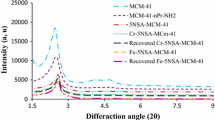
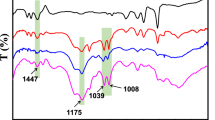
Bis(2-chloroethyl)sulfide, commonly known as mustard gas, is a highly toxic compound that acts as a vesicant, primarily by hydrolysis to produce HCl [1]. Prior to the 1960’s, this compound was synthesized and stored at various locations throughout this country and the world [2–4]. The inherent danger in storing this chemical has led to efforts to degrade it by various means. Stockpiles of mustard gas are currently destroyed by incineration, which, even when complete, can release environmentally unfavorable compounds such as sulfoxides, sulfones, carbon monoxide, and carbon dioxide into the atmosphere. Most other chemically-based techniques used to react bis(2-chloroethyl)sulfide have relied on oxidative pathways, whether using solution- or solid-based approaches [5–7]. These reactions have the same environmental problems as incineration. In addition, a highly oxidizing agent can often lead to bis(2-chloroethyl)sulfone, which is nearly as toxic as mustard gas itself [8].
Similar content being viewed by others
References
N.B. Munro S.S. Talmage G.D. Griffin L.C. Waters A.P. Watson J.F. King V. Hauschild (1999) Res. Rev. 107 933 Occurrence Handle1:CAS:528:DC%2BD3cXltFWrsw%3D%3D
L. Ember (1990) Chem. Eng. News 68 9
Y.-C. Yang J.A. Baker J.R. Ward (1992) Chem. Rev. 92 1729 Occurrence Handle1:CAS:528:DyaK38Xmtl2js70%3D
Y.-C. Yang (1999) Acc. Chem. Res. 32 109 Occurrence Handle1:CAS:528:DyaK1cXntVKgtL4%3D
L. Ember (1999) Chem. Eng. News 77 IssueID10 10
G.W. Wagner L.R. Procell R.J. O’Connor S. Munavalli C.L. Carnes P.N. Kapoor K.J. Klabunde (2001) J. Am. Chem. Soc. 123 1636 Occurrence Handle1:CAS:528:DC%2BD3MXosFSjtA%3D%3D
G.W. Wagner O.B. Koper E. Lucas S. Decker K.J. Klabunde (2000) J. Phys. Chem. B 104 5118 Occurrence Handle1:CAS:528:DC%2BD3cXivVWhurY%3D
J. Hirade A. Ninomiya (1950) J. Biochem. 37 19 Occurrence Handle1:CAS:528:DyaG3cXkslCmsQ%3D%3D
A.C. Sorensen B.L. Fuller A.G. Eklund C.C. Landry (2004) Chem. Mater. 16 2157 Occurrence Handle1:CAS:528:DC%2BD2cXjsF2qsbY%3D
C.T. Kresge M.E. Leonowicz W.J. Roth J.C. Vartuli J.S. Beck (1992) Nature 359 710 Occurrence Handle10.1038/359710a0 Occurrence Handle1:CAS:528:DyaK38Xms1entrs%3D
J.S. Beck J.C. Vartuli W.J. Roth M.E. Leonowicz C.T. Kresge K.D. Schmitt C.T.-W. Chu D.H. Olson E.W. Sheppard S.B. McCullen J.B. Higgins J.L. Schlenker (1992) J. Am. Chem. Soc. 114 10834 Occurrence Handle1:CAS:528:DyaK38Xms1entr8%3D
K.W. Gallis J.T. Araujo K.J. Duff J.G. Moore C.C. Landry (1999) Adv. Mater. 11 1452 Occurrence Handle1:CAS:528:DyaK1MXnslSgs7g%3D
K.W. Gallis A.G. Eklund S.T. Jull J.T. Araujo J.G. Moore C.C. Landry (2000) Stud. Surf. Sci. Catal. 129 747 Occurrence Handle10.1016/S0167-2991(00)80279-7 Occurrence Handle1:CAS:528:DC%2BD3cXkvFWlsr0%3D
K.W. Gallis and C.C. Landry, U.S. Patent 6,334,988 (2002).
K.W. Gallis C.C. Landry (2001) Adv. Mater 13 23 Occurrence Handle1:CAS:528:DC%2BD3MXhtVyktb8%3D
D.K. Rohrbaugh Y. Yang R.J. Ward (1988) J. Chromatogr. 417 165
D. Panayotov J.T. Yates SuffixJr. (2003) J. Phys. Chem. B 107 10560 Occurrence Handle1:CAS:528:DC%2BD3sXms12rsrk%3D
B.W.L. Jang J.J. Spivey (2000) Catal. Today 55 3 Occurrence Handle1:CAS:528:DyaK1MXnvVWis7o%3D
C. Topsøe (1984) Catal. Rev.-Sci. Eng. 26 395
E. Furminsky C.H. Amberg (1975) Can. J. Chem. 53 2542
S. Fuentes G. Diaz F. Pedraza H. Rojas N. Rosas (1988) J. Catal. 113 535 Occurrence Handle1:CAS:528:DyaL1MXpslGitA%3D%3D
C. Song K.M. Reddy (1999) Appl. Catal. A 176 1 Occurrence Handle1:CAS:528:DyaK1MXot1yjsw%3D%3D
Mustard gas has a melting point of 13 °C and a boiling point of 218 °C. CEES has a boiling point of 156 °C (melting point unavailable).
J. Laine (1982) Acta Cient. Venezolana 33 121 Occurrence Handle1:CAS:528:DyaL3sXhtFCnsLw%3D
W.P. Boone J.G. Ekerdt (2000) J. Catal. 193 96 Occurrence Handle1:CAS:528:DC%2BD3cXktVKju70%3D
R. Prins V.H.J. DeBeer G.A. Somorjai (1989) Catal. Rev.-Sci. Eng. 31 1 Occurrence Handle1:CAS:528:DyaK3cXis1amuw%3D%3D Occurrence Handle10.1080/01614948909351347
J.R. Günter O. Marks T.I. Korányi Z. Paál (1988) Appl. Catal. 39 285
Y. Morimura S. Nakata T. Takatsuka M. Nakamura (1995) Sekiyu Gakkaishi 38 192 Occurrence Handle1:CAS:528:DyaK2MXltlKrtLg%3D
T.I. Korányi V. Rozanov R. Kremó Z. Paál (1990) J. Mol. Catal. 63 31
Author information
Authors and Affiliations
Corresponding author
Additional information
To whom correspondence should be addressed.
E-mail: christopher.landry@uvm.edu
Rights and permissions
About this article
Cite this article
Sorensen, A.C., Landry, C.C. Complete reduction of 2-chloroethylethylsulfide by hydrodesulfurization using mo-doped mesoporous substrates. Catal Lett 100, 135–138 (2005). https://doi.org/10.1007/s10562-004-3441-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-004-3441-x