Abstract
Purpose
To estimate the risk of recurrent cardiovascular events in a real-world population of very high-risk Korean patients with prior myocardial infarction (MI), ischemic stroke (IS), or symptomatic peripheral artery disease (sPAD), similar to the Further cardiovascular OUtcomes Research with proprotein convertase subtilisin–kexin type 9 Inhibition in subjects with Elevated Risk (FOURIER) trial population.
Methods
This retrospective study used the Asan Medical Center Heart Registry database built on electronic medical records (EMR) from 2000 to 2016. Patients with a history of clinically evident atherosclerotic cardiovascular disease (ASCVD) with multiple risk factors were followed up for 3 years. The primary endpoint was a composite of MI, stroke, hospitalization for unstable angina, coronary revascularization, and all-cause mortality.
Results
Among 15,820 patients, the 3-year cumulative incidence of the composite primary endpoint was 15.3% and the 3-year incidence rate was 5.7 (95% CI 5.5–5.9) per 100 person-years. At individual endpoints, the rates of deaths, MI, and IS were 0.4 (0.3–0.4), 0.9 (0.8–0.9), and 0.8 (0.7–0.9), respectively. The risk of the primary endpoint did not differ significantly between recipients of different intensities of statin therapy. Low-density lipoprotein cholesterol (LDL-C) goals were only achieved in 24.4% of patients during the first year of follow-up.
Conclusion
By analyzing EMR data representing routine practice in Korea, we found that patients with very high-risk ASCVD were at substantial risk of further cardiovascular events in 3 years. Given the observed risk of recurrent events with suboptimal lipid management by statin, additional treatment to control LDL-C might be necessary to reduce the burden of further cardiovascular events for very high-risk ASCVD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death globally [1]. Patients who experience a cardiovascular event are at increased risk of further events [2,3,4,5]. Dyslipidemia is known to be a major risk factor for CVD [6, 7], and management of low-density lipoprotein cholesterol (LDL-C) levels is critical in patients at high risk of CVD [8, 9]. High LDL-C is not only a risk factor for primary CVD events, but also for secondary or recurrent CVD events in high-risk CVD patients [10,11,12]. Statins have been the mainstay of LDL-C lowering therapy, but there is still a residual risk of CVD attributable to high LDL-C. Thus, additional drugs may be needed to achieve adequate control in some patients [8].
The Further cardiovascular OUtcomes Research with proprotein convertase subtilisin–kexin type 9 (PCSK9) Inhibition in subjects with Elevated Risk (FOURIER) trial demonstrated the risk of secondary CVD events among high-risk patients and showed that adding evolocumab to statin therapy reduced the risk of recurrent cardiovascular events in patients with clinically evident atherosclerotic cardiovascular disease (ASCVD) [13]. The majority of data about the risk of cardiovascular events in patients with existing CVD and new lipid-lowering treatments come from clinical trials. A few retrospective studies have estimated the risk of cardiovascular events in very high-risk patients who met the inclusion criteria of the FOURIER trial in real-world settings (FOURIER-like populations) in Europe and the USA [14,15,16]. As such, there is a need to obtain data from routine clinical practice, in order to explore any evidence gaps between the real-world setting and clinical trials, particularly in Asian populations.
Thus, the purpose of the current study, using electronic medical records (EMR) data from a tertiary hospital, was to obtain information about the clinical characteristics and to estimate the rates of cardiovascular outcomes in very high-risk Korean patients with prior myocardial infarction (MI), ischemic stroke (IS), or symptomatic peripheral artery disease (sPAD) who met the inclusion criteria for the FOURIER trial. In addition, we tried to evaluate the real-world treatment pattern of LDL-C for the very high-risk Korean population.
Methods
Study Design
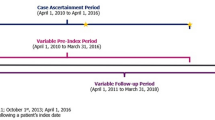
A retrospective observational cohort study was conducted using EMR data from a large tertiary hospital in Korea. The study included adult patients at high risk of cardiovascular events and receiving statin therapy, who were followed for the occurrence of subsequent cardiovascular events. The study period was from 1 January 2000 to 30 November 2016.
Data were obtained from the Asan biomedical research environment database, which is a data warehouse system [17] for the Asan Medical Center (AMC), one of the largest medical institutions in the Republic of Korea. The Asan biomedical research environment database includes de-identified information for 4 million patients and is updated every 3 days. The AMC Heart Registry (AMC-HR) was constructed using fields of structured or unstructured EMR data extracted from the Asan biomedical research environment database using structured query language queries. The AMC-HR was standardized and validated to allow EMR-based research [18]. The AMC-HR comprised 571,157 subjects who met the inclusion criteria of inpatient or outpatient encounters in the cardiology, cardiac surgery, or emergency departments for established or suspected heart disease between 1 January 2000 and 30 November 2016. Collection of data based on implied consent was approved by the AMC institutional review board. De-identification of data was performed in line with the health insurance portability and accountability act for Korea.
Study Population
The study included patients from the AMC-HR who had a history of clinically evident cardiovascular disease (MI, IS, or sPAD) and at least 1 major risk factor or at least 2 minor risk factors (similar to the criteria for the FOURIER trial; Table 1) between 1 January 2000 and 30 November 2013. The index date was defined as the date of the first diagnosis of MI, IS, or sPAD. Definitions and associated ICD-10 or claim codes for the inclusion criteria variables are provided in Online Resource 1. The study population was stratified into three subcohorts according to the index cardiovascular event (MI, IS, and sPAD cohorts) and intensity of statin, to facilitate a detailed analysis.
Patients were excluded if they were aged < 40 or > 85 years, had a recent MI or stroke within 4 weeks of the index date, did not have blood LDL-C levels available, were not receiving a statin, had uncontrolled hypertension, or had a history of hemorrhagic stroke at any time.
Outcomes
The primary endpoint was a composite of MI, stroke, hospitalization for unstable angina (UA), coronary revascularization (coronary artery bypass grafting [CABG] or percutaneous coronary intervention [PCI]), and all-cause mortality at 3-year follow-up. This mirrored the primary endpoint in the FOURIER trial except that cardiovascular-related death was replaced with all-cause mortality for the current study. The secondary endpoint was a composite of MI, stroke, and all-cause mortality.
The follow-up period continued through to 30 November 2016, to ensure that all patients had the opportunity for a 3-year follow-up evaluation. Mortality was captured through documentation of mortality in EMR based upon national health insurance information. Cardiovascular events, including MI, UA, and stroke, were captured by identifying hospitalizations with a relevant primary or secondary ICD-10 diagnosis and adjudicating the event in conjunction with related laboratory results and reports on imaging studies by cardiologists and neurologists. Repeat revascularization was identified by the presence of cost codes for CABG or PCI, with final confirmation by a cardiologist based on the notes for the procedure. Definitions and associated ICD-10 or claim codes for the individual outcome variables are provided in Online Resource 1.
Statistical Analysis
Continuous variables were presented as mean values with standard deviation and categorical variables as numbers with percentages. Continuous variables were compared using the Student t test or the Wilcoxon rank-sum test, and categorical variables were compared using χ2 statistics or Fisher’s exact test, as appropriate.
Incidence rates for the outcomes of interest were calculated as the number of first events divided by the total follow-up person-time at risk until the event or the end of 3-year follow-up, expressed as rates per 100 person-years. Cumulative incidence and survival curves were generated using the Kaplan–Meier method.
Multivariable Cox-proportional hazard regression models were applied to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary and secondary outcomes and for all-cause mortality, according to statin dose. The models included adjustment for age, gender, renal function (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2), hypertension, diabetes, current smoking, and medications (including non-statin lipid-lowing agents, aspirin or P2Y12 inhibitors, beta-blockers, and RAAS inhibitors). Cox-proportional hazard regression models were also used to identify risk factors contributing to the primary and secondary outcomes and to all-cause mortality.
A two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using R software version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org).
Results
Among subjects enrolled in the AMC-HR between 1 January 2000 and 30 November 2013, a total of 32,850 had a history of clinically evident ASCVD and at least 1 major risk factor or at least 2 minor risk factors. After screening, 17,030 of these patients were excluded because they were aged < 40 or > 85 years, had a recent MI or stroke, did not have blood LDL-C levels available, were not receiving a statin, had uncontrolled hypertension, or had a history of hemorrhagic stroke. This resulted in a final study cohort of 15,820 patients, comprising 5419 with a history of MI, 8950 with a history of IS, and 1451 with sPAD (Fig. 1).
The baseline characteristics of the population are summarized in Table 2. In general, the characteristics of the AMC-HR cohort were consistent with those of the FOURIER population, with a few exceptions. A history of MI was less common in AMC-HR than in FOURIER (34.3% versus 81.3% in the FOURIER placebo group), whereas a history of IS was more common (56.6% versus 19.2%). Most patients in AMC-HR were receiving moderate-intensity statin therapy at the time of the index event (81.8%), whereas in FOURIER most were on high-intensity statins (69.1%). Mean LDL-C level was slightly higher in the AMC-HR cohort than in the FOURIER population (104 versus 92 mg/dL), whereas triglyceride and lipoprotein(a) levels tended to be lower in the AMC-HR population. Mean body weight was lower in the AMC-HR cohort than in FOURIER (64.9 ± 14.6 kg versus 85.5 ± 17.4 kg in the FOURIER placebo group).
The baseline characteristics for subcohorts of the AMC-HR population, based on index cardiovascular event or on intensity of statin treatment are summarized in Online Resource 2. Patients in the subcohort with a history of MI were less likely to have hypertension than the subcohorts with a history of IS or sPAD (68.3% versus 78.7% and 78.3%) and more likely to be receiving beta-blockers (90.5% versus 49.7% and 68.3%) and RAAS inhibitors (79.4% versus 67.7% and 72.7%). The IS subcohort had a numerically higher proportion of women (39.4% versus 24.0% and 22.5%), a higher median LDL-C level (109 versus 99.4 and 95.4 mg/dL), and a smaller proportion on ezetimibe (9.1% versus 15.5% and 18.0%) than the MI and sPAD subcohorts. The sPAD subcohort had a greater proportion of patients with diabetes (55.8% versus 39.3% and 41.5%) and patients receiving low-intensity statins (20.1% versus 10.9% and 11.6%) compared with the MI and IS subcohorts. The subcohorts who were receiving low-/moderate-intensity statin therapy had a higher median LDL-C level (105 versus 99.8 mg/dL), lower triglyceride level (117 versus 123 mg/dL), greater proportion of patients with a history of IS (59.1% versus 45.1%), and smaller proportion on beta-blocker treatment (63.3% versus 74.7%), compared with the subcohorts who were receiving high-intensity statins or statins plus ezetimibe.
Cardiovascular Events
The cumulative incidences of the primary and secondary endpoints are summarized in Fig. 2, for the overall AMC-HR cohort and for the subcohorts with a history of MI, IS, or sPAD. For the total cohort, the 3-year cumulative incidence of the composite primary endpoint (MI, stroke, hospitalization for UA, coronary revascularization, and all-cause mortality) was 15.3%, and the 3-year cumulative incidence of the secondary composite endpoint (MI, stroke, and all-cause mortality) was 6.0%. The 3-year cumulative incidences of the composite primary and secondary endpoints were 23.5% and 6.2% in the MI subcohort, 9.5% and 4.6% in the IS subcohort, and 20.7% and 14.0% in the sPAD subcohort.
Three-year cumulative incidences of the primary and secondary endpoints in AMC-HR (for the total cohort and for the subcohorts with a history of MI, IS, or sPAD). Primary endpoint: composite of MI, stroke, hospitalization for UA, revascularization, and all-cause mortality. Secondary endpoint: composite of MI, stroke, and all-cause mortality. IS, ischemic stroke; MI, myocardial infarction; sPAD, symptomatic peripheral artery disease; UA, unstable angina
Cardiovascular event rates are presented in Table 3. For the overall AMC-HR cohort, the 3-year incidence rate for the primary composite endpoint was 5.7 (95% CI 5.5–5.9) per 100 person-years and for the secondary composite endpoint was 2.1 (95% CI 2.0–2.2) per 100 person-years. Among the individual components of the primary endpoint, 3-year incidence rates in the overall cohort ranged from 0.4 (95% CI 0.3–0.4) for MI to 2.8 (95% CI 2.6–2.9) for revascularization procedures. After stratification by subcohort, revascularization and hospitalization for UA was most frequent in the MI cohort, while stroke and all-cause mortality were the second most frequent events in the sPAD and IS cohorts, respectively.
The risk of meeting the primary or secondary endpoints during the 3 years of observation did not differ between recipients of different intensities of statin therapy (Table 4). Nonetheless, there was a trend towards a reduced risk of all-cause mortality in high-intensity treatment, as indicated by hazard ratios of 0.66 (95% CI 0.38–1.13) for high- versus low-intensity statin use and 0.73 (95% CI 0.50–1.07) for high-intensity statin or any statin plus ezetimibe versus low-/moderate-intensity statin without ezetimibe. No association between the baseline LDL-C level and recurrent cardiovascular events was observed (Table 4).
Risk Factors
Results of the analysis of potential risk factors contributing to cardiovascular outcomes within 3 years in the AMC-HR cohort are summarized in Table 5. Factors significantly associated with an increased risk of meeting the primary endpoint included male sex, diabetes, reduced renal function (eGFR < 60 mL/min/1.73 m2), and receiving non-statin lipid-lowering treatment, aspirin or P2Y12 inhibitors, beta-blockers, or RAAS inhibitors. Factors associated with an increased risk of meeting the secondary endpoint included age ≥ 65 years, diabetes, eGFR < 60 mL/min/1.73 m2, and treatment with beta-blockers or RAAS inhibitors. Factors significantly associated with an increased risk of all-cause mortality included age ≥ 65 years, diabetes, eGFR < 60 mL/min/1.73 m2, and treatment with beta-blockers.
LDL-C Follow-Up
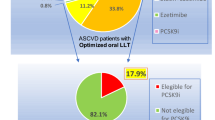
LDL-C levels were re-evaluated within 1 year of the index event in 60.8% of the AMC-HR cohort, with no marked difference seen between subgroups based on the intensity of statin treatment that was being received at the time of the index event (Table 6). LDL-C goals were only achieved in 24.4% of patients within a year; there was a trend towards a higher rate of goal achievement as statin intensity increased (from 18.7% in the low-intensity group to 34.1% in the high-intensity group). Lipid-lowering treatment was intensified in only 0.7% of patients during 1 year of follow-up.
Discussion
In the current study using EMR data, we aimed to estimate the risk of cardiovascular outcomes among very high-risk Korean patients. The main finding was that the risk of further recurrent cardiovascular events was considerable in very high-risk Korean ASCVD patients managed in a routine practice setting as a 3-year incidence rate for the primary composite endpoint of 5.7 per 100 person-years (cumulative incidence of 15.3%). No significant relationship was found between statin intensity and the risk of meeting the study’s primary or secondary endpoints, where the majority used a moderate-intensity statin. In addition, suboptimal LDL-C management during the follow-up period was observed even on statin treatment.
The 3-year cumulative incidence of 15.3% for this endpoint was consistent with that seen in the placebo group of the FOURIER clinical trial (14.6%) [13], although the primary endpoints in these studies differed slightly, in that the AMC-HR study included all-cause mortality, whereas FOURIER included only cardiovascular death. A few other retrospective studies have also assessed cardiovascular events in a FOURIER-like population in a real-world setting. In a US study, the cumulative incidence of the primary composite endpoint (MI, stroke, coronary revascularization, and cardiovascular death [but not hospitalization for UA]) after 2.2 years was 12.2%, and the cardiovascular event rate was approximately 6.0 per 100 person-years [14]. A Swedish study found that 43.8% of patients experienced one or more new events (using the same composite primary endpoint as FOURIER) during a mean follow-up of 7.3 years, with an incidence rate of 7.0 per 100 person-years [15]. A study in the UK found that 33% of patients experienced a new cardiovascular event (same composite primary endpoint as FOURIER) during a mean follow-up of 5.4 years, with an incidence rate of 7.5 per 100 person-years [16]. With previous literatures, the present study adds to the body of evidence from a real-world setting on the high risk of further cardiovascular events in patients with clinically evident ASCVD and the requirement for additional therapeutic options.
There were a few noteworthy differences between the present cohort driven from AMC-HR and the FOURIER trial population. Firstly, there was a higher prevalence of IS and a lower prevalence of MI in the present cohort compared with FOURIER. It is well-known that Asians have a higher prevalence/incidence of IS than MI [17, 18]. Of note, it can be assumed that if the prevalence of MI in the present cohort had been similar to that in FOURIER, the rate of recurrent events of all population would have been higher than was actually observed because recurrent events occurred more frequently in the MI subgroup compared with other subgroups in AMC-HR cohorts. Secondly, most patients (81.8%) in the present study were receiving moderate-intensity statin therapy at the time of the index event, whereas in FOURIER, approximately 70% of patients were receiving high-intensity statin treatment. In fact, the high prevalence of moderate-intensity statin treatment resulting in suboptimal LDL cholesterol reduction might importantly contribute to the lack of association between statin intensity and clinical outcomes in AMC-HR patients. However, interpretation should be cautious since the majority of patients received moderate statin, leading to statistical limitations for pair comparison. On the other hand, East Asian patients achieve a response to statins at a lower dose than required for Caucasians. Consequently, moderate-intensity statin therapy may be sufficient in most East Asian patients, with high-intensity statin therapy required less often [19, 20]. Hence, no significant relationship between statin intensity and the risk of endpoints in the present study may partly be explained by the ethnic disparity.
Management of LDL-C levels is important for the prevention of ASCVD; however, underutilization of lipid-lowering treatment and failure to intensify treatment is common, even for patients at high cardiovascular risk [21,22,23,24,25,26]. Measurement of LDL-C levels is often not performed in patients hospitalized with IS, even among those at high risk of future events [25, 27, 28]. Few data are available about the frequency with which lipid levels are measured during follow-up of very high-risk patients receiving lipid-lowering treatment in real-world practice. In the present study based upon EMR data, during the initial screen process, 26.9% of patients were excluded because they were non-statin users (Fig. 1). In addition, during the follow-up period, LDL-C levels were re-evaluated within 1 year in only 60.8% of patients and only 24.4% of patients achieved LDL-C goals within that time. Despite this, < 1% of patients received intensification of statin therapy. These findings suggest inadequate follow-up of LDL-C levels and highlight the need to provide better lipid monitoring. In addition, the substantially low achievement rate of LDL reduction, similarly reported in the previous investigations, indicates that new therapies such as PCSK9 inhibitors may provide an opportunity to reduce the burden of cardiovascular events among the very high ASCVD population [29, 30].
Several limitations should be noted. The first of these was inherent to the retrospective nature and observational design of the analyses. Confirmation of the results in a prospective study would be helpful. Second, although EMR data enabled reliable real-world estimates, findings from a single center cannot be generalized. Third, on analysis of risk factors for primary and secondary endpoints, cardiovascular protective medications such as aspirin, P2Y12 inhibitors, beta-blockers, or RAAS inhibitors were associated with recurrent events. These results may be due to inherent biases of retrospective analysis, but similar findings were also observed in other retrospective studies [14]. It is reasonable to assume that higher risk patients with comorbidities prone to further cardiovascular events were treated more aggressively with various kinds of cardioprotective medications. Additional methodologically rigorous studies are needed to investigate the risk factors on cardiovascular outcomes in a real-world setting. Finally, the event rate was potentially underestimated because clinical events were captured from a single-center EMR database. Linking it with national claims data or health insurance data might possibly capture the events more accurately.
Conclusion
This study in a routine practice setting in Korea found that very high-risk patients with clinically evident ASCVD were at substantial risk of further cardiovascular events within the subsequent 3 years. In addition, suboptimal LDL-C reduction, even on statin treatment, was observed. These findings may highlight the unmet need and clinical burden among very high-risk ASCVD patients treated with statin therapy and indicate the requirement for additional therapeutic options such as PCSK9 inhibitors.
Availability of Data and Material
Not applicable.
Change history
18 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10557-021-07292-x
References
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210.
Li S, Peng Y, Wang X, et al. Cardiovascular events and death after myocardial infarction or ischemic stroke in an older Medicare population. Clin Cardiol. 2019;42(3):391–9.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–40.
Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD, South London Stroke Register. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003;34(6):1457–63.
Punekar RS, Fox KM, Richhariya A, et al. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol. 2015;38(8):483–91.
Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11(5):276–89.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195–211.
Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017;23(Suppl 2):1–87.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72.
Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61.
Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39(14):1172–80.
Sabatine MS, Giugliano RP, Keech AC, FOURIER Steering Committee and Investigators, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Colantonio LD, Monda KL, Rosenson RS, et al. Characteristics and cardiovascular disease event rates among African Americans and whites who meet the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER) trial inclusion criteria. Cardiovasc Drugs Ther. 2019;33(2):189–99.
Lindh M, Banefelt J, Fox KM, et al. Cardiovascular event rates in a high atherosclerotic cardiovascular disease risk population: estimates from Swedish population-based register data. Eur Heart J Qual Care Clin Outcomes. 2019;5(3):225–32.
Toth PP, Danese M, Villa G, et al. Estimated burden of cardiovascular disease and value-based price range for evolocumab in a high-risk, secondary-prevention population in the US payer context. J Med Econ. 2017;20(6):555–64.
Shin SY, Kim WS, Lee JH. Characteristics desired in clinical data warehouse for biomedical research. Healthc Inform Res. 2014;20(2):109–16.
Ahn I, Na W, Kwon O, et al. CardioNet: a manually curated database for artificial intelligence-based research on cardiovascular diseases. BMC Med Inform Decis Mak. 2021;21(1):29.
Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99(3):410–4.
Kim HS, Lee H, Lee SH, et al. Use of moderate-intensity statins for low-density lipoprotein cholesterol level above 190 mg/dL at baseline in Koreans. Basic Clin Pharmacol Toxicol. 2017;121(4):272–8.
Ferrieres J, De Ferrari GM, Hermans MP, et al. Predictors of LDL-cholesterol target value attainment differ in acute and chronic coronary heart disease patients: results from DYSIS II Europe. Eur J Prev Cardiol. 2018;25(18):1966–76.
Tan NC, Goh CC, Goh SC, Koh YL, Koh KH. The effect of the intensity of lipid-lowering medications on the LDL cholesterol treatment goals of Asian patients with dyslipidaemia in primary care. J Clin Pharm Ther. 2016;41(6):677–83.
Nanna MG, Navar AM, Wang TY, et al. Practice-level variation in statin use and low-density lipoprotein cholesterol control in the United States: results from the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J. 2019;214:113–24.
Chiang CE, Ferrières J, Gotcheva NN, et al. Suboptimal control of lipid levels: results from 29 countries participating in the centralized pan-regional surveys on the undertreatment of hypercholesterolaemia (CEPHEUS). J Atheroscler Thromb. 2016;23(5):567–87.
Ovbiagele B, Hills NK, Saver JL, Johnston SC, CASPR Investigators. Lipid assessment and treatment patterns in hospitalized TIA and ischemic stroke patients. J Hosp Med. 2006;1(4):214–20.
Saposnik G, Fonarow GC, Pan W, AHA Get-with-the-Guidelines Stroke, et al. Guideline-directed low-density lipoprotein management in high-risk patients with ischemic stroke: findings from Get with the Guidelines-Stroke 2003 to 2012. Stroke. 2014;45(11):3343–51.
Mullard AJ, Reeves MJ, Jacobs BS, Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators, et al. Lipid testing and lipid-lowering therapy in hospitalized ischemic stroke and transient ischemic attack patients: results from a statewide stroke registry. Stroke. 2006;37(1):44–9.
Ní Chróinín D, Ní Chróinín C, Akijian L, et al. Suboptimal lipid management before and after ischaemic stroke and TIA-the North Dublin Population Stroke Study. Ir J Med Sci. 2018;187(3):739–46.
Gitt AK, Lautsch D, Ferrières J, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: results from the Dyslipidemia International Study II. Atherosclerosis. 2017;266:158–66.
Yang YS, Yang BR, Kim MS, et al. Low-density lipoprotein cholesterol goal attainment rates in high-risk patients with cardiovascular diseases and diabetes mellitus in Korea: a retrospective cohort study. Lipids Health Dis. 2020;19(5):13.
Acknowledgements
Under the direction of the authors, Kathy Croom and David P. Figgitt, PhD, ISMPP CMPP™, Content Ed Net, provided medical writing and editorial assistance in the preparation of the manuscript, with funding from Amgen Korea.
Funding
This study was funded by Amgen Korea.
Author information
Authors and Affiliations
Contributions
Osung Kwon conceived and designed the analysis, performed the analysis, and wrote the paper; Wonjun Na collected data, contributed data or analysis tools, and wrote the paper; Jaehee Hur collected data and contributed data or analysis tools; Ju Hyeon Kim contributed data or analysis tools and performed the analysis; Tae Joon Jun contributed data or analysis tools and performed the analysis; Heejun Kang contributed data or analysis tools and performed the analysis and statistical validation; Hojoon Lee performed the analysis and wrote the paper; Young-Hak Kim conceived and designed the analysis and performed the analysis.
Corresponding author
Ethics declarations
Ethics Approval/Research Involving Human Participants
For this retrospective observational study, collection of de-identified data from the AMC Heart Registry based on implied consent was approved by the Asan Medical Center institutional review board. De-identification of data was performed in accordance with the health insurance portability and accountability act in Korea.
Consent to Participate
Implied consent was provided due to participation in the AMC Heart Registry.
Consent for Publication
Not applicable.
Conflict of Interest
Hojoon Lee reports employment by Amgen Korea. All other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwon, O., Na, W., Hur, J. et al. Cardiovascular Event Rates in Statin-Treated Korean Patients with Cardiovascular Disease: Estimates from a Real-World Population Using Electronic Medical Record Data. Cardiovasc Drugs Ther 37, 129–140 (2023). https://doi.org/10.1007/s10557-021-07255-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07255-2