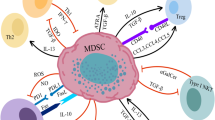
Abstract
Lung cancer and metastasis are two of the most lethal diseases globally and seldom have effective therapies. Immunotherapy is considered as one of the powerful alternatives. Regulatory T cells (Tregs) can suppress the activation of the immune system, maintain immune tolerance to self-antigens, and contribute to immunosuppression of antitumor immunity, which is critical for tumor immune evasion in epithelial malignancies, including lung cancer. The present review gives an overview of the biological functions and regulations of Tregs associated with the development of lung cancer and metastasis and explores the potentials of Treg-oriented therapeutic targets. Subsets and features of Tregs mainly include naturally occurring Tregs (nTregs) (CD4+ nTregs and CD8+ nTregs) and adaptive/induced Tregs (CD4+ iTregs and CD8+ iTregs). Tregs, especially in circulation or regional lymph nodes, play an important role in the progress and metastasis of lung cancer and are considered as therapeutic targets and biomarkers to predict the survival length and recurrence of lung cancer. Increasing understanding of Tregs’ functional mechanisms will lead to a number of clinical trials on the discovery and development of Treg-oriented new therapies. Tregs play important roles in lung cancer and metastasis, and the understanding of Tregs becomes more critical for clinical applications and therapies. Thus, Tregs and associated factors can be potential therapeutic targets for lung cancer immunotherapy.







Similar content being viewed by others
References
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet‐Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108.
Siegel, R. L., Miller, K. D., & Jemal, A. (2015). Cancer statistics, 2015. CA: A Cancer Journal for Clinicians, 65(1), 5–29.
Schwartz Albiez, R., Monteiro, R., Rodriguez, M., Binder, C., & Shoenfeld, Y. (2009). Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clinical and Experimental Immunology, 158, 43–50.
Moriya, K., Wakabayashi, A., Shimizu, M., Tamura, H., Dan, K., & Takahashi, H. (2010). Induction of tumor-specific acquired immunity against already established tumors by selective stimulation of innate DEC-205+ dendritic cells. Cancer Immunology, Immunotherapy, 59(7), 1083–1095.
Nanni, P., Nicoletti, G., Palladini, A., Croci, S., Murgo, A., Antognoli, A., Landuzzi, L., Fabbi, M., Ferrini, S., & Musiani, P. (2007). Antimetastatic activity of a preventive cancer vaccine. Cancer Research, 67(22), 11037–11044.
Zou, W. (2006). Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology, 6(4), 295–307.
Kost, S. E., Kakal, J. A., & Nelson, B. H. (2012). The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clinical Cancer Research, 18(11), 3022–3029.
Tao, H., Mimura, Y., Aoe, K., Kobayashi, S., Yamamoto, H., Matsuda, E., Okabe, K., Matsumoto, T., Sugi, K., & Ueoka, H. (2012). Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer, 75(1), 95–101.
Gershon, R. K., & Kondo, K. (1970). Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology, 18(5), 723.
Berendt, M. J., & North, R. J. (1980). T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. The Journal of Experimental Medicine, 151(1), 69.
Hori, S., Nomura, T., & Sakaguchi, S. (2003). Control of regulatory T cell development by the transcription factor Foxp3. Science’s STKE, 299(5609), 1057.
Bluestone, J. A., & Abbas, A. K. (2003). Natural versus adaptive regulatory T cells. Nature Reviews Immunology, 3(3), 253–257.
Bayer A. L., & Malek T. R. (2009). The role of IL-2 in the development and peripheral homeostasis of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Regulatory T Cells and Clinical Application, 1–20
Wing, K., Onishi, Y., Prieto-Martin, P., Yamaguchi, T., Miyara, M., Fehervari, Z., Nomura, T., & Sakaguchi, S. (2008). CTLA-4 control over Foxp3+ regulatory T cell function. Science’s STKE, 322(5899), 271.
Bombardieri, M., Alunno, A., Kelly, S., Bistoni, O., Pitzalis, C., Whyte, S., Gerli, R., Nocentini, G., & Riccardi, C. (2011). Glucocorticoid induced TNF receptor related protein (GITR) is a marker of regulatory T cells: relationship with FOXP3 expression in healthy donors and in patients with rheumatoid arthritis before and after corticosteroid therapy. Annals of the Rheumatic Diseases, 70, 685–685.
Lourenço, E. V., & La Cava, A. (2011). Natural regulatory T cells in autoimmunity. Autoimmunity, 44(1), 33.
Liu, W., Putnam, A. L., Xu-Yu, Z., Szot, G. L., Lee, M. R., Zhu, S., Gottlieb, P. A., Kapranov, P., Gingeras, T. R., de St, F., Groth, B., Clayberger, C., Soper, D. M., Ziegler, S. F., & Bluestone, J. A. (2006). CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. The Journal of Experimental Medicine, 203(7), 1701–1711.
Miyara, M., Yoshioka, Y., Kitoh, A., Shima, T., Wing, K., Niwa, A., Parizot, C., Taflin, C., Heike, T., & Valeyre, D. (2009). Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity, 30(6), 899–911.
Thornton, A. M., Korty, P. E., Tran, D. Q., Wohlfert, E. A., Murray, P. E., Belkaid, Y., & Shevach, E. M. (2010). Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. The Journal of Immunology, 184(7), 3433.
Gottschalk, R. A., Corse, E., & Allison, J. P. (2012). Expression of Helios in peripherally induced Foxp3+ regulatory T cells. The Journal of Immunology, 188(3), 976–980.
Hippen, K. L., Merkel, S. C., Schirm, D. K., Sieben, C. M., Sumstad, D., Kadidlo, D. M., McKenna, D. H., Bromberg, J. S., Levine, B. L., & Riley, J. L. (2011). Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Science Translational Medicine, 83, 83ra41–83ra41.
Shevach, E. M., & Thornton, A. M. (2014). tTregs, pTregs, and iTregs: similarities and differences. Immunological Reviews, 259(1), 88–102.
Mills, K. H. G. (2004). Regulatory T cells: friend or foe in immunity to infection? Nature Reviews Immunology, 4(11), 841–855.
Battaglia, M., Gregori, S., Bacchetta, R., & Roncarolo, M. G. (2006). Tr1 cells: from discovery to their clinical application. Seminars in Immunology, 18(2), 120–7.
Maynard, C. L., Harrington, L. E., Janowski, K. M., Oliver, J. R., Zindl, C. L., Rudensky, A. Y., & Weaver, C. T. (2007). Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nature Immunology, 8(9), 931–941.
Mandapathil, M., & Whiteside, T. L. (2011). Targeting human inducible regulatory T cells (Tr1) in patients with cancer: blocking of adenosine-prostaglandin E2 cooperation. Expert Opinion on Biological Therapy, 11(9), 1203–1214.
Brun, V., Bastian, H., Neveu, V., & Foussat, A. (2009). Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. International Immunopharmacology, 9(5), 609–613.
Santos, L., Al-Sabbagh, A., Londono, A., & Weiner, H. L. (1994). Oral tolerance to myelin basic protein induces regulatory TGF-β-secreting T cells in Peyer’s patches of SJL mice. Cellular Immunology, 157(2), 439–447.
Weiner, H. L. (2001). Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunological Reviews, 182(1), 207–214.
Carrier, Y., Yuan, J., Kuchroo, V. K., & Weiner, H. L. (2007). Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-β T cell-transgenic mice. The Journal of Immunology, 178(1), 179.
Curotto de Lafaille, M. A., & Lafaille, J. J. (2009). Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity, 30(5), 626–635.
Liu, V. C., Wong, L. Y., Jang, T., Shah, A. H., Park, I., Yang, X., Zhang, Q., Lonning, S., Teicher, B. A., & Lee, C. (2007). Tumor evasion of the immune system by converting CD4+ CD25− T cells into CD4+ CD25+ T regulatory cells: role of tumor-derived TGF-β. The Journal of Immunology, 178(5), 2883.
Al‐Qahtani, D., Anil, S., & Rajendran, R. (2011). Tumour infiltrating CD25+ FoxP3+ regulatory T cells (Tregs) relate to tumour grade and stromal inflammation in oral squamous cell carcinoma. Journal of Oral Pathology & Medicine, 40(8), 636–642.
Mayer, C. T., Floess, S., Baru, A. M., Lahl, K., Huehn, J., & Sparwasser, T. (2011). CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. European Journal of Immunology, 41(3), 716–725.
Wang, R. F. (2008). CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Human Immunology, 69(11), 811–814.
Woo, E. Y., Chu, C. S., Goletz, T. J., Schlienger, K., Yeh, H., Coukos, G., Rubin, S. C., Kaiser, L. R., & June, C. H. (2001). Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Research, 61(12), 4766–4772.
Woo, E. Y., Yeh, H., Chu, C. S., Schlienger, K., Carroll, R. G., Riley, J. L., Kaiser, L. R., & June, C. H. (2002). Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. Journal of Immunology, 168(9), 4272–4276.
Okita, R., Saeki, T., Takashima, S., Yamaguchi, Y., & Toge, T. (2005). CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncology Reports, 14(5), 1269–1273.
Meloni, F., Morosini, M., Solari, N., Passadore, I., Nascimbene, C., Novo, M., Ferrari, M., Cosentino, M., Marino, F., Pozzi, E., & Fietta, A. M. (2006). Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Human Immunology, 67(1–2), 1–12.
Wang, Y. Y., He, X. Y., Cai, Y. Y., Wang, Z. J., & Lu, S. H. (2011). The variation of CD4+CD25+ regulatory T cells in the periphery blood and tumor microenvironment of non-small cell lung cancer patients and the downregulation effects induced by CpG ODN. Targeted Oncology, 6(3), 147–154.
Su, Y. J., Ren, K., Li, H., Ren, X. B., & Wang, C. L. (2007). Clinical significance of CD4+ CD25+ regulatory T-cells detection in tumor-draining lymph nodes of nonsmall cell lung cancer patients. Zhonghua Zhong Liu Za Zhi, 29(12), 922–926.
Ju, S., Qiu, H., Zhou, X., Zhu, B., Lv, X., Huang, X., Li, J., Zhang, Y., Liu, L., Ge, Y., Johnson, D. E., & Shu, Y. (2009). CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function and increase with tumor stage in non-small cell lung cancer patients. Cell Cycle, 8(16), 2578–2585.
Karagoz, B., Bilgi, O., Gumus, M., Erikci, A. A., Sayan, O., Turken, O., Kandemir, E. G., Ozturk, A., & Yaylaci, M. (2010). CD8+CD28− cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Medical Oncology, 27(1), 29–33.
Koyama, K., Kagamu, H., Miura, S., Hiura, T., Miyabayashi, T., Itoh, R., Kuriyama, H., Tanaka, H., Tanaka, J., Yoshizawa, H., Nakata, K., & Gejyo, F. (2008). Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clinical Cancer Research, 14(21), 6770–6779.
Wang W., Hodkinson P., McLaren F., Mackinnon A., Wallace W., Howie S., & Sethi T. (2012). Small cell lung cancer tumour cells induce regulatory T lymphocytes, and patient survival correlates negatively with FOXP3(+) cells in tumour infiltrate. Int J Cancer, 15;131(6):E928–37.
Hasegawa, T., Suzuki, H., Yamaura, T., Muto, S., Okabe, N., Osugi, J., Hoshino, M., Higuchi, M., Ise, K., & Gotoh, M. (2014). Prognostic value of peripheral and local forkhead box P3+ regulatory T cells in patients with non-small-cell lung cancer. Molecular and Clinical Oncology, 2(5), 685–694.
Hanagiri, T., Shigematsu, Y., Shinohara, S., Takenaka, M., Oka, S., Chikaishi, Y., Nagata, Y., Iwata, T., Uramoto, H., & So, T. (2013). Clinical significance of the frequency of regulatory T cells in regional lymph node lymphocytes as a prognostic factor for non-small-cell lung cancer. Lung Cancer, 81(3), 475–479.
Black, C. C., Turk, M. J., Dragnev, K., & Rigas, J. R. (2013). Adenocarcinoma contains more immune tolerance regulatory T-cell lymphocytes (versus squamous carcinoma) in non-small-cell lung cancer. Lung, 191(3), 265–270.
Kinoshita, T., Ishii, G., Hiraoka, N., Hirayama, S., Yamauchi, C., Aokage, K., Hishida, T., Yoshida, J., Nagai, K., & Ochiai, A. (2013). Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Science, 104(4), 409–415.
Li, J.-Y., Duan, X.-F., Wang, L.-P., Xu, Y.-J., Huang, L., Zhang, T.-F., Liu, J.-Y., Li, F., Zhang, Z., & Yue, D.-L. (2014). Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. Journal of Immunology Research, 2014, 286170. doi:10.1155/2014/286170.
Teng, M. W. L., Ritchie, D. S., Neeson, P., & Smyth, M. J. (2011). Biology and clinical observations of regulatory T cells in cancer immunology. Cancer Immunology and Immunotherapy, 61–95.
Peggs, K. S., Quezada, S. A., Korman, A. J., & Allison, J. P. (2006). Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current Opinion in Immunology, 18(2), 206–213.
Keilholz, U. (2008). CTLA-4: negative regulator of the immune response and a target for cancer therapy. Journal of Immunotherapy, 31(5), 431.
Ackerman, A., Klein, O., McDermott, D.F., Wang, W., Ibrahim, N., Lawrence, D.P., Gunturi, A., Flaherty, K.T., Hodi, F.S., Kefford, R., Menzies, A.M., Atkins, M.B., Long, G.V., & Sullivan, R.J. (2014). Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 1;120(11):1695–701.
Erfani, N., Mehrabadi, S. M., Ghayumi, M. A., Haghshenas, M. R., Mojtahedi, Z., Ghaderi, A., & Amani, D. (2012). Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer, 77(2), 306–11.
Karabon, L., Pawlak, E., Tomkiewicz, A., Jedynak, A., Passowicz-Muszynska, E., Zajda, K., Jonkisz, A., Jankowska, R., Krzakowski, M., & Frydecka, I. (2011). CTLA-4, CD28, and ICOS gene polymorphism associations with non-small-cell lung cancer. Human Immunology, 72(10), 947–54.
Mellor, A. L., & Munn, D. H. (2004). IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunology, 4(10), 762–774.
Salvi S., Fontana V., Boccardo S., Merlo D. F., Margallo E., Laurent S., Morabito A., Rijavec E., Dal Bello M. G., & Mora M. (2012). Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunology, Immunotherapy, 1–10.
Leach, D. R., Krummel, M. F., & Allison, J. P. (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science, 271(5256), 1734–1736.
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., Gonzalez, R., Robert, C., Schadendorf, D., & Hassel, J. C. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine, 363(8), 711–723.
Robert, C., Thomas, L., Bondarenko, I., O’Day, S., Weber, J., Garbe, C., Lebbe, C., Baurain, J.-F., Testori, A., & Grob, J.-J. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine, 364(26), 2517–2526.
Lynch, T. J., Bondarenko, I., Luft, A., Serwatowski, P., Barlesi, F., Chacko, R., Sebastian, M., Neal, J., Lu, H., & Cuillerot, J.-M. (2012). Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. Journal of Clinical Oncology, 30(17), 2046–2054.
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A., & Formenti, S. C. (2013). An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunology Research, 1(6), 365–372.
Reck, M., Bondarenko, I., Luft, A., Serwatowski, P., Barlesi, F., Chacko, R., Sebastian, M., Lu, H., Cuillerot, J.-M., & Lynch, T. (2013). Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Annals of Oncology, 24(1), 75–83.
Kamphorst, A. O., & Ahmed, R. (2013). Manipulating the PD-1 pathway to improve immunity. Current Opinion in Immunology, 25(3), 381–388.
Waki, K., Yamada, T., Yoshiyama, K., Terazaki, Y., Sakamoto, S., Matsueda, S., Komatsu, N., Sugawara, S., Takamori, S., & Itoh, K. (2014). PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Science, 105(10), 1229–1235.
Topalian, S. L., Drake, C. G., & Pardoll, D. M. (2012). Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Current Opinion in Immunology, 24(2), 207–212.
Zhang, Y., Huang, S., Gong, D., Qin, Y., & Shen, Q. (2010). Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+T lymphocytes in human non-small cell lung cancer. Cellular & Molecular Immunology, 7(5), 389–395.
Lipson, E. J., Sharfman, W. H., Drake, C. G., Wollner, I., Taube, J. M., Anders, R. A., Xu, H., Yao, S., Pons, A., & Chen, L. (2013). Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clinical Cancer Research, 19(2), 462–468.
Gettinger, S., Horn, L., Gandhi, L., Spigel, D., Antonia, S., Rizvi, N., Powderly, J., Heist, R., Carvajal, R., & Jackman, D. (2014). Long-term survival, clinical activity, and safety of nivolumab (anti-PD-1; BMS-936558, ONO-4538) in patients (pts) with advanced non-small cell lung cancer (NSCLC). International Journal of Radiation Oncology Biology Physics, 90(5), S34.
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., Powderly, J. D., Carvajal, R. D., Sosman, J. A., & Atkins, M. B. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine, 366(26), 2443–2454.
Antonia S. J., Gettinger S., Chow L. Q., Juergens R., Borghaei H., Shen Y., Harbison C., Chen A. C., Ready N. E., & Rizvi N. A. (2014). Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line non-small cell lung cancer (NSCLC): interim phase 1 results. Journal of clinical oncology, 32,5s.
Okamura, T., Fujio, K., Sumitomo, S., & Yamamoto, K. (2012). Roles of LAG3 and EGR2 in regulatory T cells. Annals of the Rheumatic Diseases, 71(Suppl 2), i96–i100.
Gandhi, M. K., Lambley, E., Duraiswamy, J., Dua, U., Smith, C., Elliott, S., Gill, D., Marlton, P., Seymour, J., & Khanna, R. (2006). Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood, 108(7), 2280.
Prigent, P., Dréano, M., & Triebel, F. (1999). Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. European Journal of Immunology, 29(12), 3867–3876.
Brignone, C., Grygar, C., Marcu, M., Perrin, G., & Triebel, F. (2007). IMP321 (sLAG-3) safety and T cell response potentiation using an influenza vaccine as a model antigen: a single-blind phase I study. Vaccine, 25(24), 4641–4650.
Brignone, C., Escudier, B., Grygar, C., Marcu, M., & Triebel, F. (2009). A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clinical Cancer Research, 15(19), 6225–6231.
Brignone, C., Gutierrez, M., Mefti, F., Brain, E., Jarcau, R., Cvitkovic, F., Bousetta, N., Medioni, J., Gligorov, J., & Grygar, C. (2010). First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. Journal of Translational Medicine, 8(1), 71.
Camisaschi, C., Casati, C., Rini, F., Perego, M., De Filippo, A., Triebel, F., Parmiani, G., Belli, F., Rivoltini, L., & Castelli, C. (2010). LAG-3 expression defines a subset of CD4+ CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. The Journal of Immunology, 184(11), 6545–6551.
Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y., & Sakaguchi, S. (2002). Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nature Immunology, 3(2), 135–142.
Cohen, A. D., Schaer, D. A., Liu, C., Li, Y., Hirschhorn-Cymmerman, D., Kim, S. C., Diab, A., Rizzuto, G., Duan, F., & Perales, M. A. (2010). Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. Plos One, 5(5), e10436.
Zhou, P., L’italien, L., Hodges, D., & Schebye, X. M. (2007). Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. The Journal of Immunology, 179(11), 7365–7375.
Houot, R., & Levy, R. (2009). T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood, 113(15), 3546–3552.
Cohen, A. D., Diab, A., Perales, M. A., Wolchok, J. D., Rizzuto, G., Merghoub, T., Huggins, D., Liu, C., Turk, M. J., & Restifo, N. P. (2006). Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Research, 66(9), 4904.
Imai, N., Ikeda, H., Tawara, I., Wang, L., Nishikawa, H., Kato, T., & Shiku, H. (2009). Glucocorticoid-induced tumor necrosis factor receptor stimulation enhances the multifunctionality of adoptively transferred tumor antigen-specific CD8+ T cells with tumor regression. Cancer Science, 100(7), 1317–1325.
Mitsui, J., Nishikawa, H., Muraoka, D., Wang, L., Noguchi, T., Sato, E., Kondo, S., Allison, J. P., Sakaguchi, S., & Old, L. J. (2010). Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clinical Cancer Research, 16(10), 2781.
Pruitt, S. K., Boczkowski, D., de Rosa, N., Haley, N. R., Morse, M. A., Tyler, D. S., Dannull, J., & Nair, S. (2011). Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. European Journal of Immunology, 41(12), 3553–63.
Stephens, G. L., McHugh, R. S., Whitters, M. J., Young, D. A., Luxenberg, D., Carreno, B. M., Collins, M., & Shevach, E. M. (2004). Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+ CD25+ T cells. The Journal of Immunology, 173(8), 5008–5020.
Côté, A. L., Zhang, P., O’Sullivan, J. A., Jacobs, V. L., Clemis, C. R., Sakaguchi, S., Guevara-Patiño, J. A., & Turk, M. J. (2011). Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens. The Journal of Immunology, 186(1), 275.
Joetham, A., Ohnishi, H., Okamoto, M., Takeda, K., Schedel, M., Domenico, J., Dakhama, A., & Gelfand, E. W. (2012). Loss of T regulatory cell suppression following signaling through glucocorticoid-induced tumor necrosis receptor (GITR) is dependent on c-Jun N-terminal kinase activation. Journal of Biological Chemistry, 287(21), 17100–17108.
Haskó, G., Linden, J., Cronstein, B., & Pacher, P. (2008). Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Reviews Drug Discovery, 7(9), 759–770.
Mandapathil, M., Hilldorfer, B., Szczepanski, M. J., Czystowska, M., Szajnik, M., Ren, J., Lang, S., Jackson, E. K., Gorelik, E., & Whiteside, T. L. (2010). Generation and accumulation of immunosuppressive adenosine by human CD4+ CD25highFOXP3+ regulatory T cells. Journal of Biological Chemistry, 285(10), 7176.
Sitkovsky, M., Lukashev, D., Deaglio, S., Dwyer, K., Robson, S., & Ohta, A. (2008). Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. British Journal of Pharmacology, 153(S1), S457–S464.
Häusler S. F. M., Montalbán del Barrio I., Strohschein J., Anoop Chandran P., Engel J. B., Hönig A., Ossadnik M., Horn E., Fischer B., & Krockenberger M. (2011). Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunology, Immunotherapy, 1–14.
Clayton, A., Al-Taei, S., Webber, J., Mason, M. D., & Tabi, Z. (2011). Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. The Journal of Immunology, 187(2), 676.
Hughes, P. D., Belz, G. T., Fortner, K. A., Budd, R. C., Strasser, A., & Bouillet, P. (2008). Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity, 28(2), 197–205.
Janssens, W., Carlier, V., Wu, B., VanderElst, L., Jacquemin, M. G., & Saint-Remy, J. M. R. (2003). CD4+ CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. The Journal of Immunology, 171(9), 4604.
Strauss, L., Bergmann, C., Gooding, W., Johnson, J. T., & Whiteside, T. L. (2007). The frequency and suppressor function of CD4+ CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clinical Cancer Research, 13(21), 6301–6311.
Chen, A., Liu, S., Park, D., Kang, Y., & Zheng, G. (2007). Depleting intratumoral CD4+ CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Research, 67(3), 1291.
Gritzapis, A. D., Voutsas, I. F., Lekka, E., Papamichail, M., & Baxevanis, C. N. (2010). Peptide vaccination breaks tolerance to HER-2/neu by generating vaccine-specific FasL+ CD4+ T cells: first evidence for intratumor apoptotic regulatory T cells. Cancer Research, 70(7), 2686.
Reardon, C., Wang, A., & McKay, D. M. (2008). Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death. The Journal of Immunology, 180(12), 8316–8326.
Kontani, K., Sawai, S., Hanaoka, J., Tezuka, N., Inoue, S., & Fujino, S. (2001). Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. European Journal of Surgical Oncology, 27(2), 180–186.
Cao, X., Fehniger, T. A., Cai, S. F., & Ley, T. J. (2006). The unique roles of murine granzymes (Gzm) A and B for NK-dependent tumor cell killing and regulatory T cell control of NK cells. Proceedings of the American Association for Cancer Research, 2006(1), 149.
Ashley, C. W., & Baecher-Allan, C. (2009). Cutting edge: responder T cells regulate human DR+ effector regulatory T cell activity via granzyme B. The Journal of Immunology, 183(8), 4843–4847.
Soriano, C., Mukaro, V., Hodge, G., Ahern, J., Holmes, M., Jersmann, H., Moffat, D., Meredith, D., Jurisevic, C., & Reynolds, P. N. (2012). Increased proteinase inhibitor-9 (PI-9) and reduced granzyme B in lung cancer: mechanism for immune evasion? Lung Cancer, 77(1), 38–45.
Czystowska, M., Strauss, L., Bergmann, C., Szajnik, M., Rabinowich, H., & Whiteside, T. L. (2010). Reciprocal granzyme/perforin-mediated death of human regulatory and responder T cells is regulated by interleukin-2 (IL-2). Journal of Molecular Medicine, 88(6), 577–588.
Mader, J. S., Ewen, C., Hancock, R. E. W., & Bleackley, R. C. (2011). The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in regulatory T cells. Journal of Immunotherapy, 34(3), 229.
Bruder, D., Probst‐Kepper, M., Westendorf, A. M., Geffers, R., Beissert, S., Loser, K., von Boehmer, H., Buer, J., & Hansen, W. (2004). Frontline: neuropilin-1: a surface marker of regulatory T cells. European Journal of Immunology, 34(3), 623–630.
Mizui, M., & Kikutani, H. (2008). Neuropilin-1: the glue between regulatory T cells and dendritic cells? Immunity, 28(3), 302–303.
Glinka, Y., & Prud’homme, G. J. (2008). Neuropilin-1 is a receptor for transforming growth factor β-1, activates its latent form, and promotes regulatory T cell activity. Journal of Leukocyte Biology, 84(1), 302–310.
Battaglia, A., Buzzonetti, A., Monego, G., Peri, L., Ferrandina, G., Fanfani, F., Scambia, G., & Fattorossi, A. (2008). Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology, 123(1), 129–138.
Jubb, A. M., Strickland, L. A., Liu, S. D., Mak, J., Schmidt, M., & Koeppen, H. (2012). Neuropilin-1 expression in cancer and development. The Journal of Pathology, 226(1), 50–60.
Pyzik, M., & Piccirillo, C. (2007). TGF-ß1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. Journal of Leukocyte Biology, 82(2), 335–346.
Ni, X., Sui, H., Liu, Y., Ke, S., Wang, Y., & Gao, F. (2012). TGF-β of lung cancer microenvironment upregulates B7H1 and GITRL expression in dendritic cells and is associated with regulatory T cell generation. Oncology Reports, 28(2), 615.
Roman, C. D., Morrow, J., Whitehead, R., & Beauchamp, R. D. (2002). Induction of cyclooxygenase-2 and invasiveness by transforming growth factor-β 1 in immortalized mouse colonocytes expressing oncogenic ras. Journal of Gastrointestinal Surgery, 6(3), 304–309.
Takizawa, H., Tanaka, M., Takami, K., Ohtoshi, T., Ito, K., Satoh, M., Okada, Y., Yamasawa, F., Nakahara, K., & Umeda, A. (2001). Increased expression of transforming growth factor-β 1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). American Journal of Respiratory and Critical Care Medicine, 163(6), 1476–1483.
Uva, V., Sfondrini, L., Triulzi, T., Casalini, P., Tagliabue, E., & Balsari, A. (2015). FOXP3 expression in tumor cells and its role in cancer progression. Cancer Research, 75(8), 1703–13.
Um, S. W., Lee, S. H., Kim, H., Kwon, O. J., Kang, J. S., & Lee, W. J. (2011). The regulation of FOXP3 expression by the treatment of TGF-β and the modification of DNA methylation in lung cancer cell lines. Tuberculosis and Respiratory Diseases, 70(3), 206–217.
Marconi, P., Patel, K., Thimothy, L., Buchanan, S., Liptay, M., Coon, J., Bonomi, P., & Borgia, J. (2010). Modulation of the epithelial-to-mesenchymal-like transition by BMP7 and TGF-β in non-small cell lung cancer cell lines in vitro. Journal of Clinical Oncology, 28(15), e21016.
Cao, M., Seike, M., Soeno, C., Mizutani, H., Kitamura, K., Minegishi, Y., Noro, R., Yoshimura, A., Cai, L., & Gemma, A. (2012). MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. International Journal of Oncology, 41(3), 869–75.
Halder, S. K., Cho, Y. J., Datta, A., Anumanthan, G., Ham, A. J. L., Carbone, D. P., & Datta, P. K. (2011). Elucidating the mechanism of regulation of transforming growth factor β type II receptor expression in human lung cancer cell lines. Neoplasia, 13(10), 912.
Sharma, S., Stolina, M., Lin, Y., Gardner, B., Miller, P. W., Kronenberg, M., & Dubinett, S. M. (1999). T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. The Journal of Immunology, 163(9), 5020.
Hatanaka, H., Abe, Y., Kamiya, T., Morino, F., Nagata, J., Tokunaga, T., Oshika, Y., Suemizu, H., Kijima, H., & Tsuchida, T. (2000). Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Annals of Oncology, 11(7), 815–819.
Shih, C. M., Lee, Y. L., Chiou, H. L., Hsu, W. F., Chen, W. E., Chou, M. C., & Lin, L. Y. (2005). The involvement of genetic polymorphism of IL-10 promoter in non-small cell lung cancer. Lung Cancer, 50(3), 291–297.
Huang, M., Sharma, S., Mao, J. T., & Dubinett, S. M. (1996). Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. The Journal of Immunology, 157(12), 5512–5520.
Mocellin, S., Marincola, F. M., & Young, H. A. (2005). Interleukin-10 and the immune response against cancer: a counterpoint. Journal of Leukocyte Biology, 78(5), 1043–1051.
Soria, J. C., Moon, C., Kemp, B. L., Liu, D. D., Feng, L., Tang, X., Chang, Y. S., Mao, L., & Khuri, F. R. (2003). Lack of interleukin-10 expression could predict poor outcome in patients with stage I non-small cell lung cancer. Clinical Cancer Research, 9(5), 1785–1791.
Miotto, D., Cascio, N. L., Stendardo, M., Querzoli, P., Pedriali, M., De Rosa, E., Fabbri, L., Mapp, C., & Boschetto, P. (2010). CD8+ T cells expressing IL-10 are associated with a favourable prognosis in lung cancer. Lung Cancer, 69(3), 355–360.
Castellani, M., Anogeianaki, A., Felaco, P., Toniato, E., De Lutiis, M., Shaik, B., Fulcheri, M., Vecchiet, J., Tetè, S., & Salini, V. (2010). IL-35, an anti-inflammatory cytokine which expands CD4+ CD25+ Treg cells. Journal of Biological Regulators and Homeostatic Agents, 24(2), 131.
Bardel, E., Larousserie, F., Charlot-Rabiega, P., Coulomb-L’Herminé, A., & Devergne, O. (2008). Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. The Journal of Immunology, 181(10), 6898–6905.
Collison, L. W., Pillai, M. R., Chaturvedi, V., & Vignali, D. A. A. (2009). Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. The Journal of Immunology, 182(10), 6121–6128.
Collison, L. W., Chaturvedi, V., Henderson, A. L., Giacomin, P. R., Guy, C., Bankoti, J., Finkelstein, D., Forbes, K., Workman, C. J., & Brown, S. A. (2010). IL-35-mediated induction of a potent regulatory T cell population. Nature Immunology, 11(12), 1093–1101.
Koh, H. S., Lee, C., Lee, K. S., Ham, C. S., Seong, R. H., Kim, S. S., & Jeon, S. H. (2008). CD7 expression and galectin-1-induced apoptosis of immature thymocytes are directly regulated by NF-κB upon T-cell activation. Biochemical and Biophysical Research Communications, 370(1), 149–153.
Miller, M. C., Nesmelova, I. V., Platt, D., Klyosov, A., & Mayo, K. H. (2009). The carbohydrate-binding domain on galectin-1 is more extensive for a complex glycan than for simple saccharides: implications for galectin–glycan interactions at the cell surface. Biochemical Journal, 421(Pt 2), 211.
Wang, J., Lu, Z. H., Gabius, H. J., Rohowsky-Kochan, C., Ledeen, R. W., & Wu, G. (2009). Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. The Journal of Immunology, 182(7), 4036–4045.
Blaskó, A., Fajka-Boja, R., Ion, G., & Monostori, É. (2011). How does it act when soluble? Critical evaluation of mechanism of galectin-1 induced T-cell apoptosis. Acta Biologica Hungarica, 62(1), 106–111.
Liu, F. T., & Rabinovich, G. A. (2005). Galectins as modulators of tumour progression. Nature Reviews Cancer, 5(1), 29–41.
Ito K., & Ralph S. J. (2012). Inhibiting galectin-1 reduces murine lung metastasis with increased CD4+ and CD8+ T cells and reduced cancer cell adherence. Clinical and Experimental Metastasis, 1–12.
Kuo, P.-L., Hung, J.-Y., Huang, S.-K., Chou, S.-H., Cheng, D.-E., Jong, Y.-J., Hung, C.-H., Yang, C.-J., Tsai, Y.-M., & Hsu, Y.-L. (2011). Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. The Journal of Immunology, 186(3), 1521–1530.
Brandt, B., Abou-Eladab, E., Tiedge, M., & Walzel, H. (2010). Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death & Disease, 1(2), e23.
Baratelli, F., Lin, Y., Zhu, L., Yang, S.-C., Heuzé-Vourc’h, N., Zeng, G., Reckamp, K., Dohadwala, M., Sharma, S., & Dubinett, S. M. (2005). Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. The Journal of Immunology, 175(3), 1483–1490.
Sharma, S., Yang, S.-C., Zhu, L., Reckamp, K., Gardner, B., Baratelli, F., Huang, M., Batra, R. K., & Dubinett, S. M. (2005). Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Research, 65(12), 5211–5220.
Baratelli, F., Lee, J. M., Hazra, S., Lin, Y., Walser, T. C., Schaue, D., Pak, P. S., Elashoff, D., Reckamp, K., & Zhang, L. (2010). PGE2 contributes to TGF-β induced T regulatory cell function in human non-small cell lung cancer. American Journal of Translational Research, 2(4), 356.
Soontrapa, K., Honda, T., Sakata, D., Yao, C., Hirata, T., Hori, S., Matsuoka, T., Kita, Y., Shimizu, T., & Kabashima, K. (2011). Prostaglandin E2–prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proceedings of the National Academy of Sciences, 108(16), 6668–6673.
Yukawa, T., Shimizu, K., Maeda, A., Yasuda, K., Saisho, S., Okita, R., & Nakata, M. (2015). Cyclooxygenase-2 genetic variants influence intratumoral infiltration of Foxp3-positive regulatory T cells in non-small cell lung cancer. Oncology Reports, 33(1), 74–80.
Dohadwala, M., Yang, S.-C., Luo, J., Sharma, S., Batra, R. K., Huang, M., Lin, Y., Goodglick, L., Krysan, K., & Fishbein, M. C. (2006). Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E2 induces transcriptional repressors ZEB1 and Snail in non-small cell lung cancer. Cancer Research, 66(10), 5338–5345.
Dohadwala, M., Batra, R. K., Luo, J., Lin, Y., Krysan, K., Põld, M., Sharma, S., & Dubinett, S. M. (2002). Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. Journal of Biological Chemistry, 277(52), 50828–50833.
Krysan, K., Reckamp, K. L., Dalwadi, H., Sharma, S., Rozengurt, E., Dohadwala, M., & Dubinett, S. M. (2005). Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Research, 65(14), 6275–6281.
Kim, J. I., Lakshmikanthan, V., Frilot, N., & Daaka, Y. (2010). Prostaglandin E2 promotes lung cancer cell migration via EP4-βArrestin1-c-Src signalsome. Molecular Cancer Research, 8(4), 569–577.
Horn, L., Milne, G., Sandler, A., Morrow, J., Carbone, D., Shyr, Y., Hayes, A., Campbell, N., & Johnson, D. H. (2009). Urine PGE-M to assess prostaglandin E2 (PGE2) levels in non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 27, e19026.
Acknowledgments
The work was supported by Zhongshan Distinguished Professor Grant (XDW), The National Nature Science Foundation of China (91230204, 81270099, 81320108001, 81270131, 81300010), The Shanghai Committee of Science and Technology (12JC1402200, 12431900207, 11410708600, 14431905100), Operation Funding of Shanghai Institute of Clinical Bioinformatics, and Ministry of Education, Academic Special Science and Research Foundation for PhD Education (20130071110043).
Authors’ contributions
DZ contributed to the collection of data, discussion, writing, and review of figures, and ZHC, DCW, and XDW contributed to the revision, structure design, manuscript preparation, and discussion.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ding Zhang and Zhihong Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, D., Chen, Z., Wang, D.C. et al. Regulatory T cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev 34, 277–290 (2015). https://doi.org/10.1007/s10555-015-9566-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-015-9566-0