Abstract
The value of cardiovascular magnetic resonance (CMR) in assessing and predicting acute right ventricular (RV) dysfunction in patients with anterior ST-segment elevation myocardial infarction (STEMI) remains ascertained. Eighty eight patients with anterior STEMI were prospectively recruited and underwent CMR examinations within one week following the coronary intervention. Patients with RV ejection fraction (RVEF) less than 2 standard deviations below the average at the center (RVEF ≤ 45.0%) were defined as having RV dysfunction. The size of infarction, segmental wall motion, and T1 and T2 mapping values of global myocardium and the interventricular septum (IVS) were measured. Predictive performance was calculated using receiver-operating characteristic curve analysis and logistic regression test. Twenty two patients presented with RV dysfunction. The RV dysfunction group had a larger IVS infarct extent (54.28 ± 10.35 vs 33.95 ± 15.09%, P < 0.001) and lower left ventricle stroke volume index (33.93 ± 7.96 vs 42.46 ± 8.14 ml/m2, P < 0.001) compared to the non-RV dysfunction group. IVS infarct extent at 48.8% best predicted the presence of RV dysfunction with an area under the curve of 0.864. Left ventricular stroke volume index (LVSVI) and IVS infarct extent were selected by stepwise multivariable logistic regression analysis. Lower LVSVI (odds ratio [OR] 0.90; 95% confidence interval [CI], 0.79 to 0.99; P = 0.044) and higher IVS infarct extent (OR 1.16; 95% CI 1.05 to 1.33; P = 0.01) were found to be independent predictors for RV dysfunction. In patients with anterior STEMI, those with larger IVS infarct extent and worse LV function are more likely to be associated with RV dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior ST-segment elevation myocardial infarction (STEMI), caused by the occlusion of the left anterior descending (LAD) artery, is associated with a poor prognosis due to a large extent of myocardial damage [1]. Although the infarction of the anterior wall results in left ventricular (LV) dysfunction, the focus on LV dysfunction has often led to overlooking right ventricular (RV) dysfunction. RV dysfunction has been identified as a risk factor for heart failure and mortality in patients with STEMI [2]. Previous studies have noted that RV is more tolerant to ischemia than LV is, but the prevalence of RV dysfunction has been reported to vary widely from studies. An autopsy study by Andersen et al. [3]. found that 84% of hearts with coronary heart disease had RV scars. While RV function is a predictor of post-infarction adverse events [4, 5], accurately sizing and calculating the function of the RV is difficult to achieve via echocardiography owing to its complex geometry [6, 7]. Therefore, it would be beneficial to identify additional indicators of RV injury and predictors of RV dysfunction to better evaluate RV involvement in patients experiencing anterior STEMI.
Studies have demonstrated that LV dysfunction is closely correlated with the presence of RV dysfunction [8, 9]. The interaction between the two ventricles through the interventricular septum (IVS) has been proposed as a possible explanation for this interrelated injury, particularly in patients with anterior myocardial infarction [10]. Therefore, LV parameters could be useful in alerting healthcare providers about the presence of RV dysfunction. Furthermore, significant advancements have been made in quantifying biventricular function, and cardiovascular magnetic resonance (CMR) is widely recognized as the gold standard for noninvasive assessment of cardiac function, anatomy, and myocardial characterization. Compared to echocardiography, CMR is superior with regard to the ability to accurately visualize the endo- and epicardium [11, 12], providing a more precise tool for quantifying RV function and risk stratification [13]. Additionally, multiple CMR sequences can be used to characterize LV tissue, with proven correlation to myocardial pathology results [14]. CMR can also detect subclinical changes in patients with preserved LV ejection fraction (LVEF) and predict the prognosis for patients with cardiomyopathy [15].
The relationship between RV and LV morphology is complex [7], and the correlation between CMR-derived LV parameters and RV dysfunction has not been systematically investigated heretofore. The aim of this study is to utilize CMR technology to investigate the occurrence, clinical characteristics, and clinical correlates of RV dysfunction in patients with acute anterior STEMI.
Materials and methods
Study group
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of West China Hospital, Sichuan University. All participants provided written informed consent prior to their inclusion in the study. The confidentiality of personal data was maintained and only used for the purpose of this trial.
Data were prospectively collected from our center from September 2015 to March 2017. A total of 121 consecutive patients referred to the chest pain center were initially recruited for this study. Patients who were diagnosed with first-time anterior STEMI based on the STEMI clinical guidelines [16] and received primary percutaneous coronary intervention (PPCI) were included in the study. The culprit infarct-related artery was the LAD. Successful PCI was defined as a residual stenosis of less than 20% and thrombolysis in myocardial infarction (TIMI) grade 3 flow, without any serious complications.
All patients included in the study underwent CMR examination within one week after PPCI. The exclusion criteria were as follows: (1) RV infarction, as defined by an ST-segment elevation of 0.1 mV or greater in leads V3R and V4R on ECG at presentation [16] and/or any RV wall motion abnormality detected by echocardiography; (2) previous coronary artery bypass surgery or revascularization intervention; (3) any cardiomyopathy, valvular disease, or congenital heart disease; (4) severe diseases that independently affect RV function, such as old inferior wall infarction, old RV infarction, severe chronic obstructive pulmonary disease, interstitial lung disease, pulmonary embolism, and primary pulmonary hypertension; (5) contraindication to CMR (e.g., pacemaker and claustrophobia) or insufficient image quality; (6) pregnancy; (7) inability to lie in a supine position; (8) an estimated glomerular filtration rate of 30 ml/(min·1.73 m2) or less; (9) patients with significant stenosis (> 50%) affecting RV branch or right coronary artery (RCA) proximal to RV branch; and (10) consent refusal.
Basic characteristics such as age, sex, body mass index (BMI), and body surface area (BSA), as well as cardiovascular risk factors like smoking history, underlying diseases including hypertension, diabetes mellitus, hypercholesterolemia, and relevant laboratory findings (triglyceride, cholesterol, serum creatinine, cardiac troponin [cTnT] peak, B-type natriuretic peptide [BNP] peak, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], white blood cell [WBC] count, and platelet [PLT] count) were collected from the patient’s history recordings. Peri-PPCI variables including pre- and post- TIMI flow, pain to device time (time to PCI-mediated reperfusion), and whether thrombectomy was performed were also recorded. BSA was calculated using the formula: BSA (m2) = 0.0061 × height (cm) + 0.0128 × weight (kg) − 0.1529.
Magnetic resonance imaging
CMR examinations were conducted using a 3.0 T MR scanner (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany). The examinations were performed by an MRI trainee technician, who was supervised by a senior technician with at least 5 years of experience in CMR imaging. The patients were placed in a supine position, and an 18-element body phased array coil was used during the examination. A standard electrocardiographic triggering device was used for heart rate triggering and monitoring.
A balance steady-state free precession sequence was used to acquire contiguous short-axis (SAX) slices encompassing the whole LV, as well as standard two-, three-, and four-chamber long-axis (LAX) cine images during repeated breath holds. The parameters for this sequence were: repetition time (TR), 40.25 ms; echo time (TE), 1.2 ms; flip angle, 40°; field of view (FOV), 340 × 285 mm2; matrix, 208 × 139; slice thickness, 8 mm; and number of phases, 25. T2 mapping was acquired with a T2-prepared steady-state free precession sequence, with the following parameters: TR, 272.95 ms; TE, 1.06 ms; flip angle, 35°; matrix, 192 × 116, FOV: 360 × 289 mm2; and slice thickness, 8 mm. Native T1 mapping was performed using a modified look-locker inversion recovery sequence, with the following parameters: TR, 319.83 ms; TE, 1.17 ms; flip angle, 35°; matrix, 256 × 145; field of view, 360 × 307 mm2; and slice thickness, 8 mm. A 5s(3s)3s modified sampling protocol was used. Standard SAX slices at apical, middle, and basal level of the LV, as well as two-, three-, and four-chamber colored native T1 and T2 maps, were generated after motion correction of the set of images acquired at different inversion times. A dose of 0.1 mmol/kg gadolinium (gadodiamide, 469.01 mg/ml; gadopentetic acid dimeglumine salt injection, Bayer, Germany) was injected at a flow rate of 2.5–3.0 ml/s. Late gadolinium enhancement (LGE) images were then acquired 10–15 min after contrast administration using a phase-sensitive inversion recovery sequence, with the following parameters: TR, 824 ms; TE, 3.5 ms; flip angle, 40°; matrix, 256 × 127, FOV, 400 × 275 mm; and slice thickness, 8 mm.
Image post-processing
Image post-processing and analysis were performed by two radiologists who were blinded to the clinical data, using commercially available software (Cvi42; Circle Cardiovascular Imaging, Inc., Calgary, Canada). In case of any discrepancy or disagreement between the two radiologists, a third senior radiologist with over 10 years of experience was consulted. Global cardiac function indexes, including LV/RV end-diastolic volume (EDV) and end-systolic volume (ESV), as well as ejection fraction (EF), were analyzed in the SAX view following the standardized protocol of Society of Cardiovascular Magnetic Resonance post-processing guideline [17]. LV/RV stroke volume (SV) was calculated as LV/RV EDV-LV/RV ESV. The LV/RV EDV index (EDVI), ESV index (ESVI) and SV index (SVI) was calculated as LV/RV EDV, ESV and SV/BSA. RV dysfunction was defined as RV ejection fraction (RVEF) of less than 45% [18].
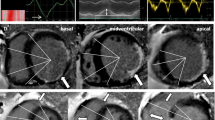
Subsequently, the infarction area was defined on the LGE SAX images as an area with a signal intensity greater than 5 standard deviations (SD) above the average intensity of the region of interest (ROI) drawn at the remote normal myocardium [19]. Microvascular obstruction (MVO) area was determined by visually recognizing the core area inside the infarcted myocardium without enhancement on the baseline LGE images. The overall size and volume of IVS were outlined and analyzed separately, with an extra ROI placed to calculate the infarction size at the IVS region (Fig. 1). The native T1 and T2 of the IVS were measured. End-diastolic wall thickness (EDWT) and end-systolic wall thickness (ESWT) were calculated by referring to the 16-AHA segmentation method. Systolic wall thickening was calculated by using the formula: (ESWT − EDWT)/EDWT × 100%.
Post-processing of the CMR images. A The high signal intensity in the left ventricular anterior wall and the IVS on LGE imaging indicates infarction area, while the low signal intensity (white arrows) inside the infarction area indicates MVO. B Exhibited the infarction area and MVO using red and blue color. The infarct extent is calculated as the area of red and blue as a percentage of the total myocardial mass. C The area (white arrows) between the anterior (blue point) and posterior (red point) right ventricle insertion points indicates IVS. The infarction area as demonstrated by the red and blue color inside IVS was recorded as the infarction size in IVS (CMR cardiovascular magnetic resonance, LGE late gadolinium enhancement, MVO microvascular obstruction, IVS interventricular septum)
Myocardial strain analysis was performed in a semi-automated manner using the Cvi42 5.12.1 software (Tissue Tracking, Circle Cardiovascular Imaging Inc., CVI42® 5.12.1 software, Calgary, Alberta). A stack of SAX images between the LV apex and the mitral valve plane, as well as two- and four-chamber LAX cine images were used for the analysis. Endocardial and epicardial borders were manually contoured, and both the anterior and posterior RV insertion points were manually determined at the end-diastolic phase. In addition, 2D global longitudinal strain (GLS) was acquired from the two LAX slices, while 2D global circumferential strain (GCS) and 2D global radial strain (GRS) were acquired from the SAX slices. The strain analysis was performed by the software in a semi-automated manner.
Statistical analysis
The patients were divided into two groups, RV dysfunction and non-RV dysfunction, to compare the CMR data. Continuous measures were compared using either a two-sided non-paired Student’s t-test or Mann–Whitney U-test as appropriate. Categorical measures were compared using a chi-square test. Pearson’s linear regression analyses were used to determine the relationships between RVEF and LV CMR variables. The linear regression coefficients (beta), correlation coefficients, and P-values were reported. Optimal cut-off values for predicting RV dysfunction with CMR indexes were determined by receiver operating characteristic (ROC) curve analysis, where sensitivity and specificity intersected. Univariate logistic regression analysis was used to test the associations between LV CMR variables and RV dysfunction. Stepwise multiple logistic regression analysis was performed to identify clinical and imaging variables associated with RV dysfunction. The optimal threshold was determined by Youden index (Youden index = sensitivity + specificity − 1) [20]. Variables with a P-value of less than 0.05 on bivariable analysis were considered candidate variables in the multivariate logistic regression model. In all analyses, a P-value of less than 0.05 was considered statistically significant, and all the analyses were performed using the R project V 3.3.1 (R Project for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics of the study population
Out of the 122 patients with PPCI who were included in the study, 24 and nine were excluded due to occlusion of the RCA and left circumflex (LCx) arteries, respectively. One patient was excluded from the study due to unsatisfactory image quality. Finally, 88 patients [age: 57.34 ± 11.18 years; males/females: 80/8] were included in the analysis (Fig. 2). Among the included patients, 77 (87.5%) were current smokers. Additionally, 41 out of 88 (46.6%) had hypertension, and 19 (21.6%) had diabetes mellitus (Table 1). The majority of the patients (n = 83, 94.3%) presented with a TIMI flow grade of 0 or I before the intervention, and all patients achieved a TIMI flow grade of III after the intervention. None of the patients died during hospitalization.
A total of 22 out of 88 (25%) patients presented with RV dysfunction. Patients with RV dysfunction had slightly higher peak BNP levels (2734.50 [1207.25, 3305.75] pg/ml vs 1401.50 [796.50, 2512.00], P = 0.056) and significantly higher peak cTnT levels (8036.50 [5577.25, 10001.00] vs 4588.00 [1875.50, 6781.00], P < 0.001) than patients without RV dysfunction. Additionally, heart rate was significantly higher for patients with RV dysfunction than for patients without the same (90.00 [76.25, 100.75] vs 76.00 [67.25, 85.00], P = 0.002). A poor pre-PCI TIMI flow grade was found in patients with RV dysfunction. However, no prominent difference was found between the two groups concerning cardiovascular risk factors and PPCI-related variables (Table 1).
CMR characteristics between the patients with and without RV dysfunction
In the RV dysfunction group, a total of 21 out of 22 (95.5%) patients presented with MVO, which was significantly higher than that in the non-RV dysfunction group (95.5% vs 50.0%, P < 0.001). Patients with RV dysfunction had a significantly larger infarction extent (45.40% ± 14.63% vs 29.03% ± 14.37%, P < 0.001), reduced LVEF (39.17% ± 10.61% vs 49.64% ± 9.29%, P < 0.001), lower LVSVI (33.93 ± 7.96 ml/m2 vs 42.46 ± 8.14 ml/m2, P < 0.001), as well as decreased GLS [− 9.11 (− 10.39, − 7.07)% vs − 11.15 (− 12.24, − 9.18)%, P = 0.006], GCS (− 10.73% ± 3.45% vs − 14.18% ± 3.32%, P = 0.0010), and GRS [13.09 (11.65, 18.24)% vs 19.28 (15.17, 22.70)%, P < 0.001]. Between-group differences were found in RVEDVI and RVSVI but not RVESVI (Table 2).
Regarding the IVS measurements, patients with RV dysfunction had a larger infarction area in the IVS region (all P < 0.01) than in the non-IVS region. Additionally, significantly lower T2 [43.98 (41.05, 46.61) vs 46.79 (44.14, 49.01), P = 0.006] was observed in patients with RV dysfunction compared with those without it, while the native T1 was not significant (1395.55 ± 71.98 vs 1426.07 ± 82.862, P = 0.0063). No significant differences existed in any of the IVS’s EDWT and ESDW between patients with RV dysfunction and those without the same. However, systolic wall thickening was lower in patients with RV dysfunction than in those without it [37.92 (26.08, 49.14) % vs 51.77 (37.98, 62.63) %, P = 0.015].
Correlations of RV dysfunction with LV CMR variables
The cTnT peak, presence of MVO, global LGE percentage, LVEF, LVSVI, LV-GCS and GRS, IVS’s infarction size, and IVS T2 were all found to be associated with RV dysfunction. However, only LVSVI (odds ratio [OR], 0.90 [95% CI 0.79 to 0.99; P = 0.044) and IVS’s infarct extent (OR 1.16 [95% CI 1.05 to 1.33; P = 0.01) were identified as independent predictors for RV dysfunction (Table 3).
The diagnostic accuracy of RV dysfunction using different CMR variables is presented in Fig. 3. The single cut-off that maximised sensitivity and specificity (Youdens index) for LVSVI was 37.567 ml/m2 (AUC, 0.783) and IVS infarct extent was 48.8% (AUC, 0.864) were found to be the best predictors of RV dysfunction during the acute phase. The positive predictive value (PPV) and negative predictive value (NPV) at Youdens index for LVSVI were 50.0% and 89.2%, respectively, whilst the PPV and NPV for IVS infarct extent were 62.1% and 93.2%, respectively.
Discussion
Patients with anterior STEMI combined with RV dysfunction are at a higher risk of in-hospital and long-term mortality [5, 21]. Anterior STEMI with RV involvement may have its clinical manifestations obscured by LV dysfunction. Inappropriate management can precipitate severe hemodynamic derangements, potentially culminating in mortality. Early identification of RV function amidst LV ischemia post-myocardial infarction holds considerable significance for guiding clinical diagnosis, therapeutic interventions, and prognostic evaluation. Our study showed that approximately one-fourth of patients with anterior STEMI had RV dysfunction. To our knowledge, this is the first CMR study that has demonstrated the correlation between RV dysfunction and CMR imaging parameters of LV and IVS for patients with anterior STEMI. Our results suggest that LV dysfunction accompanied by primarily IVS-related infarction might indicate RV dysfunction and should be considered a potential risk factor for RV dysfunction when assessing patients with anterior STEMI.
The percentage of patients presenting with RV abnormalities in our study was 25%, which is within the range reported by previous studies [18]. Masci et al. [22] reported the highest rate at approximately 50%, when measured by myocardial edema. In contrast, we defined RV dysfunction based only on RVEF and reported a lower rate of RV dysfunction. However, we assumed that more patients may have RV injury but no significant functional abnormality. Matthias et al. [23] reported a similar rate of RV dysfunction at 16.5%, for which the definition of RV injury required both myocardial edema and reduced wall motion. Given the irregular geometry of the RV and measurement variance, it has been difficult to reach a common consensus on the definition of RV dysfunction. A review reported less than 45% as the incidence of RV dysfunction in patients with non-ischemic disease [24, 25], while no definite cut-off for patients with ischemic diseases has been determined. In this study, we used RVEF < 45% as the standard to avoid measurement variance from other centers. RV dysfunction defined using RVEF has been proven to be associated with adverse clinical outcomes for patients with ischemic heart disease [26].However, the clinical significance of subclinical RV dysfunction in the absence of a reduced RVEF necessitates further validation [27]. RV strain could potentially serve as a valuable tool in aiding our understanding of this condition.
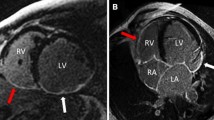
We found that patients with RV dysfunction had a larger reduction in LVEF and LVSVI, more infarction size at IVS, and a higher frequency of MVO. Consistent with an echocardiographic study [21], all patients with RV dysfunction in our study presented with LV dysfunction, except for one patient without the presence of MVO but with a large involvement of the IVS (54.4% area of the whole IVS; Fig. 4 and Supplemental Fig. 1). Tricuspid annular plane systolic excursion, a quantitative index of RV systolic function, has been demonstrated to positively correlate with LVEF in an echocardiographic study, implying that a reduction in LVEF may adversely affect RV systolic function [5, 21]. Another study also found that impaired LV diastolic function resulting from anterior STEMI contributes to RV dysfunction six months later [28].
Case presentation: A 67-year old female with left anterior descending artery occlusion. A Infarction at the anterior and interventricular septum (IVS) was detected by late gadolinium enhancement (black arrows). The anterior (blue) and posterior (red) right ventricle insertion points were manually marked. The myocardium was then divided into six segments for the middle layer. B Native T1 mapping showed higher intensity at IVS (black arrow). C T2 mapping showed higher intensity at IVS and the anterior wall (black arrows)
LGE imaging can provide additional prognostic information in patients with myocardial infarction [29, 30]. In our study, we found that patients with RV dysfunction had a larger infarction size of the whole heart. However, our regression analysis indicated that the global LGE percentage was not statistically significant in the multivariable analysis. The result demonstrated a positive correlation between IVS involvement and the presence of RV dysfunction, suggesting a joint role that IVS potentially plays in the interaction between RV with LV [23]. IVS is the “engine” of RV, as the torsions of the IVS help RV ejection, and the alternating pattern of torsions helps the rapid filling of RV. The left coronary artery is tasked with supplying the anterior two-thirds of the myocardium in the IVS. Consequently, when the left coronary artery is stenosed, the blood supply to the anterior septum diminishes, impairing the IVS’s mobility and, as a result, compromising the overall functional capacity of the RV. Our study contributes to this understanding by demonstrating that IVS’s T1 and T2 mapping values, rather than wall motion indexes, were reduced in patients with RV dysfunction. Native T1 and T2 values have been observed to increase in myocardial inflammation and edema and to decrease in the presence of MVO for STEMI patients [31,32,33]. We believe that the reduction of T1 and T2 in IVS regions for patients with RV dysfunction reflects the presence of MVO. We further noted that within the cohort exhibiting RV dysfunction, who may have a greater extent of IVS infarction, the T2 values of the IVS were somewhat reduced. This reduction could be attributed to the potential occurrence of MVO in certain patients with extensive infarct size. MVO is characterized by a diminished signal on T2-weighted imaging, which corresponds with our observed reduction in T2 values for the IVS in patients with RV dysfunction [34]. Thus, our results suggest that it might be the severity of injury at IVS that affects RV function, rather than simply taking part in the RV myocardium movement.
Nevertheless, several limitations should be noted in our study. First, this was a single-center study with a limited number of patients. Therefore, further multiple center studies should be performed to validate the predictors of RV dysfunction. Second, neither of the finding of LV dysfunction or large IVS infarction size necessarily indicate RV dysfunction. The research undertaken thus far, along with the evidence gathered, has not reached the level of sufficiency required to conclusively establish a cause-and-effect relationship between LV dysfunction and RV dysfunction. The NPV was high (85.2–93.2%) while the PPV was relatively low. Thus, our study only suggests that LV dysfunction and large involvement of the IVS could be used as screening tool for alerting RV dysfunction. Other parameters should be closely measured and documented to decide if the patients would develop RV dysfunction. Third, parametric mapping measures were not performed for RV myocardium. Due to the thickness of RV myocardium and artifacts, it was difficult to ensure reproducibility in measuring native T1 and T2 values on RV myocardium. Therefore, this analysis was not performed in our study. In future studies, RV dysfunction should be assessed from multiple aspects rather than RVEF.
Conclusion
RV dysfunction occurs in approximately one-fourth of patients with anterior STEMI. Patients with a larger IVS infarction and reduced LV function are more likely to be associated with RV dysfunction. Almost all cases of RV dysfunction are accompanied by LV dysfunction. However, the prognostic and clinical value of these associated factors in patients with RV dysfunction remains to be ascertained. Further studies are required to validate these findings and to explore the clinical implications of RV dysfunction in patients with anterior STEMI.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CMR:
-
Cardiovascular magnetic resonance
- EDVI:
-
End-diastolic volume index
- EDWT:
-
End-diastolic wall thickness
- ESVI:
-
End-systolic volume index
- ESWT:
-
End-systolic wall thickness
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- GRS:
-
Global radial strain
- IVS:
-
Interventricular septum
- LAD:
-
Left anterior descending
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVEF:
-
LV ejection fraction
- MVO:
-
Microvascular obstruction
- RV:
-
Right ventricular
- STEMI:
-
ST-segment elevation myocardial infarction
- TIMI:
-
Thrombolysis in myocardial infarction
References
Reindl M, Holzknecht M, Tiller C et al (2020) Impact of infarct location and size on clinical outcome after ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Int J Cardiol 301:14–20. https://doi.org/10.1016/j.ijcard.2019.11.123
Houston BA, Brittain EL, Tedford RJ (2023) Right ventricular failure. N Engl J Med 388(12):1111–1125. https://doi.org/10.1056/NEJMra2207410
Andersen HR, Falk E, Nielsen D (1987) Right ventricular infarction: frequency, size and topography in coronary heart disease: a prospective study comprising 107 consecutive autopsies from a coronary care unit. J Am Coll Cardiol 10(6):1223–1232. https://doi.org/10.1016/s0735-1097(87)80122-5
Stiermaier T, Backhaus SJ, Matz J et al (2020) Frequency and prognostic impact of right ventricular involvement in acute myocardial infarction. Heart. https://doi.org/10.1136/heartjnl-2020-317184
Keskin M, Uzun AO, Hayıroğlu Mİ, Kaya A, Çınar T, Kozan Ö (2019) The association of right ventricular dysfunction with in-hospital and 1-year outcomes in anterior myocardial infarction. Int J Cardiovasc Imaging 35(1):77–85. https://doi.org/10.1007/s10554-018-1438-6
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–788. https://doi.org/10.1016/j.echo.2010.05.010
Mauger C, Gilbert K, Lee AM et al (2019) Right ventricular shape and function: cardiovascular magnetic resonance reference morphology and biventricular risk factor morphometrics in UK Biobank. J Cardiovasc Magn Reson 21(1):41. https://doi.org/10.1186/s12968-019-0551-6
Desai RV, Meyer P, Ahmed MI et al (2011) Relationship between left and right ventricular ejection fractions in chronic advanced systolic heart failure: insights from the best trial. Eur J Heart Fail 13(4):392–397. https://doi.org/10.1093/eurjhf/hfq206
Friedberg MK (2018) Imaging right–left ventricular interactions. JACC Cardiovasc Imaging 11(5):755–771. https://doi.org/10.1016/j.jcmg.2018.01.028
Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ (1985) Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation 71(3):473–480. https://doi.org/10.1161/01.cir.71.3.473
Erley J, Tanacli R, Genovese D et al (2020) Myocardial strain analysis of the right ventricle: comparison of different cardiovascular magnetic resonance and echocardiographic techniques. J Cardiovasc Magn Reson 22(1):51. https://doi.org/10.1186/s12968-020-00647-7
Gavara J, Marcos-Garces V, Lopez-Lereu MP et al (2022) Magnetic resonance assessment of left ventricular ejection fraction at any time post-infarction for prediction of subsequent events in a large multicenter STEMI registry. J Magn Reson Imaging 56(2):476–487. https://doi.org/10.1002/jmri.27789
Miszalski-Jamka T, Klimeczek P, Tomala M et al (2010) Extent of RV dysfunction and myocardial infarction assessed by CMR are independent outcome predictors early after STEMI treated with primary angioplasty. JACC Cardiovasc Imag 3(12):1237–1246. https://doi.org/10.1016/j.jcmg.2010.09.018
Zhao S (2020) Letter to the editor: is it time for imaging to level with pathology? Int J Cardiovasc Imag 36(11):2249–2250. https://doi.org/10.1007/s10554-020-01936-z
Scatteia A, Baritussio A, Bucciarelli-Ducci C (2017) Strain imaging using cardiac magnetic resonance. Heart Fail Rev 22(4):465–476. https://doi.org/10.1007/s10741-017-9621-8
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60(16):1581–1598. https://doi.org/10.1016/j.jacc.2012.08.001
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): board of trustees task force on standardized post-processing. J Cardiovasc Magn Reson 22(1):19. https://doi.org/10.1186/s12968-020-00610-6
Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R (2019) Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 73(12):1463–1482. https://doi.org/10.1016/j.jacc.2018.12.076
Bodi V, Monmeneu JV, Ortiz-Perez JT et al (2016) Prediction of reverse remodeling at cardiac MR imaging soon after first ST-segment-elevation myocardial infarction: results of a large prospective registry. Radiology 278(1):54–63. https://doi.org/10.1148/radiol.2015142674
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1):32–35
Radwan HI, Alhoseeny AMA, Ghoniem SM, Nashy BNE, Shehata IE (2023) Early right ventricular dysfunction after primary percutaneous coronary intervention in anterior versus isolated inferior myocardial infarction assessed by tissue doppler imaging and speckle tracking echocardiography. Heart Fail Rev 28(2):407–417. https://doi.org/10.1007/s10741-022-10278-y
Masci PG, Francone M, Desmet W et al (2010) Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction: characterization with cardiovascular magnetic resonance. Circulation 122(14):1405–1412. https://doi.org/10.1161/CIRCULATIONAHA.110.940254
Grothoff M, Elpert C, Hoffmann J et al (2012) Right ventricular injury in ST-elevation myocardial infarction: risk stratification by visualization of wall motion, edema, and delayed-enhancement cardiac magnetic resonance. Circ Cardiovasc Imaging 5(1):60–68. https://doi.org/10.1161/CIRCIMAGING.111.967810
Gulati A, Ismail TF, Jabbour A et al (2013) The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 128(15):1623–1633. https://doi.org/10.1161/CIRCULATIONAHA.113.002518
Pueschner A, Chattranukulchai P, Heitner JF et al (2017) The prevalence, correlates, and impact on cardiac mortality of right ventricular dysfunction in nonischemic cardiomyopathy. JACC Cardiovasc Imag 10(10):1225–1236. https://doi.org/10.1016/j.jcmg.2017.06.013
Sabe MA, Sabe SA, Kusunose K, Flamm SD, Griffin BP, Kwon DH (2016) Predictors and prognostic significance of right ventricular ejection fraction in patients with ischemic cardiomyopathy. Circulation 134(9):656–665. https://doi.org/10.1161/CIRCULATIONAHA.116.022339
Di Bella G, Siciliano V, Aquaro GD et al (2015) Right ventricular dysfunction: an independent and incremental predictor of cardiac deaths late after acute myocardial infarction. Int J Cardiovasc Imag 31(2):379–387. https://doi.org/10.1007/s10554-014-0559-9
Azevedo PS, Cogni AL, Farah E et al (2012) Predictors of right ventricle dysfunction after anterior myocardial infarction. Can J Cardiol 28(4):438–442. https://doi.org/10.1016/j.cjca.2012.01.009
Yang Z, Fu H, Li H et al (2021) Late gadolinium enhancement is a risk factor for major adverse cardiac events in unrecognised myocardial infarction without apparent symptoms: a meta-analysis. Clin Radiol 76(1):79.e1-79.e11. https://doi.org/10.1016/j.crad.2020.07.038
Zhao CX, Wei L, Dong JX et al (2023) Nomograms referenced by cardiac magnetic resonance in the prediction of cardiac injuries in patients with ST-elevation myocardial infarction. Int J Cardiol 385:71–79. https://doi.org/10.1016/j.ijcard.2023.05.009
von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA et al (2013) Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 15(1):53. https://doi.org/10.1186/1532-429X-15-53
Alam MH, Auger D, Smith GC et al (2015) T1 at 1.5T and 3T compared with conventional T2* at 1.5T for cardiac siderosis. J Cardiovasc Magn Reson. https://doi.org/10.1186/s12968-015-0207-0
Cameron D, Siddiqi N, Neil CJ et al (2016) T₁ mapping for assessment of myocardial injury and microvascular obstruction at one week post myocardial infarction. Eur J Radiol 85(1):279–285. https://doi.org/10.1016/j.ejrad.2015.10.008
Demirkiran A, Everaars H, Amier RP et al (2019) Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. Eur Heart J Cardiovasc Imag 20(7):723–734. https://doi.org/10.1093/ehjci/jez094
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (No. 82200553), Key Research and Development Programs of Sichuan Province (No. 2023YFG0276), the Key Research and Development Programs of Sichuan Province (No. 2022YFS0357).
Author information
Authors and Affiliations
Contributions
LSC, CS, DKY and HY participated in the initiation and design of the study. LSC, CS and MM selected the anterior STEMI patients according to guidelines. XCC and DKY (experienced radiologist) performed the CMR analysis. LSC, CS and BYL collected the patients’ information. SFF and HLT helped in the statistic analysis. LSC, CS and BYL processed all data and prepared the manuscript. All authors revised it critically and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of West China Hospital, Sichuan University.
Consent to participate
The consents were signed by all patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10554_2024_3166_MOESM1_ESM.tif
Supplementary file1 (TIF 97434 KB) The American Heart Association’s 16-segment plot for the Case presentation. (A) Native T1 mapping; (B) T2 mapping
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, S., Chen, S., Bai, Y. et al. Interventricular septum involvement is related to right ventricular dysfunction in anterior STEMI patients without right ventricular infarction: a cardiovascular magnetic resonance study. Int J Cardiovasc Imaging (2024). https://doi.org/10.1007/s10554-024-03166-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10554-024-03166-z