can also be applied to optically active aminoacid derivatives without racemization (Eq. 5) [28].

D. Devanne showed that the same catalyst RuCl2(PMe3)(p-cymene) could perform the addition of carboxylic acids and aminoacids to propargylic alcohols to produce β-oxopropylesters without racemization (Eq. 6) [29]. The formation of these esters is consistent with external initial addition of the carboxylate to the substituted carbon of the coordinated triple bond.

This synthesis of enol esters was applied by M. Neveux and C. Bruneau for the production of enol formates that were revealed as excellent formylation reagents under mild conditions [30]. They also showed that oxalic acid add to alkynes to form enol oxalyl esters which easily gave access to 1,2-dicarbonyl derivatives [31]. With B. Seiller they revealed that the [Ru(O2CH)(CO)2(PPh3)]2 catalyst could perform tandem catalysis offering the direct access to 1,3-dioxan-4-ones, via enol esters arising from addition of α-hydroxy carboxylic acids to alkynes followed by intramolecular catalytic addition of the hydroxyl group to the enol double bond [32, 33].
[Ru(O2CH)(CO)2(PPh3)]2 was found by C. Darcel and C. Bruneau to be the most efficient catalyst to activate propargylic alcohols for the formation of β-ketoesters in the presence of carboxylic acid and has allowed to produce optically active β-ketoesters with retention of configuration at the stereogenic carbon from chiral propargylic alcohols obtained by S. M. Roberts via enzymatic hydrolysis of their acetate [34]. Moreover they used the [Ru(O2CH)(CO)2(PPh3)]2 catalyst for the stereoselective transformation at C(17) of steroids of propargylic derivatives into steroids with β-oxopropyl ester functionality [35].
It is noteworthy that the ruthenium catalytic addition of carboxylic acids to terminal alkynes and propargylic alcohols actually inhibited ruthenium–vinylidene formation. It was suggested that this inhibition was brought by the concomitant protonation of the ruthenium center and external addition of the carboxylate to the coordinated triple bond [33, 34].
All the previous examples were consistent with Markovnikov addition of carboxylate to the triple bond of terminal alkynes. However, H. Doucet, B. Martin-Vaca, N. Derrien and C. Bruneau have demonstrated that the use of Ru(methallyl)2(diphosphine) catalyst precursors has allowed to produce anti-Markovnikov addition of carboxylic acids to terminal alkynes and to isopropenylacetylene affording preferentially the Z-enol- or dienol-esters with high Z-stereoselectivity (>95 %) (Eq. 7) [36–39].

In that case H. Doucet showed that the catalyst precursors react with carboxylic acids to give Ru(O2CR)2(diphosphine) and isobutene inhibiting ruthenium protonation and favouring activation of alkynes via vinylidene intermediate. The nature of the diphosphine was crucial as bis(diphenylphosphino)butane (dppb) favours formation of Z-enol esters at low temperature whereas bis(diphenylphosphino)ethane (dppe) leads to better selectivity with bulky terminal alkynes (Eq. 7) [36–39]. These Z-enol esters were found to be useful for the access to (E)-enamines and to α-cyanoesters on reaction with amines and KCN/HSiEt3, respectively [40].
4 Catalytic Synthesis of Cyclic Carbonates and Carbamates
Our interest in directly incorporating CO2 derivatives into alkynes, by routes avoiding the use of phosgene, led J. Fournier and C. Bruneau to discover an “organocatalytic” synthesis of cyclic carbonates and carbamates by a route different from the usual insertion of CO2 into oxiranes and oxetanes.
During the study of the ruthenium catalyzed synthesis of β-oxoalkylcarbamates by reaction of CO2, HNR2 and propargylic alcohol with RuCl2(PBu3)(arene) (Eq. 3), to understand the mechanism different ratios of Ru(II) catalyst and PBu3 were used and by changing the amine by NEt3 or H2NR we observed that (i) PBu3 alone catalyzed the reaction, (ii) with tertiary amine NEt3 cyclic carbonate was partially formed, and iii) with primary amine H2NR α-methylene oxazolidinones were produced. Thus, tertiary propargylic alcohols with CO2 (50 bar) in an inert autoclave with 8 mol % of PBu3 led to the isolation (>95 %) of α-methylene cyclic carbonates, in the absence of other solvent than the propargylic alcohol. PBu3 appeared to be more efficient than PPh3 and PCy3 (Eq. 8) [41, 42].
The formation of these cyclic carbonates can be explained by the addition of the phosphine to the triple bond, transfer of alcohol proton to the alkenyl carbon, addition of CO2 to the alcoholate and addition of the resulting carbonate oxygen to the alkenyl carbon with release of PBu3.

However, under the same conditions with primary amines, but in the presence of both ruthenium(II) catalyst and PBu3, the N,N′-disubstituted ureas were selectively formed [43].
This easy formation of cyclic carbonates opened the route to many derivatives on addition of simple reagents such as primary amines leading to β-oxopropylcarbamates and to their oxazolidinones, as on addition of aminoacids [44], and to opened carbonates by addition of alcohols [45, 46] or to functional enones [47].
The PBu3 catalyzed activation of propargylic alcohols was applied by J. Fournier and J. M. Joumier to the direct formation of α-methylene oxazolidinones via reaction of primary amine, CO2 and a variety of tertiary propargylic alcohols (Eq. 9) [48].

The phosphine-catalyzed synthesis of α-methylene cyclic carbonates constituted actually a catalytic platform [49] for the fast access to functional allyl or alkynyl carbonates, and to allenyl alcohols and esters. C. Darcel developed this platform by palladium catalyzed transformations of these cyclic carbonates (Scheme 2).
Cyclic carbonates were previously used for the Pd-catalyzed allylation of isocyanate and aldehydes leading to heterocycles [50, 51] We showed (Scheme 2) that these carbonates were straightforward precursors of allylcarbonates and of functional enones via Pd-catalyzed allylation of nucleophiles (a,b) [47]. The useful formation of cyclic alkynyl carbonates (c) [45, 46] offered the direct access to allylcarbonates on catalytic reduction with ammonium formate in the presence of Ru(methallyl)2(dppe) catalyst (d) [38], and to α-allenyl alcohols via catalytic hydrogenolysis with HCO2 − HNEt +3 and Pd(dba)2/diphosphine catalyst (e) [52]. The reaction is expected to result from the formation of allenyl-Pd(II) species followed by hydride transfer from the coordinated formate. The cyclic alkynyl carbonates on reaction with terminal alkyne with Pd(PPh3)4/CuI catalyst offer a route to alkynyl allenyl alcohols (f) [53] which was applied to the modification of the C17 side chain of steroids [53]. C. Darcel showed that cyclic alkynyl carbonates can also be selectively carbonylated with carbon monoxide in alcohol to afford allenyl esters with Pd(dba)2/PBu3 catalyst (g) [54]. (Scheme 2).
A dihydrofuran synthesis was found by serendipity on Pd-catalyzed reaction of alkynylcarbonates with acrylic esters and acrylamides. However the reaction is very sentive to the conditions to get excellent yield as it requires the use of Pd(OAc)2/PPh3 catalyst in the presence of NBu4Br with NEt3–H2O in DMF or THF solvent. (Scheme 3) [55].
5 Activation of Alkynes Via Oxidative Coupling Versus Vinylidene with Cp*Ru(X)L2 Catalysts
5.1 Oxidative Couplings of C≡C and C=C bonds
The catalytic synthesis of vinylcarbamates, via a vinylidene–ruthenium(II) active intermediate, resulted from alkyne activation with a 16 electron ruthenium(II) species for example of type [RuX(PR3)(arene)]+Y−. We decided to explore the activation of terminal alkynes with ruthenium(II) catalyst precursors capable to provide a coordinatively unsaturated 14 electron catalytic species, by displacement of weakly coordinating ligands, but at the same time more electron-rich ruthenium(II) sites to favour oxidative couplings between two functional unsaturated molecules.
Initial results by B. M. Trost already showed that CpRuCl(COD), (Cp=C5H5), in the presence of NH4PF6 allowed the regioselective oxidative coupling of an alkyne and a functional alkene and led to the formation of a 1,4-diene with branched derivative as a major isomer (Eq. 10) [56, 57].

B. M. Trost showed also that the same catalyst CpRuCl(COD) could offer the access to unsaturated ketones from terminal alkyne and substituted allylic alcohols. In that case the oxidative coupling preferentially afforded the linear isomer [58].
Sylvie Dérien, as Rennes assistant professor, explored the reaction of terminal alkynes with simple allyl alcohol using a more sterically hindered and electron-rich catalyst Cp*RuCl(COD) with labile COD which led to a high regioselective oxidative coupling. Only γ,δ-unsaturated aldehydes were selectively obtained (Eq. 11) [59, 60] and the branched isomer was only formed, due to the bulkiness of the Cp* = C5Me5 ligand which favours the head-to-tail coupling of the triple and double bonds to give a metallacyclopentene. The exocyclic beta-elimination and reductive elimination explain the product formation. A vinylidene intermediate would have led to vinyl allyl ether. This reaction showed that the [Cp*RuX] moiety favours oxidative coupling versus vinylidene intermediate and that the bulkiness of the C5Me5 ligand drives the coupling regioselectivity.

This reaction applied to a propargyl alcohol as terminal alkyne and the allyl alcohol with the same catalyst Cp*RuCl(COD) led selectively to 2-alkoxy-5-methylenetetrahydropyranes resulting also from head-to-tail coupling and formation of a hemiacetal (Eq. 12) [61, 62]

In the mean time Mitsudo used Cp*RuCl(COD) for the catalytic coupling of a strained alkene (norbornene, norbornadiene) with an internal alkyne. In that case the ruthenacyclopentene intermediate cannot offer a beta-elimination process, thus the cyclobutene derivative was selectively obtained via reductive elimination (Eq. 13) [63]. Later this reaction was applied for the [2+2] cycloaddition of a variety of substituted norbornenes and norbornadienes with alkynes [64–66]

The catalytic precursor based on Cp*RuXL2 with labile L ligands offers till now the best catalytic system for regioselective oxidative couplings of alkyne with an alkene. Many useful applications in organic synthesis have been found later via intramolecular oxidative couplings of triple and double bonds by several groups especially by Trost’s group [67–70] and gathered in a 2004 review [71].
5.2 Ruthenium–Vinylidene Intermediates with Cp*RuXL2 Catalysts
The development of vinylidene-metal intermediates has led to many applications in synthesis and we have gathered these discoveries in several reviews [15, 72, 73] Special efforts have been also made to generate vinylidene active species arising from the terminal alkyne by activation with Cp*RuXL2 catalysts. However the examples were reported only with Cp*RuXL2 derivatives containing strong Ru–L bond such as Ru–PR3 bond, thus affording the 16 electron cationic activating species Cp*RuL2 +. First B. M. Trost showed that using the CpRu(PPh3)2Cl/NH4PF6 catalytic system, the addition of allylic alcohols to terminal alkynes was possible and took place affording β,γ-unsaturated ketones, via addition of allyl alcohol to the vinylidene intermediate and C–O bond cleavage and allyl migration (Eq. 14) [74–77].

Ruthenium vinylidene intermediates were also proposed by M. Murakami using a similar catalytic system CpRu(PPh3)2Cl/NaPF6 for the coupling of unactivated alkenes with terminal alkynes to afford 1,3-dienes as a mixture of two isomers, the linear one being favoured (Eq. 15) [78].
The same catalytic system has allowed later the useful regioselective ruthenium-catalyzed alkenylation at C2 position of pyridine with terminal alkyne [79].

We have discovered with F. Jérome, a post doctor fellow, and PhD student J. Monnier another application of vinylidene intermediate arising from propargylic alcohol: the hydrophosphination of propargylic alcohols on addition of HPPh2, in the presence of Cp*RuCl(COD) catalyst. This reaction affords regioselectively the Z-hydroxyl alkenylphosphine as the kinetic product which isomerizes easily into the E isomer (Eq. 16) [80].

The formation of the vinylidene intermediate results from the initial displacement of the COD ligand by two HPPh2 ligands and the activation of the terminal propargylic alcohol with the 16 electron intermediate before addition of the secondary phosphine to the vinylidene carbon (Scheme 4) [80].
The power of CpRu(Cl)L2 catalysts for the generation of vinylidene–ruthenium or alkylidene–ruthenium intermediates in synthesis has been illustrated in several reviews [71, 81].
5.3 Oxidative Coupling of Terminal Alkynes with Cp*RuXL2 Catalysts as a Route to Functional 1,3-Dienes
It is noteworthy that the Cp*RuXL2 catalysts in the presence of both an alkyne and an alkene offer the cross oxidative coupling of the two different unsaturated bonds as shown before (Sect. 5.1) [71]. However when the alkene is absent then head-to-head oxidative addition of two molecules of terminal alkyne takes place. The isolated intermediate surprisingly was shown by Singleton et al. as early as 1986 [82] to contain a “bis-carbene”-ruthenium structure A (Eq. 17), rather than the expected ruthenacyclopentadiene which is observed only on addition of a phosphine to the ruthenium [83].
S. Dérien decided to explore the “bis-carbene” properties of the in situ produced intermediate from terminal alkyne and Cp*RuCl(COD) and addition of pronucleophiles to alkynes was attempted. The first example reported PhD student Jacques Le Paih showed the addition of a proton at one carbene carbon and the addition of nucleophile at the other carbene carbon, thus creating 3 bonds leading to the diene product B (Eq. 17) [84].

Thus the simple addition of carboxylic acids to arylacetylenes quantitatively and stereoselectively led at room temperature in one step to 1,3-dienes, containing a carboxylate group at carbon C1 (Eq. 18) [84].

The catalytic addition mechanism has been elucidated by DFT calculations with the help of Odile Eisenstein and Eric Clot (Scheme 5) [85]. It shows that the metallacyclic intermediate that behaves as a mixed Fischer-Schrock-type biscarbene-ruthenium complex, allowing protonation first at the Schrock type carbene carbon, not at the metal, to give the intermediate C, followed by nucleophilic addition of the carboxylate at the Fischer type carbene carbon in D. The possibility to form the expected mixed alkyl allyl intermediate instead of C was ruled out. Another approach has been proposed recently for which the carboxylate coordinates to the ruthenium site before addition to the carbon C1 [86].
Later Min Zhang showed that the addition of alcohols and water to the head-to-head dimerized alkyne ruthenium species can also take place, however subtle catalyst modifications have to be done. As alcohols are less acidic than carboxylic acids the cationic catalyst Cp*Ru(CH3CN) +3 PF6 − has to be used to form dienyl alkyl ethers (Eq. 19) [87]. For the addition of water, catalytic amount of a strong acid such as paratoluenesulfonic acid p-TSA needs to be used to protonate the biscarbene intermediate to lead to the quantative formation of dienol and the unsaturated ketone (Eq. 19) [87].

The easy synthesis of unsaturated ketones from alkynes allowed to perform sequential catalysis. Thus after formation of the ketone, addition of copper(II) catalyst in air atmosphere allowed its transformation into furan derivative (Eq. 19) [87]. Alternatively the ruthenium catalyzed formation of unsaturated ketone followed by silver catalysis allow the hydroarylation of the double bond with electron rich arenes [88].
Recently this catalytic 1,4-addition of a proton and anion on the biscarbene intermediate was used by S. Dérien for the preparation of 1,3-dienylchlorides and bromides by addition of camphorsulfonic acid (AH) and a chloride or bromide salt, using Cp*RuCl(COD) catalyst (Eq. 20) [89]. This reaction led preferentially to the 1Z,3E dienes

The above dimerization of alkynes via biscarbene-ruthenium intermediate has potential for the formation of a variety of functional dienes, via 1,4-addition, but also for molecular materials preparation, by catalytic modification of currently used alkyne group for electronic communication, and for oligomerization/polymerization of rigid conjugated bridged diynes.
The ability of Cp*RuXL system to accommodate carbene has led to develop the chemistry of Cp*RuX(=CHY) intermediates, often via the activation of diazoalkanes. These intermediates behave very differently of the isoelectronic Grubbs catalysts of type RuX2(=CHY)L2 as they inhibit alkene metathesis processes [81].
Profit of Cp*RuX(=CHY) intermediates has recently been made for the synthesis of functional dienes from alkynes [90], and from propargylic carboxylates [91], and for the access to alkenyl bicyclic derivatives (Scheme 6) [92].
6 Concluding Remark
Our initial results in homogeneous catalysis presented above have deeply fed our further research topics till now. First the search for evidence of active vinylidene–ruthenium species in the ruthenium(II) activation of terminal alkynes has been the first step to develop the topic of carbon-rich ruthenium systems with D. Touchard [93] and A. Romero, via the characterization of vinylidene- allenylidene- and cumulenylidene-ruthenium complexes [94–96]. Among these we reported in 1998 the evidence for the first allenylidene-ruthenium catalytic precursors for alkene metathesis [72, 97], and R. Castarlenas demonstrated that the resulting catalytic species was the corresponding indenylidene–ruthenium complex easily generated on protonation of the allenylidene-ruthenium parents [98, 99]. Indenylidene-ruthenium catalysts are now commonly used in catalysis based on their efficiency and the easiness of preparation from simple propargylic alcohols rather than from diazoalkanes [72, 73]. Moreover the Rennes allenylidene- and indenylidene-ruthenium catalysts led us to develop many applications in alkene metathesis [100–103] some of them in cooperation with IFP [103–105] and Arkema [106, 107] companies, and this topic is still a strong research topic in Rennes.
Our research topic on enantioselective catalysis, in cooperation with the company Oril-Servier, deeply involved the ruthenium-catalyzed hydrogenation of cyclic carbonates and oxazolidinones for the production of optically active diols from α-methylenedioxolanones [108] and of the optically active N-acyloxazolidinones, the Evans reagents, from α-methylene cyclic carbonates [109].
Finally the use of Cp*RuXL2 catalysts which revealed both the oxidative coupling of alkynes into biscarbene intermediate in catalysis, and the stabilization of Cp*Ru-alkylidene and their vinylalkylidene-RuCp* species has later allowed to discover novel syntheses of functional dienes and cyclopropyl and bicyclic derivatives [81].
The above examples of both activation of alkynes and ruthenium-catalyzed syntheses have contributed over the years to make of ruthenium an attractive and tamed metal for catalysis for the synthesis of complex architectures and molecular materials. It is no longer a matter of “connoisseurs”.
References
Sasaki Y, Dixneuf PH (1986) A novel catalytic synthesis of vinyl carbamates from carbon dioxide, diethylamine and alkyne in the presence of Ru3(CO)12. J Chem Soc Chem Commun 10:790–791
Mahé R, Dixneuf PH, Lécolier S (1986) One-step synthesis of vinyl carbamates, catalyzed by mononuclear ruthenium complexes, via addition of carbon dioxide and amine to terminal alkynes. Tetrahedron Lett 27:6333–6336
King RB, Saran MS (1972) Metal complexes with terminal dicyanomethylenecarbene ligands formed by chlorine migration reactions. J Chem Soc Chem Commun 19:1053–1054
Jolly PW, Pettit R (1968) 3-(ð-cyclopentadienyliron dicarbonyl)propyne. J Organomet Chem 12:491–495
Chisholm MH, Clark HC (1972) Cationic acetylenic platinum(ii) compounds and their derivatives. Reactions of platinum(II) stabilized carbonium ions. J Am Chem Soc 94:1532–1539
Bruce MI, Swincer AG, Wallis RC (1979) Cyclopentadienyl-ruthenium and osmium chemistry. Some reactions of substituted vinylidene complexes. J Organomet Chem 171:C5–C8
Davidson A, Selegue JP (1978) Stable dimethyl, methyl, and unsubstituted vinylidene complexes. J Am Chem Soc 100:7763–7765
Abbott S, Davies SG, Warner P (1983) Disubstituted vinylidene complexes of iron and ruthenium: nucleophilic properties of acetylide ligands. J Organomet Chem 246:C65–C68
Bruce MI, Humphrey MG, Liddell MJ (1987) Reactions of transition metal σ-acetylides. Preparation of some aryldiazovinylidene complexes. J Organomet Chem 321:91–102
Bruce MI, Koutsantonis GA, Liddell MJ (1987) Reactions of transition metal ó-acetylides. Synthesis and X-ray structure of [Ru(C=CIPh)(PPh3)2(C5H5)][I3]. J Organomet Chem 301:217–227
Silvestre J, Hoffmann R (1985) Hydrogen migration in transition metal alkyne and related complexes. Helv Chim Acta 68:1461–1506
De los Rios I, Jimenez Tenorio M, Puerta MC, Valerga P (1997) Alternative mechanisms to the alkyne to vinylidene isomerization promoted by half-sandwich ruthenium complexes. J Am Chem Soc 119:6529–6538
Bruce MI, Swincer AG (1983) Vinylidene and propadienylidene (allenylidene) metal complexes. Adv Organomet Chem 22:59–128
Bruce MI (1991) Organometallic chemistry of vinylidene and related unsaturated carbenes. Chem Rev 91:197–257
Bruneau C, Dixneuf PH (1999) Metal vinylidenes in catalysis. Acc Chem Res 32:311–323
Ouzzine K, Le Bozec H, Dixneuf PH (1986) Arene carbene ruthenium complexes via vinylidene–ruthenium intermediates. Organomet Chem 317:C25–C27
Sasaki Y, Dixneuf PH (1987) Ruthenium-catalyzed synthesis of vinyl carbamates from carbon dioxide, acetylene and secondary amines. J Org Chem 52:314–315
Bruneau C, Dixneuf PH, Lécolier S (1988) Acetylene in catalysis: a one-step synthesis of vinylcarbamates with (RuCl2(norbornadiene))n. J Mol Catal 44:175–178
Höfer J, Doucet H, Bruneau C, Dixneuf PH (1991) Ruthenium catalyzed regioselective synthesis of O-1-(1,3-dienyl) carbamates directly from CO2. Tetrahedron Lett 32:7409–7410
Mahe R, Sasaki Y, Bruneau C, Dixneuf PH (1989) Catalytic synthesis of vinylcarbamates from carbon dioxide and alkynes with ruthenium complexes. J Org Chem 54:1518–1523
Bruneau C, Dixneuf PH (1987) Catalytic synthesis of O-beta-oxoalkyl-carbamates. Tetrahedron Lett 28:2005–2008
Sasaki Y, Dixneuf PH (1987) Ruthenium-catalyzed reaction of carbon dioxide, amine and acetylenic alcohol. J Org Chem 52:4389–4391
Ruppin C, Dixneuf PH (1986) Synthesis of enol esters from terminal alkynes catalyzed by ruthenium complexes. Tetrahedron Lett 27:6323–6324
Ruppin C, Dixneuf PH, Lécolier S (1988) Regioselective synthesis of isopropenyl esters by ruthenium catalyzed addition of N-protected amino-acids to propyne. Tetrahedron Lett 29:5365–5368
Kabouche Z, Bruneau C, Dixneuf PH (1991) Enol esters as intermediates for the facile conversion of amino acids into amides and dipeptides. Tetrahedron Lett 32:5359–5362
Rotem M, Shvo Y (1983) Addition of carboxylic acids to alkynes catalyzed by ruthenium complexes. Vinyl ester formation. Organometallics 2:1689
Mitsudo T, Hori Y, Yamakawa Y, Watanabe Y (1987) Ruthenium-catalyzed selective addition of carboxylic acids to alkynes. A novel synthesis of enol esters. J Org Chem 52:2230
Philippot K, Devanne D, Dixneuf PH (1990) General Synthesis of 2-acyloxy-1,3-dienes in one step from carboxylic acids and butenyne derivatives. J Chem Soc Chem Commun 17:1199–1200
Devanne D, Ruppin C, Dixneuf PH (1988) Synthesis or b-oxopropyl esters by catalytic addition of carboxylic acids and N-protected amino-acids to propargyl alcohol. J Org Chem 53:925–928
Neveux M, Bruneau C, Dixneuf PH (1991) Enol formates: ruthenium catalysed formation and formylating reagents. J Chem Soc Perkin Trans I 5:1197–1199
Neveux M, Bruneau C, Lécolier S, Dixneuf PH (1993) Novel synthesis of oxamides, oxamates and oxalates from diisopropenyl oxalate. Tetrahedron 49:2629–2640
Neveux M, Seiller B, Hagedorm F, Bruneau C, Dixneuf PH (1993) Novel ruthenium-catalyzed synthesis of 1,3-dioxolan-4-ones from α-hydroxy acids and terminal alkynes via enol esters. J Organomet Chem 451:133–138
Bruneau C, Neveux M, Kabouche Z, Ruppin C, Dixneuf PH (1991) Ruthenium-catalysed additions to alkynes: synthesis of activated esters and their use in acylation reactions. Synlett 11:755–763
Darcel C, Bruneau C, Dixneuf PH, Roberts SM (1997) Stereoselective synthesis of α-ketoesters from prop-2-yn-1-ols. Tetrahedron 53:9241–9252
Darcel C, Bruneau C, Dixneuf PH, Neef G (1994) Concomitant catalytic transformations of geminal ethynyl and hydroxy groups of steroids into acetyl and ester functions with retention of configuration by [Ru(O2CH)(CO)2(PPh3)]2. J Chem Soc Chem Commun 3:333–334
Doucet H, Höfer J, Bruneau C, Dixneuf PH (1993) Stereoselective synthesis of Z-enol esters catalyzed by bis(2-methylpropenyl)(bis(diphenylphosphino)alkane)ruthenium complexes. J Chem Soc Chem Commun 10:850–851
Doucet H, Martin-Vaca B, Bruneau C, Dixneuf PH (1995) A general synthesis of Z-alk-1-en-1-yl esters via ruthenium-catalyzed anti-markovnikov trans-addition of carboxylic acids to terminal alkynes. J Org Chem 60:10901–10912
Doucet H, Höfer J, Derrien N, Bruneau C, Dixneuf PH (1996) Preparation of tuneable (Ph2P(CH2)nPPh2)Ru(2-methylpropenyl)2 catalysts and their use for the stereoselective synthesis of dienyl esters. Bull Soc Chim Fr 133:939–944
Doucet H, Derrien N, Kabouche Z, Bruneau C, Dixneuf PH (1997) Powerful control by organoruthenium catalysts of the regioselective addition to C(1) or C(2) of the prop-2-ynyl ethers triple bond. J Organomet Chem 551:151–157
Doucet H, Bruneau C, Dixneuf PH (1997) Novel two step stereoselective synthesis of (E)-enamines and 1-amino-1,3-dienes from terminal alkynes. Synlett 7:807–808
Fournier J, Bruneau C, Dixneuf PH (1989) Phosphine catalysed synthesis of unsaturated cyclic carbonates from carbon dioxide and propargylic alcohols. Tetrahedron Lett 30:3981–3982
Bruneau C, Dixneuf PH (1992) Catalytic incorporation of CO2 into organic substrates: synthesis of unsaturated carbamates, carbonates and ureas. J Mol Catal 74:97–107
Fournier J, Bruneau C, Dixneuf PH, Lécolier S (1991) Ruthenium catalyzed synthesis of symmetric N, N′-disubstituted ureas directly from carbon dioxide and amines. J Org Chem 56:4456–4458
Joumier JM, Grainger R, Bruneau C, Dixneuf PH (1993) Synthesis of functional oxazolidin-2-ones and oxadiazin-2-ones in two steps from CO2 via Cyclic α-methylene carbonates. Synlett 6:423–424
Joumier JM, Fournier J, Bruneau C, Dixneuf PH (1991) Functional carbonates: cyclic α-methylene and β-oxopropyl carbonates from prop-2-ynyl alcohol derivatives and CO2. J Chem Soc Perkin Trans I 12:3271–3274
Joumier JM, Bruneau C, Dixneuf PH (1993) Direct access to β-oxopropyl carbonates from bulky alcohols. J Chem Soc Perkin Trans I 15:1749–1751
Joumier JM, Bruneau C, Dixneuf PH (1992) A new route to functional α-enones via prop-2-ynyl alcohol derivatives and carbonates. Synlett 5:453–454
Fournier J, Bruneau C, Dixneuf PH (1990) A simple synthesis of oxazolidinones in one step from carbon dioxide. Tetrahedron Lett 31:1721–1722
Bruneau C, Darcel C, Dixneuf PH (1997) Selective palladium-catalyzed transformations of cyclic alk-2-ynyl carbonates. Curr Org Chem 1:197–218
Ohe K, Matsuda H, Morimoto T, Ogoshi T, Chatani N, Murai S (1994) Nucleophilic substitution at the central allyl carbon atom of a (π-ally1)platinum complex. J Am Chem Soc 116:4125
Inoue Y, Matsushita K, Yen YF, Imaizumi S (1991) α-Methylene cyclic carbonate as a conjunctive agent for aromatic aldehydes. Chem Lett 8:1377–1378
Darcel C, Bartsch S, Bruneau C, Dixneuf PH (1994) Selective catalytic transformations of alkynyl cyclic carbonates into either homopropargylic or α-allenyl alcohols. Synlett 1994:457–458
Darcel C, Bruneau C, Dixneuf PH (1994) Palladium(0), copper(I) catalyzed synthesis of conjugated alkynyl α-allenols from alkynyl cyclic carbonates and terminal alkynes. J Chem Soc Chem Commun 16:1845–1846
Darcel C, Bruneau C, Dixneuf PH (1996) New synthesis of heterocycles via palladium-catalyzed double carbonylation of cyclic alk-1-ynyl carbonates. Synlett 1996:218–220
Darcel C, Bruneau C, Albert M, Dixneuf PH (1996) Synthesis of alkenyl-2,5-dihydrofurans via palladium-catalysed reaction of cyclic alkynyl carbonates. J Chem Soc Chem Commun 8:919–920
Trost BM, Indolese AF, Müller TJJ, Treptow B (1995) A Ru catalyzed addition of alkenes to alkynes. J Am Chem Soc 117:615
Trost BM, Indolese A (1993) Ruthenium-catalyzed addition of alkenes to acetylenes. J Am Chem Soc 115:4361–4362
Trost BM, Martinez JA, Kulawiec RJ, Indolese AF (1993) Ruthenium-catalyzed addition of allyl alcohols and acetylenes. A simple synthesis of gamma, delta-unsaturated ketones. J Am Chem Soc 115:10402
Dérien S, Dixneuf P-H (1994) Ruthenium catalysed synthesis of unsaturated acetals and aldehydes via C–C bond coupling of alkynes with allyl alcohol. J Chem Soc Chem Commun 22:2551–2552
Dérien S, Jan D, Dixneuf P-H (1996) Ruthenium-catalysed coupling of allyl alcohol with alkynes: a new route to unsaturated acetals and aldehydes. Tetrahedron 52:5511–5524
Dérien S, Gomez Vicente B, Dixneuf P-H (1997) Ruthenium catalysed formation of 2-alkoxy-5-methylenetetrahydropyrans. J Chem Soc Chem Commun 15:1405–1406
Dérien S, Ropartz L, Le Paih J, Dixneuf P-H (1999) Synthesis of 2-alkoxy-5-methylenetetrahydropyrans: a regioselective ruthenium-catalyzed C–C coupling reaction of prop-2-ynols with allyl alcohol. J Org Chem 64:3524–3531
Mitsudo T-A, Naruse H, Kondo T, Ozaki Y, Watanabe Y (1994) [2+2]Cycloaddition of norbornenes with alkynes catalyzed by ruthenium complexes. Angew Chem Int Ed Engl 33:580–581
Jordan RW, Tam W (2002) Reactivity of the alkene component in the ruthenium-catalyzed [2+2] cycloaddition between an alkene and an alkyne. Tetrahedron Lett 43:6051–6054
Jordan RW, Tam W (2001) Study on the reactivity of the alkene component in ruthenium-catalyzed [2+2] cycloadditions between an alkene and an alkyne. Org Lett 3:2367–2370
Jordan RW, Tam W (2000) Ruthenium-catalyzed [2+2] cycloadditions of 2-substituted norbornenes. Org Lett 2:3031
Trost BM, Toste FD (2002) Mechanitic dichotomy in CpRu(CH3CN)3PF6 catalyzed enyne cyclotrimerizations. J Am Chem Soc 124:5025
Mendez M, Paz Munoz M, Nevado C, Cardenas DJ, Echavarren AM (2001) Cyclizations of enynes catalyzed by PtCl2 or other transition metal chlorides: divergent reaction pathways. J Am Chem Soc 123:10511
Trost BM, Shen HC (2001) Constructing tricyclic compounds containing a seven-membered ring by ruthenium-catalyzed intramolecular [5+2] cycloaddition. Angew Chem Int Ed Engl 40:2313–2316
Mori M, Saito N, Tanaka D, Takimoto M, Sato Y (2003) Novel alkenylative cyclization using a ruthenium catalyst. J Am Chem Soc 125:5606
Derien S, Monnier F, Dixneuf PH (2004) Ruthenium-catalyzed C–C bond formation. In: Bruneau C, Dixneuf P (eds) Ruthenium catalysts and fine chemistry. Topics in organometallic chemistry, vol 11. Springer, Berlin, p 1–44
Bruneau C, Dixneuf PH (2006) Metal vinylidenes and allenylidenes in catalysis. Applications in anti-Markovnikov additions to terminal alkynes and alkene metathesis. Angew Chem Int Ed 45:2176–2203
Bruneau C, Dixneuf PH (eds) (2008) Metal vinylidenes and allenylidenes in catalysis. Wiley, Weinheim
Trost BM, Dyker G, Kulawiec RJ (1990) A ruthenium-catalyzed reconstitutive condensation of acetylenes and allyl alcohols. J Am Chem Soc 112:7809–7811
Trost BM, Kulawiec RJ (1992) On the mechanism of the ruthenium-catalyzed reconstitutive condensation of allylic alcohols and terminal alkynes. J Am Chem Soc 114:5579–5584
Trost BM, Kulawiec RJ, Hammes A (1993) Ruthenium catalyzed reconstitutive condensation. Application to functionalized steroid side chains. Tetrahedron Lett. 34:587–590
Trost BM, Flygare JA (1992) A novel ruthenium-catalyzed tandem cyclization-reconstitutive addition of propargyl alcohols with allyl alcohols. J Am Chem Soc 114:547–5477
Murakami M, Ubukata M, Ito Y (1998) Ruthenium-catalyzed coupling of unactivated olefins with unactivated alkynes. Tetrahedron Lett 39:7361
Murakami M, Hori S (2003) Ruthenium-mediated regio- and stereoselective alkenylation of pyridine. J Am Chem Soc 125:4720–4721
Jerome F, Monnier F, Lawicka H, Derien S, Dixneuf PH (2003) Ruthenium catalyzed regioselective hydrophosphination of propargyl alcohols. Chem. Commun 6:696–697
Vovard-Le Bray C, Dérien S, Dixneuf PH (2010) Cp*RuCl(COD) in catalysis: a unique role in the addition of diazoalkane carbene to alkynes. C R Acad Sci 13:292–303
Albers MO, de Waal DJA, Liles DC, Robinson DJ, Singleton E, Wiege MB (1986) J Chem Soc Chem Commun 1986:1680
Yi CS, Torres-Luhian JR, Liu N, Rheingold AL, Guzei IA (1998) Organometallics 17:1257
Le Paih J, Derien S, Dixneuf PH (1999) Ruthenium-catalysed multiple component transformations: one-step stereoselective synthesis of functional dienes from alkynes and carboxylic acids. Chem Commun 15:1437–1438
Le Paih J, Monnier F, Derien S, Dixneuf PH, Clot E, Eisenstein O (2003) Biscarbene-ruthenium complexes in catalysis: novel stereoselective synthesis of (1E,3E)-1,4-disubstituted-1,3-dienes via head-to-head coupling of terminal alkynes and addition of carboxylic acids. J Am Chem Soc 125:11964–11975
Yamamoto Y (2013) Theoretical study on ruthenium-catalyzed hydrocarboxylative dimerization of phenylacetylene with acetic acid leading to (1E,3E)-1,4-diphenyl-1,3-butadienyl acetate. Organometallics 32:5201–5211
Zhang M, Jiang H-F, Neumann H, Beller M, Dixneuf PH (2009) Sequential synthesis of furans from alkynes: successive ruthenium(II)- and copper(II)-catalyzed processes. Angew Chem Int Ed 48:1681–1684
Zhang M, Jiang H, Dixneuf PH (2009) Ruthenium(II)-catalyzed regioselective synthesis of allylketones from alkynes and their silver(I)-catalyzed hydroarylation into functionalized ketones. Adv Synth Catal 351:1488–1494
Klein H, Roisnel T, Bruneau C, Derien S (2012) One-step synthesis of 1-halo-1,3-butadienes via ruthenium-catalysed hydrohalogenative dimerisation of alkynes. Chem Commun 48:11032–11034
Le Paih J, Vovard-Le Bray C, Dérien S, Dixneuf PH (2010) Ruthenium-catalyzed synthesis of functional conjugated dienes via addition of two carbene units to alkynes. J Am Chem Soc 132:7391–7397
Vovard-Le Bray C, Dérien S, Dixneuf PH (2009) Ruthenium-catalyzed synthesis of functionalized dienes from propargylic esters: formal cross-coupling of two carbenes. Angew Chem Int Ed 121:1467–1470
Monnier F, Vovard-Le Bray C, Castillo D, Aubert V, Derien S, Dixneuf PH, Toupet L, Ienco A, Mealli C (2007) Selective ruthenium-catalyzed transformations of enynes with diazoalkanes into alkenylbicyclo[3.1.0]hexanes. J Am Chem Soc 129:6037–6049
Touchard D, Dixneuf PH (1998) A new class of carbon-rich organometallics. The C3, C4 and C5 metallacumulenes Ru=(C=) n CR2. Coord Chem Rev 178–180(1):409–429
Romero A, Vegas A, Dixneuf PH (1990) Ruthenium containing cumulenes: generation of 2,3,4-pentatrienylidene- and 3-oxo-1,4-pentadienyl-complexes. Angew Chem Int Ed Engl 29:215–216
Pirio N, Touchard D, Dixneuf PH, Fettouhi M, Ouahab L (1992) Metallacumulenes: activation of diynes and access to a novel bis (alkenylallenylidene) metal complex R2C=C=C=Ru=C=C=CR2 ++. Angew Chem Int Ed Engl 31:651–653
Touchard D, Haquette P, Daridor A, Toupet L, Dixneuf PH (1994) First isolable pentatetraenylidene metal complex containing the Ru=C=C=C=C=CPh2 assembly. A key intermediate to provide functional allenylidene complexes. J Am Chem Soc 116:11157–11158
Fürstner A, Picquet M, Bruneau C, Dixneuf PH (1998) Cationic ruthenium allenylidene complexes as a new class of performing catalysts for ring closing metathesis. J Chem Soc Chem Commun 12:1315–1316
Castarlenas R, Dixneuf PH (2003) Highly active catalysts in alkene metathesis: first observed transformation of allenylidene into indenylidene via alkenylcarbyne: ruthenium species. Angew Chem Int Ed 42:4524–4527
Castarlenas R, Vovard C, Fischmeister C, Dixneuf PH (2006) Allenylidene to indenylidene rearrangement in arene-ruthenium complexes: a key step to highly active catalysts for olefin metathesis reactions. J Am Chem Soc 128:4079–4089
Fischmeister C, Bruneau C (2011) Enyne cross-metathesis with ruthenium carbene catalysts. Beilstein J Org Chem 7:156–166
Osipov SN, Artyushin OI, Kolomiets AF, Bruneau C, Picquet M, Dixneuf PH (2001) Synthesis of fluorine-containing cyclic α-amino acid and α-amino phosphonate derivatives by alkene metathesis. Eur J Org Chem 20:3891–3897
Cetinkaya B, Demir S, Ozdemir I, Semeril D, Bruneau C, Dixneuf PH (2003) η6-Mesityl, imidazolinylidene carbene-ruthenium(II) complexes: catalytic activity of their allenylidene derivatives in alkene metathesis and cycloisomerization reactions. Chem Eur J 9:2323–2330
Semeril D, Olivier-Bourbigou H, Bruneau C, Dixneuf PH (2002) Alkene metathesis catalysis in ionic liquids with ruthenium allenylidene salts. Chem Commun 12:146–147
Thurier C, Fischmeister C, Bruneau C, Olivier-Bourbigou H, Dixneuf PH (2007) Ionic imidazolium containing ruthenium complexes and olefin metathesis in ionic liquids. J Mol Catal A Chem 268:127–133
Thurier C, Fischmeister C, Bruneau C, Olivier-Bourbigou H, Dixneuf PH (2008) Ethenolysis of methyl oleate in room-temperature ionic liquid. ChemSusChem 1:118–122
Miao X, Fischmeister C, Dixneuf PH, Bruneau C, Dubois J-L, Couturier J-L (2012) Polyamide precursors from renewable 10-undecenenitrile and methyl acrylate via olefin cross-metathesis. Green Chem 14:2179–2183
Miao X, Bidange J, Dixneuf PH, Fischmeister C, Bruneau C, Dubois J-L, Couturier J-L (2012) Ruthenium-benzylidenes and -indenylidenes as efficient catalysts for the hydrogenation of aliphatic nitriles into primary amines. ChemCatChem 4:1911–1916
Le Gendre P, Braun T, Bruneau C, Dixneuf PH (1996) Preparation of optically active cyclic carbonates and 1,2-diols via enantioselective hydrogenation of α-methylene dioxolanones catalyzed by chiral ruthenium(II) complexes. J Org Chem 61:8453–8455
Le Gendre P, Thominot P, Bruneau C, Dixneuf PH (1998) A new preparation of optically active N-acyloxazolidinones via ruthenium-catalyzed enantioselective hydrogenation. J Org Chem 63:1806–1809
Acknowledgments
All the students and researchers who started homogeneous catalysis in Rennes, whose the names appear in the references, are warmly acknowledged for their hard and enthusiastic work and for successfully paving the roads for their successors who innovated more recently in catalysis and organometallic chemistry. PHD thanks the industrial companies SNPE, Oril industrie, IFP, Firmenich, Arkema for their financial and scientific support. PHD also thanks the CNRS, French Ministry of research, University of Rennes, Region of Bretagne and European Networks who continuously supported his research on catalysis and Institut Universitaire de France for membership.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Michael I. Bruce and Barry M. Trost who inspired our early steps in catalysis by their pioneered works, respectively on vinylidene-metal complexes and ruthenium catalysis for synthesis.
Rights and permissions
About this article
Cite this article
Dixneuf, P.H. Early Steps of Homogeneous Catalysis in Rennes: Carbon Dioxide Incorporation, Alkyne Activation and Ruthenium Catalysis. Catal Lett 145, 360–372 (2015). https://doi.org/10.1007/s10562-014-1444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1444-9