Abstract
Advanced cardiac imaging techniques such as cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) are widely used in clinical practice in patients with acute myocarditis and chronic inflammatory cardiomyopathies (I-CMP). We aimed to provide a review article with practical recommendations from the European Society of Cardiovascular Radiology (ESCR), in order to guide physicians in the use and interpretation of CMR and PET in clinical practice both for acute myocarditis and follow-up in chronic forms of I-CMP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced cardiac imaging techniques such as cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) are widely used in clinical practice in patients with acute myocarditis and chronic inflammatory cardiomyopathies (I-CMP). I-CMPs are characterised by inflammatory cell infiltration into the myocardium in association with cardiac dysfunction, ventricular remodelling and have both infectious and non-infectious aetiology (Fig. 1) [1]. Virally mediated cardiac injury is the most common cause of acute myocarditis. A complex interplay of genetic, autoimmune, and environmental factors contributes to the highly variable risk of deteriorating cardiac function, acute heart failure, and arrhythmia as well as chronic dilated cardiomyopathy and its sequelae [2]. The reason why some patients with myocardial inflammation recover without residual myocardial injury whereas others develop dilated cardiomyopathy remains unclear.

Adapted from [2]
Aetiology of inflammatory cardiomyopathies. HIV = Human Immunodeficiency Virus; CMV = Cytomegalovirus; EBV = Epstein-Barr Virus; VZV = Varicella Zoster Virus; DRESS = Drug Reaction with Eosinophilia and Systemic Symptoms; SLE = Systemic Lupus Erythematosus; RA = Rheumatoid Arthritis. NB: The term “Autoimmune” embraces auto-inflammatory and immune-mediated inflammatory diseases (IMID). Connective tissue disease is also known as autoimmune rheumatic disease. Churg-Strauss syndrome is also known as eosinophilic granulomatosis with polyangiitis (EGPA).
CMR and PET have become key tools to non-invasively diagnose acute myocarditis and I-CMPs, visualize and understand pathophysiological mechanisms and to better identify patients at risk of developing heart failure and dilated cardiomyopathy. They have largely reduced the need for endomyocardial biopsy (EMB) in hemodynamically stable patients given its limitations such as invasiveness, availability, costs and sampling error due to the predominantly subepicardial and mid-myocardial wall involvement in acute myocarditis and I-CMP [1, 3].
We aimed to provide a review article with practical recommendations to guide physicians in the use, and interpretation of CMR and PET in clinical practice both for acute myocarditis and follow-up in chronic forms of I-CMP.
Diagnosis of acute myocarditis and inflammatory cardiomyopathy
The diagnosis of acute myocarditis and I-CMP is based on a combination of clinical history, electrocardiogram, blood tests, cardiac imaging, and where necessary, EMB.
Endomyocardial biopsy
EMB should be considered in all cases with presumed giant cell myocarditis and fulminant myocarditis (severe heart failure/cardiogenic shock), malignant ventricular arrhythmia or high-grade atrioventricular block (II° or III°). Given the limited diagnostic accuracy of CMR in identifying the specific aetiology of myocardial inflammation, EMB may also be indicated in patients with a presumed cardiac sarcoidosis, eosinophilic myocarditis or systemic inflammatory disease for which there are specific treatment options available apart from general heart failure treatment [4,5,6,7,8]. Of note, myocardial inflammation often involves the (sub-)epicardial and mid-myocardial walls and the left ventricle whereas EMB preferentially is done from samples of the (sub-)endocardial layers of the right ventricle. EMB may therefore lead to sampling errors, the sensitivity of EMB has been reported to be higher for giant cell myocarditis (80–93%) than for sarcoidosis (25%) and lymphocytic myocarditis (35%) [9, 10].
CMR
Although EMB is still the reference standard to prove a diagnosis of myocarditis and its etiology, there has been a notable shift for the diagnosis of myocarditis over the last decades towards a non-invasive approach. CMR offers non-invasive imaging that can accurately assess myocardial inflammation and is now considered the first-line modality to confirm suspected inflammatory myocardial disease. CMR is basically recommended [4] to confirm diagnosis in clinically suspected acute myocarditis (onset of symptoms < 30 days, mostly infarct-like presentation) or [5] to evaluate the presence of chronic myocarditis or chronic inflammatory cardiomyopathy (I-CMP) in patients with persistent cardiac symptoms (onset of symptoms > 30 days, persistent troponinaemia, mostly presentation with heart-failure-like symptoms or unexplained arrhythmias) [4, 5].
CMR is recommended in clinically stable patients with acute symptoms to confirm clinical suspicion of acute myocarditis by demonstrating inflammatory necrosis and myocardial oedema [1, 4]. The diagnostic accuracy of CMR following Lake Luis Criteria (LLC) is high for acute infarct-like presentations (a diagnostic accuracy up to 90% can be achieved, Fig. 2) [11, 12]. However, the sensitivity of CMR in biopsy-proven acute myocarditis depends on the type of clinical presentation and is lower for chronic cardiomyopathic, and very low for arrhythmic patterns (sensitivities: 40-57%) [11]. In most stable patients with presumed myocarditis, CMR will be sufficient for confirming diagnosis. In high-risk patients with cardiogenic shock or fulminant clinical course, EMB should be first and foremost performed [1, 4]. Nevertheless, in experienced medical centers with interdisciplinary teams of radiologists, anesthesiologists, and cardiologists, CMR can be performed even in intubated intensive care patients (if mechanical circulatory support is not required) to guide subsequent EMB [13]. Myocardial mapping techniques have further improved diagnostic accuracies over the last years, especially for the detection of diffuse myocardial oedema and inflammatory processes [12, 14, 15]. Moreover, CMR offers prognostic value by the assessment of disease activity and severity including ventricular remodeling and function, myocardial inflammation (oedema and necrosis), and myocardial fibrosis [16].
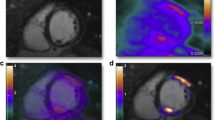
Original and 2018 Lake Louise criteria (LLC) in a 24-year-old man with acute myocarditis. Original LLC consisted of three main criteria: regional high T2 signal intensities on T2-weighted images (white arrows) or increased global T2 signal intensity ratio, increased early gadolinium enhancement ratio on T1-weighted images, and areas with high signal intensities in nonischemic distribution pattern on late gadolinium enhancement (LGE) images (white arrows). 2018 LLC consist of two main criteria (T1-based criterion and T2-based criterion). T1-based criterion is considered to be positive if increase of native T1 relaxation times, increase of extracellular volume (ECV), or positive LGE (white arrows) exist. T2-based criterion is positive in cases of increased T2 relaxation times or in cases with regional high T2 signal intensities on T2-weighted images (white arrows) or increased global T2 signal intensity ratio. Gd = gadolinium, SI = signal intensity, STIR = short tau inversion recovery.Reprinted from [12]. No changes were made
In the work-up of patients with unexplained heart-failure symptoms or ventricular arrhythmias, CMR is recommended to exclude chronic inflammatory myocardial disease [4, 5]. CMR can help to differentiate between ischaemic or non-ischaemic myocardial disease by visualization of the pattern of myocardial scarring (I.e., subendocardial scarring with matching a coronary artery territory, as a sign for ischaemic injury) and fibrosis and work as a gatekeeper for potential EMB. Due to their higher sensitivity for the detection of diffuse myocardial edema and fibrosis, the application of T1 and T2 mapping can be of particular value in patients with chronic myocarditis or chronic I-CMP [17]. High-sensitivity cardiac troponin (hs-cTn) assays are very sensitive but non-specific markers of myocyte injury and almost invariably elevated in patients with acute or ongoing myocardial inflammation [18] Myocardial inflammation may rarely occur with normal hs-cTn levels e.g. in patients treated with immune checkpoint inhibitors (probably only the presence of myocardial oedema without myocyte injury) [19]. Therefore, hs-cTn assays may be used to exclude ongoing myocardial inflammation in the vast majority of patients [8]. EMB should be considered to exclude low-grade myocardial inflammation in patients with negative CMR result but refractory cardiac symptoms and persistent suspicion of chronic inflammation.
Furthermore, CMR can be indicated to evaluate adverse effects of different treatments, e.g. traditional and new anticancer therapies, in patients with suspected cardiotoxicity including immune checkpoint inhibitor myocarditis [5, 20,21,22].
PET
Positron emission tomography (PET) with 2-deoxy-2-[18 F]-fluoro-D-glucose (18 F-FDG) has gained interest in the last years, owing to its capability to reveal focal or diffuse patterns of inflammation as seen in myocarditis.
Glucose is a normal metabolic substrate of myocardium, which is normally used in clinical practice for exploring myocardial viability [23]. Due to the physiologic 18 F-FDG uptake within the myocardium, a specific patient’s preparation is needed to assess the presence of inflammatory foci. Therefore, long fasting, low-carbohydrates and high-fat meal and/or fractionated/unfractionated heparin administration before 18 F-FDG injection are commonly used to suppress physiological radiotracer uptake and increase PET specificity [24,25,26].
To date, 18 F-FDG PET has been suggested for the noninvasive diagnosis of myocarditis, to guide EMB, and for monitoring treatment response. However, as large clinical trials are lacking so far, the use of 18 F-FDG PET as standalone modality in the diagnostic workup cannot be recommended, possibly with the exception of cardiac sarcoidosis [2].
Recently, new hybrid PET-CMR scanners became available in clinical practice, and they represent an attractive imaging modality for the evaluation of myocarditis and I-CMP. In fact, PET-CMR has the advantage of allowing simultaneous acquisition of both CMR and PET combining the morphological and ventricular functional data, tissue characterization, and metabolic information in the same examination [27]. However, due to higher costs and limited availability compared to standalone modalities, the use hybrid PET/MR imaging is not widespread yet.
Imaging findings
CMR
CMR is ideal to illustrate most of the historical hallmarks of inflammation: 1: rubor/calor (Oedema sequences), 2: dolor (patient history), 3: tumor (transient elevated myocardial mass/“hypertroph” due to oedema), 4: function laesa (Ejection fraction, regional wall motion abnormalities). Furthermore, it enables a multiparametric assessment that combines the evaluation of myocardial tissue abnormalities, the impairment of the contractile function and the pericardial involvement. The presence of myocardial oedema, hyperaemia, necrosis and/or fibrosis represents the typical features of inflammatory damage and allow to assess the extent and degree of activity of the myocardial injury (Fig. 3).
Myocardial oedema
Myocardial oedema, defined as an increase in water content in myocardial tissue due to the expansion of the interstitial fluid, can be depicted by T2 weighted images (double or triple inversion recovery with blood and fat suppression) [28] as implemented signal intensity (SI) areas as compared to the not injured myocardium [29]. The distribution of these tissue abnormalities is mostly confined to the mid-myocardium and subepicardium but may also occur transmurally or subendocardially. Myocardial oedema may occur globally but more frequently in a regional pattern and in association with occurrence of acute late gadolinium enhancement. In contrast to ischaemia-associated myocardial damage, oedema in myocarditis typically does not occur in a coronary artery pattern [30].
The intrinsic limitations in evaluating the myocardial edema when the T2 SI is diffusely increased can be overcome by the semi-quantitative analysis based on the normalization of myocardial SI to that of the skeletal muscle. A myocardial-to-skeletal muscle T2 signal ratio > 2 may be considered consistent for the presence of edema [31]. New approaches rely on relaxometric sequences: T2 mapping is highly specific for edema detection (area under the curve (AUC) 0.85–0.91 [32]) since those sequences are based on direct calculation of T2 relaxation times [33], and therefore highly specific for the acute setting of the disease [14]. T1 mapping sequences can also reveal the presence of edema (AUC 0.94–0.95 [34, 35]), but with lower specificity than T2 mapping, due to different mechanisms associated to an increase in T1 values [14, 33, 36]. T1 and T2 mapping sequences are able to detect edema even if diffuse and not easily evaluated with conventional sequences mainly based on the visual assessment of the disease (Fig. 4) [33].

Reprinted under the terms of the Creative Commons Attribution 4.0 International License from [33]. No changes were made
Typical appearance of T1, T2, T2*, and ECV maps in healthy subjects and in patients with myocardial disease. Arrows denote relative change in respective parametric maps.
Hyperaemia and capillary leak
Hyperaemia reflects the increased permeability of the vessels associated to the inflammatory response. The detection of this phenomenon results to be the most difficult and challenging for CMR [37]. According to the old Lake Louise criteria (LLC), hyperemia and capillary leakage were evidenced by increased SI with T1-weighted spin echo (T1-SE) sequences acquired early after contrast media administration compared to pre-contrast T1 - SE ones [31]. Historically, the semi-quantitative analysis defined the presence of hyperemia when SI ratio is > 4 as compared to the skeletal muscle or when the absolute myocardial enhancement is > 45% [31]. However, by definition, hyperemia is a dynamic process theoretically influenced by time-variations of tissue enhancement and therefore the technique for its evaluation, based on static images obtained with long acquisition times, suffers from low robustness and accuracy. Consequently, this criterion was excluded from the revised LLC, based on the low AUC demonstrated in several studies, ranging from 0.62 to 0.93 (Fig. 3) [14]. A promising prospective is offered by the measurement of early T1 shortening, measured by the percentage of T1 value reduction on T1 maps acquired early after administration of contrast medium (sensitivity/specificity of 93%/95% for early T1 shortening ≧ 70%) [38].
Necrosis and fibrosis
Necrosis, and subsequent fibrosis, represents the irreversible step of myocardial injury induced by the inflammatory cascade and are both associated with alteration of the permeability of the sarcolemma resulting in a myocardial accumulation of gadolinium [14].
LGE imaging has proven to be a valid tool for the detection of such damage, showing a high specificity [39] through the identification of common patterns of the regional distribution of non-ischemic myocardial injury [40]. Myocarditis-associated LGE lesions usually involve subepicardium and mid-wall and tends to favor basal to mid-inferolateral wall in a non-coronary artery pattern [41]. Nevertheless, severe inflammation can rarely lead to the extension of LGE area to the entire myocardial wall [31]. The solitary use of LGE for diagnosing myocarditis, however, is not recommended, due to its low specificity for acute inflammation. In this regard, Radunski et al. demonstrated a better diagnostic accuracy of T1 and ECV mapping, which increase the sensibility of CMR in the detection and quantification of diffuse myocardial fibrosis compared to LGE images [42].
Furthermore, the LGE areas may persist even in the chronic phase, when the inflammatory activity subsides, with possible shrinkage of the areas of enhancement, in relation to scar remodeling phenomena. It should be noted that in the acute phase it is often impossible to say whether LGE is a sign of focal (irreversible/chronic) fibrosis or oedema. In this situation, FDG PET may aid in the diagnostic definition.
Ventricular geometry and functional abnormalities
Myocardial inflammation may lead to regional or global left ventricle (LV) and right ventricle (RV) dysfunction (function laesa) [31]. However, wall motion abnormalities in myocarditis are often focal and can be compensated by a hypercontractility of the surrounding myocardium, which can mask the dysfunction [14]. Even with significant tissue injury, there may be remarkably little impact on cardiac contractility, as the endocardial myocytes, which tend to be prime movers in normal ventricular function are often relatively spared in acute myocarditis [8]. In this regard, myocardial strain can be helpful in detecting subtle wall motion abnormalities, resulting in an increased sensitivity of CMR [43].
However, alterations in regional contractility or global systolic function can underlie multiple pathological conditions, not necessarily related to a direct myocardial insult. Therefore, functional abnormalities should be considered as an ancillary criterion for the diagnosis of myocarditis [14].
Pericardial involvement
Pericarditis and myocarditis often coexist, due to the common etiologic agents and overlapping pathophysiological mechanisms. Although pericardial effusion is a common finding in patients with myocarditis, its presence alone is not sufficient for the diagnosis of pericarditis or myo-pericarditis [44].
Acute pericardial inflammation may be depicted by CMR as thickening of pericardial layers in high-resolution fast spin echo (FSE) T1 images, hyperintensity of the pericardial layers on T2-weighted, or pericardial enhancement on ECG-gated Dixon fat-water separation sequences or LGE images [45].
PET
Typically, areas of active inflammation present with increased 18 F-FDG uptake, which could be focal, diffuse, or focal on diffuse depending on the underlying nature of the disease [46]. Such areas of increased 18 F-FDG uptake may show a resolution after treatment, thus holding the potential for monitoring treatment response [47].
A retrospective study featuring 29 symptomatic patients showed that there is an excellent correlation between 18 F-FDG PET/CT and EMB from left ventricular posterior wall. Of note, the authors suggested that the best timing of imaging is within 14 days after the onset of clinical symptoms [46]. Moreover, another paper by Perel-Winkler et al. using 18 F-FDG-PET/CT in patients with systemic lupus erythematosus [48] showed diffuse myocardial 18 F-FDG uptake in those patients with chest pain, dyspnea and/or impairment of left ventricular ejection fraction (LVEF). Similar results were reported by Besenyi et al. [49], wherein patients with systemic sclerosis showed both visually and semi-quantitatively higher myocardial 18 F-FDG uptake compared to healthy subjects. Hence, there is a strong rationale to suggest that the presence of areas of increased myocardial 18 F-FDG uptake in symptomatic patients is highly suggestive for active inflammation, as it can be seen in myocarditis.
Using a hybrid PET/MR approach, images normally show focal or diffuse 18 F-FDG uptake, corresponding to MR alterations (Fig. 5). In a prospective study, a good agreement between the two techniques and feasibility of hybrid imaging has been demonstrated [50]. Of note, preliminary data also show potential incremental role of PET/MR over CMR alone. In fact, LGE may not detect myocardial damage if scattered, and mild borderline myocarditis can be often challenging to reveal with LGE due to the absence of relevant myocardial necrosis [33]. Hence, in selected patients the 18 F-FDG PET component may increase the sensitivity of CMR by providing metabolic information (Fig. 6) [51].

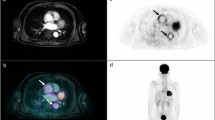
Reprinted with permission of Springer from [50]
PET/MRI examination in a 32-year-old male patient presenting with dyspnea, mild ventricular dysfunction (51% LFEV), and a history of recent systemic viral disease. A shows patchy intramyocardial late gadolinium enhancement in the lateral and inferior wall as well as pericardial effusion. B shows significantly increased T2 signal in the lateral wall representing myocardial edema. C (PET) and D (fusion between T2-weighted MR image and PET) show diffusely increased FDG uptake in the lateral, anterolateral, and inferolateral wall. Histopathological assessment after endomyocardial biopsy showed acute myocarditis with lymphocytic infiltration and moderate myocyte apoptosis. The patient demonstrated elevated levels of C-reactive protein (4.1 mg/dl) as well as elevated myocardiocytolysis serum markers (Troponin-I: 0.42 ng/ml). PCR and immunohistochemical analysis did not detect specific infectious agents such as viruses, bacteria, or fungi.

Reprinted with permission of Springer from [50]
PET/MRI examination in a 30-year-old male patient presenting with chest pain, dyspnea, palpitations, severely limited exercise capacity, mild ventricular dysfunction (54% LVEF), and mild ECG abnormalities (T-wave flattening in II, III, aVF, and V6). The patient demonstrated neither elevated levels of C-reactive protein nor elevated myocardiocytolysis serum markers. LGE images (A, B) did not reveal any signs of myocardial necrosis. PET images (C, F) demonstrated focal FDG uptake in the lateral wall. T2-weighted imaging (E) showed mild myocardial edema (T2 ratio: 2.0). Diagnosis of borderline myocarditis was confirmed by histopathological assessment after endomyocardial biopsy demonstrating sparse inflammatory infiltrates but no myocardial necrosis (D). PCR and immunohistochemical analysis did not detect specific infectious agents such as viruses, bacteria, or fungi.
Specific conditions
Specific conditions are summarized in Table 1.
CMR follow-up in myocarditis
Most patients (up to 84% in some series) with acute myocarditis have a benign course with full recovery of ventricular function and resolution of myocardial oedema without sequelae (healed myocarditis) [16, 71, 72]. Occasionally, acute myocarditis may induce significant morbidity and mortality, especially in severe forms presenting with ventricular functional impairment [73]. A 5 - year mortality rate of almost 20% in severe forms of acute myocarditis and up to 10% of sudden cardiac death in young adults has been described [74, 75]. Persistent inflammation, often subclinical, with an autoimmune substrate, may lead to dilated cardiomyopathy [71]. Up to 30% of biopsy–proven myocarditis can progress to dilated cardiomyopathy with an associated poor prognosis [1]. Known predictors of poor outcome include viral infection or evidence of immunohistological signs of inflammation on EMB, poor New York Heart Association (NYHA) functional class, impaired LV function or presence/extent of LGE [76].
Most patients with acute myocarditis have a good short-to-midterm clinical evolution with complete resolution of myocardial edema, improvement of LVEF, and reduction of left ventricular mass index (LVMi), being a marker of global myocardial inflammatory infiltration (Table 2). In the follow up reduction or disappearance of LGE in a follow-up CMR scan might refelct reversible injury. CMR with parametric mapping can effectively distinguish healed from active myocarditis [42, 77,78,79]. After the acute presentation, T1 native and T2 values decreased progressively in the early follow up period, both representing progressive resolution of the myocardial edema [78]. In fact, several studies have revealed a steady decline on T1 native and ECV values from the acute phase to chronic convalescent phase, but being higher than in controls [42, 72, 78,79,80]. Higher T1 and T2 AUC (0.947 and 0.931, respectively) have been described for discriminating between acute from healed myocarditis compared to LGE and T2 STIR (0.809 and 0.884, respectively) [77]. Also, ECV was the most robust parameter for differentiating healed myocarditis form healthy controls (AUC: 0.925; ECV > 26%, 85.2% sensitivity and 100% specificity) [77]. Malek et al. showed that patients with persistent myocardial inflammation (up to 28%), usually asymptomatic, had myocardial oedema or LGE on the initial CMR scan [81]. Moreover, LGE extent has been associated with adverse remodeling (increased LVEDV index and LV systolic volume index), lower LVEF and occurrence of major cardiovascular events (MACEs) [41, 79, 82, 83]. Because subclinical persistent ongoing inflammation and LGE can lead to dilated cardiomyopathy, heart failure and ventricular arrythmias, several authors have suggested that a CMR follow up may be adequate in patients with acute myocarditis [16, 81].
Several studies evaluated the prognostic value of CMR surveillance in the long – term follow up in patients with acute myocarditis (Table 3). Chopra et al. showed that LVEF was lower in patients with MACEs compared to those free of MACEs (48.9 ± 11.5% vs. 57 ± 8.0%; p < 0.05) [84]. Other authors showed that LVEF constituted the best independent predictor of adverse clinical events, incomplete recovery and lower LVEF at follow up [16, 73, 74, 85]. Larger LVEDV index at the initial CMR was associated with lower LVEF at follow up CMR (85.9 ± 21.7 ml/m2 vs. 71.8 ± 17.1 ml/m2 LVEDV index for reduced and preserved LVEF, respecrively; p = 0.02) [85]. Higher extension of reversible myocardial damage was seen in patients without MACES, being an independent predictor of LVEDV and LVEF improvement at follow up (reverse remodeling) [85,86,87]. LGE extent, presence of LGE without myocardial edema and septal pattern on LGE were independent predictors of MACEs and hospitalization due to heart failure in the follow up.
Although advantages have been described in the literature regarding the value of CMR in tissue characterization and risk stratification in the surveillance of I-CMP, there is currently no consensus on the use and timing of CMR during I-CMP follow up. Follow up CMR in I-CMP may be considered in patients with adverse cardiac remodeling (increased LVEDV or LVSV index), impaired LVEF (< 50%), extensive reversible myocardial damage or abundant LGE (in particular with septal or ring-like LGE pattern) [8, 16, 90, 91]. Unless recurrent flares occur, oedema tends to decline 4 weeks after disease onset [88]. Myocardial LGE often appears to be less extensive in follow-up CMRs or may even disappear completely at 6 months (healed myocarditis) when it had been expression of oedema and not fibrosis [16]. In order to improve risk stratification and differentiation of convalescent myocarditis from healthy individuals, CMR in the follow may include parametric T1 and T2 mapping with calculated ECV values whenever possible.
Future directions in myocarditis diagnostics
Although parametric mapping techniques have further increased the sensitivity of CMR to diagnose myocardial inflammation, the diagnostic accuracy in low-grade or chronic inflammatory disease might be hampered. Therefore, there is still a need for additional imaging markers to further improve diagnostic accuracy and risk stratification in patients with inflammatory myocardial disease.
CMR fingerprinting is a technique that allows for rapid and simultaneous acquisition of multiple, fully co-registered parametric maps within a single scan by matching complex signal measurements to a dictionary of simulated signals [92, 93]. It has the potential to improve diagnostic accuracy of myocardial maps by increasing the resolution and anatomic coverage, as well as substantially improving the reproducibility (enabling more reproducible measurements independent of center-specific hardware and patient physiology) [94, 95]. Moreover, it could extend myocardial tissue characterization beyond traditional mapping techniques by incorporating new parametric maps (e.g., diffusion or perfusion maps) and be used for comprehensive machine learning applications [96]. There are several other promising quantitative CMR techniques in preclinical evaluation that could help improve diagnosis of inflammatory myocardial disease in the future: cardiac diffusion-weighted imaging (cDWI) showed promising correlation between apparent diffusion coefficient (ADC) and LGE in chronic myocardial infarction [98]. In vivo studies of cardiac diffusion tensor imaging (cDTI) have shown its ability for microstructural and functional assessment of the myocardium, that might open up the road for detection of myocardial fiber remodeling also in inflammatory cardiomyopathy [97].
Artificial intelligence (AI) incorporated with machine learning and deep learning algorithms is going to revolutionize medical healthcare and in particular cardiovascular imaging. CMR lends itself to AI applications because it is based on complex image acquisition, reconstruction, segmentation/quantification, as well as image analysis and diagnostic reporting, which can be markedly improved by machine learning applications [98]. First AI applications for automated cardiac function analysis have already found their way into clinical use [99]. The aforementioned pre-clinical quantitative CMR techniques can benefit tremendously from machine learning algorithms. CMR fingerprinting directly profits from machine learning, as faster and more robust acquisition and reconstruction algorithms facilitate the generation of reproducible and unbiased maps needed for the development of machine learning applications [100]. Deep learning-based segmentation of LGE scars and parametric mapping could extend myocardial tissue characterization by improving reproducibility and sensitivity [101]. Furthermore, deep learning algorithms could decisively improve CMR techniques that are on the cusp of routine clinical application, such as functional strain analysis, by further improving its accuracy and reproducibility [102].
Machine learning approaches and big data analysis gave rise to another promising field, termed radiomics. Radiomics reflects a conversion of medical images into high-dimensional data and enables the extraction of various features (e.g., texture or filter features) that go beyond the conventional visual approach [98]. First radiomics and texture analysis applications in CMR using T2 mapping-derived texture features analysis showed superior diagnostic performance in patients with infarct-like and heart failure-like myocarditis [103, 104]. These novel texture analysis concepts could significantly improve the current challenges in diagnosis of low-grade or chronic myocardial inflammation or inflammatory cardiomyopathy (e.g., detection of subtle, diffuse or even visually non-assessable myocardial alterations).
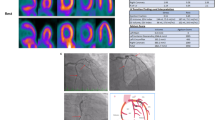
Furthermore, alternative imaging modalities such as spectral dual-energy and photon-counting CT could allow early detection of myocardial inflammation in routine clinical practice, where CT is typically performed before CMR. Hybrid imaging using PET, with its ability to detect focal and chronic inflammation, could be specifically incorporated into diagnostic algorithms for myocarditis and could further improve by the development of new tracers [50, 105, 106]. A proposal for a diagnostic algorithm is provided in Fig. 7.
Clinical scenarios where acute myocarditis/acute myocardial inflammation may be suspected with a summary of guidance of diagnostic multi-modality imaging assessments, general treatment and surveillance recommendations. Clinical and CMR findings are proposed when a follow-up CMR scan should be evaluated
Conclusion
CMR represents an invaluable tool in the diagnostic work-up of acute myocarditis and chronic i-CMP. In some cases, adding 18 F-FDG may help in differentiating between acute and chronic i-CMP, thus allowing to choose the most effective therapeutic approach. Scarce data are available on hybrid PET/MR imaging, but combining the information coming from both morphologic and metabolic assessment may yield improved accuracy in selected cases, wherein the diagnosis is not clear.
References
Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB (2013) u. a. current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648
Ismail TF, Hua A, Haaf P, Giorgetti A, Infection (2021) Myocarditis. In: Caobelli F (ed) Imaging of inflammation and infection in Cardiovascular Diseases. Springer, Cham
Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A (2010) u. a. comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 122:900–909
Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, Pedrotti P, Rimoldi OE, Schultheiss HP, Tschöpe C, Cooper LT Jr, Camici PG (2020) Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail 13:e007405
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726
Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zlotoff DA, Murphy SP, Stone JR, Golden DLA, Alvi RM, Rokicki A, Jones-O’Connor M, Cohen JV, Heinzerling LM, Mulligan C, Armanious M, Barac A, Forrestal BJ, Sullivan RJ, Kwong RY, Yang EH, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Coelho-Filho OR, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Mercurio V, Mahmoudi M, Lawrence DP, Reynolds KL, Weinsaft JW, Baksi AJ, Ederhy S, Groarke JD, Lyon AR, Fradley MG, Thavendiranathan P, Neilan TG (2020) Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 41:1733–1743
Tschöpe C, Cooper LT, Torre-Amione G, Van Linthout S (2019) Management of myocarditis-related cardiomyopathy in adults. Circ Res 124:1568–1583
Ismail TF, Hua A, Plein S, D’Cruz DP, Fernando MMA, Friedrich MG, Zellweger MJ, Giorgetti A, Caobelli F, Haaf P (2022) The role of cardiovascular magnetic resonance in the evaluation of acute myocarditis and inflammatory cardiomyopathies in clinical practice - a comprehensive review. Eur Heart J Cardiovasc Imaging 23(4):450–464
Shields RC, Tazelaar HD, Berry GJ, Cooper LT (2002) The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J Card Fail 8:74–78
Hauck AJ, Kearney DL, Edwards WD (1989) Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 64:1235–1245
Francone M, Chimenti C, Galea N et al (2014) CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging 7:254–263
Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, Sprinkart AM, Thomas D (2019) Comparison of original and 2018 Lake Louise Criteria for diagnosis of Acute Myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging 1:e190010
Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, Elliott MD, Ferrari VA, Friedrich MG, Lawton C, Manning WJ, Ordovas K, Plein S, Powell AJ, Raman SV, Carr J (2020) Society for Cardiovascular magnetic resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson 22:26
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert Recommendations. J Am Coll Cardiol 72:3158–3176
Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, Block W, Andrié R, Stehning C, Fimmers R, Gieseke J, Thomas DK, Schild HH, Naehle CP (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17:154–161
Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G (2019) Cardiac magnetic Resonance” Working Group of the italian society of Cardiology. Prognostic value of repeating Cardiac magnetic resonance in patients with Acute Myocarditis. J Am Coll Cardiol 74:2439–2448
Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM (2020) Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging 13:1221–1234
Heymans S (2007) Myocarditis and heart failure: need for better diagnostic, predictive, and therapeutic tools. Eur Heart J 28:1279–1280
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM et al (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755–1764
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document Group (2016) ;. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801
Faron A, Isaak A, Mesropyan N, Reinert M, Schwab K, Sirokay J, Sprinkart AM, Bauernfeind FG, Dabir D, Pieper CC, Heine A, Kuetting D, Attenberger U, Landsberg J, Luetkens JA (2021) Cardiac MRI depicts Immune Checkpoint inhibitor-induced myocarditis: a prospective study. Radiology 301:602–609
Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zlotoff DA, Murphy SP, Stone JR, Golden DLA, Alvi RM, Rokicki A, Jones-O’Connor M, Cohen JV, Heinzerling LM, Mulligan C, Armanious M, Barac A, Forrestal BJ, Sullivan RJ, Kwong RY, Yang EH, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Coelho-Filho OR, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Mercurio V, Mahmoudi M, Lawrence DP, Reynolds KL, Weinsaft JW, Baksi AJ, Ederhy S, Groarke JD, Lyon AR, Fradley MG, Thavendiranathan P, Neilan TG (2020) Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 41:1733–1743
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S et al (2016) ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 23:1187–1226
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I et al (2016) The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial (18)F-fuorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol 23(2):244–252
Giorgetti A, Marras G, Genovesi D, Filidei E, Bottoni A, Mangione M et al (2018) Effect of prolonged fasting and low molecular weight heparin or warfarin therapies on 2-deoxy-2-[18F]- fuoro-D-glucose PET cardiac uptake. J Nucl Cardiol 25(4):1364–1371 7 Infection: Myocarditis 232
Popescu CE, Caobelli F (2021) Challenges in Patient Preparation. In: Caobelli F (ed) Imaging of inflammation and infection in Cardiovascular Diseases. Springer, Cham
Nazir MS, Ismail TF, Reyes E, Chiribiri A, Kaufmann PA, Plein S (2018) Hybrid positron emission tomography-magnetic resonance of the heart: current state of the art and future applications. Eur Heart J Cardiovasc Imaging 19:962–974
Simonetti OP, Finn JP, White RD, Laub G, Henry DA (1996) Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 199:49–57
Carbone I, Friedrich MG (2012) Myocardial edema imaging by cardiovascular magnetic resonance: current status and future potential. Curr Cardiol Rep 14:1–6
Abdel-Aty H, Simonetti O, Friedrich MG (2007) T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging 26:452–459
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P (2009) International Consensus Group on Cardiovascular magnetic resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53:1475–1487
Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD (2018) Diagnostic accuracy of Cardiovascular magnetic resonance in Acute Myocarditis: a systematic review and Meta-analysis. JACC Cardiovasc Imaging 11:1583–1590
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular magnetic resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19:75 Erratum in: J Cardiovasc Magn Reson. 2018;20:9
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:42
Ferreira VM, Holloway CJ, Piechnik SK, Karamitsos TD, Neubauer S (2013) Is it really fat? Ask a T1-map. Eur Heart J Cardiovasc Imaging 14:1060
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB, Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology (2013) Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular magnetic resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 15:92
Esposito A, Francone M, Faletti R, Centonze M, Cademartiri F, Carbone I, De Rosa R, Di Cesare E, La Grutta L, Ligabue G, Lovato L, Maffei E, Marano R, Midiri M, Pontone G, Natale L, De Cobelli F ; Working Group of the Italian College of Cardiac Radiology by SIRM. Lights and shadows of cardiac magnetic resonance imaging in acute myocarditis. Insights Imaging ;7:99–110
Palmisano A, Vignale D, Tadic M, Moroni F, De Stefano D, Gatti M, Boccia E, Faletti R, Oppizzi M, Peretto G, Slavich M, Sala S, Montorfano M, Agricola E, Margonato A, De Cobelli F, Gentile F, Robella M, Cortese G, Esposito A (2022) Myocardial late contrast enhancement CT in troponin-positive acute chest Pain Syndrome. Radiology 302:545–553
Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J (2005) Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 45:1815–1822
Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, Sadoul N, Karcher G, Aliot E, Marie PY (2007) Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging 25:957–964
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590
Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K (2014) CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 7:667–675
Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, Guensch DP, Huber AT, Greulich S, Aghayev A, Steigner M, Blankstein R, Kwong RY, Gräni C (2020) Feature tracking myocardial strain incrementally improves prognostication in Myocarditis Beyond Traditional CMR Imaging features. JACC Cardiovasc Imaging 13:1891–1901
Di Bella G, Imazio M, Bogaert J, Pizzino F, Camastra G, Monti L, Dellegrottaglie S, Donato R, Moro C, Pepe A, Lanzillo C, Pontone G, Marra MP, Fusco A, Scatteia A, Pingitore A, Aquaro GD (2019) Clinical value and prognostic impact of Pericardial involvement in Acute Myocarditis. Circ Cardiovasc Imaging 12:e008504
Bogaert J, Francone M (2009) Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson 11:14
Ozawa K, Funabashi N, Daimon M, Takaoka H, Takano H, Uehara M et al (2013) Determination of optimum periods between onset of suspected acute myocarditis and 18F-fuorodeoxyglucose positron emission tomography in the diagnosis of infammatory left ventricular myocardium. Int J Cardiol 169:196–200
Moriwaki K, Dohi K, Omori T, Tanimura M, Sugiura E, Nakamori S et al (2017) A survival case of fulminant right-side dominant eosinophilic myocarditis. Int Heart J 58:459–462
Perel-Winkler A, Bokhari S, Perez-Recio T, Zartoshti A, Askanase A, Geraldino-Pardilla L (2018) Myocarditis in systemic lupus erythematosus diagnosed by 18F-fuorodeoxyglucose positron emission tomography. Lupus Sci Med 5:e000265
Besenyi Z, Ágoston G, Hemelein R, Bakos A, Nagy FT, Varga A et al (2019) Detection of myocardial infammation by 18F-FDG-PET/CT in patients with systemic sclerosis without cardiac symptoms: a pilot study. Clin Exp Rheumatol 37:88–96
Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J et al (2018) Feasibility of FDG PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol 25:785–794
Chen W, Jeudy J (2019) Assessment of Myocarditis: Cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep 21:76
Huber AT, Bravetti M, Lamy J, Bacoyannis T, Roux C, de Cesare A et al (2018) Non-invasive differentiation of idiopathic inflammatory myopathy with cardiac involvement from acute viral myocarditis using cardiovascular magnetic resonance imaging T1 and T2 mapping. J Cardiovasc Magn Reson 20:11
Sebai F, Brun S, Petermann A, Ribes D, Prevot G, Cariou E et al (2019) Cardiac magnetic resonance imaging with late gadolinium enhancement in acute myocarditis: towards differentiation between immune-mediated and viral-related aetiologies. Arch Cardiovasc Dis 112:559–566
Ferrero P, Piazza I, Lorini LF, Senni M (2020) Epidemiologic and clinical profiles of bacterial myocarditis. Report of two cases and data from a pooled analysis. Indian Heart J 72:82–92
Inayat F, Ali NS, Riaz I, Virk HUH (2017) From the gut to the heart: Campylobacter jejuni Enteritis leading to Myopericarditis. Cureus 9:e1326
Mustafa K, Hillyard J, Nowak E, Slowikowski J, Okogbue I, Garner D (2021) Toxoplasma myocarditis: an atypical case in an immunocompetent patient. IDCases 26:e01273
Patel RA, DiMarco JP, Akar JG, Voros S, Kramer CM (2005) Chagas myocarditis and syncope. J Cardiovasc Magn Reson 7:685–688
Duran-Crane A, Rojas CA, Cooper LT, Medina HM (2020) Cardiac magnetic resonance imaging in Chagas’ disease: a parallel with electrophysiologic studies. Int J Cardiovasc Imaging 36:2209–2219
Buchanan CE, Kakkar E, Dreskin SC, Allen LA, Groves DW, Altman NL (2020) Allergy and the heart: eosinophilic myocarditis with Biventricular Thrombi. JACC Case Rep 2:1942–1946
Hameed A, Lashin H, Khanji MY, Spiritoso R (2020) Eosinophilic myocarditis secondary to T-Cell lymphoma complicated by left ventricular Thrombus and tear. JACC Case Rep 2:1954–1958
Mavrogeni S, Koutsogeorgopoulou L, Markousis-Mavrogenis G, Bounas A, Tektonidou M, Lliossis SC et al (2018) Cardiovascular magnetic resonance detects silent heart disease missed by echocardiography in systemic lupus erythematosus. Lupus 27:564–571
Kikuchi N, Watanabe E, Nagao M, Yoshizawa S, Kobashigawa T, Hagiwara N (2021) Acute myocarditis complicating systemic Lupus Erythematosus: detection and evolution of transmural spiral late gadolinium enhancement on Cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 14:e011319
De Luca G, Campochiaro C, De Santis M, Sartorelli S, Peretto G, Sala S et al (2020) Systemic sclerosis myocarditis has unique clinical, histological and prognostic features: a comparative histological analysis. Rheumatology (Oxford) 59:2523–2533
Rosenbohm A, Buckert D, Gerischer N, Walcher T, Kassubek J, Rottbauer W et al (2015) Early diagnosis of cardiac involvement in idiopathic inflammatory myopathy by cardiac magnetic resonance tomography. J Neurol 262:949–956
Yang S, Chen X, Li J, Sun Y, Song J, Wang H et al (2021) Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Fail 8:2320–2327
Tacke CE, Kuipers IM, Groenink M, Spijkerboer AM, Kuijpers TW (2011) Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging 4:712–720
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS et al (2014) HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 11:1305–1323
Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH (2013) Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging 6:1080–1091
Onderko L, Starobin B, Riviere AE, Hohl PK, Phillips CT, Morgan RB et al (2021) Myocarditis in the setting of recent COVID-19 vaccination. Case Rep Cardiol 2021:6806500
Kim YJ, Bae JI, Ryoo SM, Kim WY (2019) Acute fulminant myocarditis following influenza vaccination requiring extracorporeal membrane oxygenation. Acute Crit Care 34:165–169
Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, Radziwolek L, Avanesov M, Tahir E, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K (2017) Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging 18:744–751
Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, McCrohon J, Higgins DM, Carr-White G, Mayr M, Nagel E, Puntmann VO (2015) Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 8:37–46
Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, Sechtem U, Mahrholdt H (2014) Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson 16:14
Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H (2012) Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol 59:1604–1615
Fabre A, Sheppard MN (2006) Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 92:316–320
Lee JW, Jeong YJ, Lee G, Lee NK, Lee HW, Kim JY, Choi BS, Choo KS (2017) Predictive value of Cardiac magnetic resonance imaging-derived myocardial strain for poor outcomes in patients with Acute Myocarditis. Korean J Radiol 18:643–654
Li H, Zhu H, Yang Z, Tang D, Huang L, Xia L (2021) Application of Multiparametric quantitative Cardiac magnetic resonance for detection and monitoring of Myocardial Injury in patients with fulminant myocarditis. Acad Radiol 28:e35–e43
von Knobelsdorff-Brenkenhoff F, Schüler J, Dogangüzel S, Dieringer MA, Rudolph A, Greiser A, Kellman P, Schulz-Menger J (2017) Detection and monitoring of Acute Myocarditis applying quantitative Cardiovascular magnetic resonance. Circ Cardiovasc Imaging 10:e005242
Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, Scherff F, Schmied C, Templin C, Lüscher TF, Heidecker B, Manka R (2017) Cardiac magnetic resonance imaging in Myocarditis reveals Persistent Disease Activity despite normalization of Cardiac enzymes and inflammatory parameters at 3-Month Follow-Up. Circ Heart Fail 10:e004262
Luetkens JA, Homsi R, Dabir D, Kuetting DL, Marx C, Doerner J, Schlesinger-Irsch U, Andrié R, Sprinkart AM, Schmeel FC, Stehning C, Fimmers R, Gieseke J, Naehle CP, Schild HH, Thomas DK (2016) Comprehensive Cardiac magnetic resonance for short-term Follow-Up in Acute Myocarditis. J Am Heart Assoc 5:e003603
Małek ŁA, Kamińska H, Barczuk-Falęcka M, Ferreira VM, Wójcicka J, Brzewski M, Werner B (2020) Children with Acute Myocarditis often have persistent subclinical changes as revealed by Cardiac magnetic resonance. J Magn Reson Imaging 52:488–496
Faletti R, Gatti M, Baralis I, Bergamasco L, Bonamini R, Ferroni F, Imazio M, Stola S, Gaita F, Fonio P (2017) Clinical and magnetic resonance evolution of “infarct-like” myocarditis. Radiol Med 122:273–279
Ammirati E, Moroni F, Sormani P, Peritore A, Milazzo A, Quattrocchi G, Cipriani M, Oliva F, Giannattasio C, Frigerio M, Roghi A, Camici PG, Pedrotti P (2017) Quantitative changes in late gadolinium enhancement at cardiac magnetic resonance in the early phase of acute myocarditis. Int J Cardiol 231:216–221
Chopra H, Arangalage D, Bouleti C et al (2016) Prognostic value of the infarct- and non-infarct like patterns and cardiovascular magnetic resonance parameters on long-term outcome of patients after acute myocarditis. Int J Cardiol 212:63–69
Sanguineti F, Garot P, Mana M et al (2015) Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson 17:78
Vermes E, Childs H, Faris P, Friedrich MG (2014) Predictive value of CMR criteria for LV functional improvement in patients with acute myocarditis. Eur Heart J Cardiovasc Imaging 15:1140–1144
Zagrosek A, Abdel-Aty H, Boyé P et al (2009) Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging 2:131–138
Gräni C, Eichhorn C, Bière L et al (2019) Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J Cardiovasc Magn Reson 21:14
Mavrogeni S, Spargias C, Bratis C et al (2011) Myocarditis as a precipitating factor for heart failure: evaluation and 1-year follow-up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail 13:830–837
Georgiopoulos G, Figliozzi S, Sanguineti F, Aquaro GD, di Bella G, Stamatelopoulos K, Chiribiri A, Garot J, Masci PG, Ismail TF (2021) Prognostic impact of late Gadolinium Enhancement by Cardiovascular magnetic resonance in myocarditis: a systematic review and Meta-analysis. Circ Cardiovasc Imaging 14:e011492
Muser D, Nucifora G, Muser D, Nucifora G, Pieroni M, Castro SA, Casado Arroyo R, Maeda S, Benhayon DA, Liuba I, Sadek M, Magnani S, Enriquez A, Liang JJ, Sassone B, Desjardins B, Dixit S, Deo R, Garcia FC, Callans DJ, Frankel DS, Selvanayagam JB, Marchlinski FE, Santangeli P (2021) Prognostic Value of Nonischemic Ringlike Left ventricular scar in patients with apparently idiopathic nonsustained ventricular arrhythmias. Circulation 143:1359–1373
Ma D, Gulani V, Seiberlich N et al (2013) Magnetic resonance fingerprinting. Nature 495(7440):187–192
Hamilton JI, Jiang Y, Ma D, Lo WC, Gulani V, Griswold M, Seiberlich N (2018) Investigating and reducing the effects of confounding factors for robust T1 and T2 mapping with cardiac MR fingerprinting. Magn Reson Imaging 53:40–51
Fang Z, Chen Y, Hung SC, Zhang X, Lin W, Shen D (2020) Submillimeter MR fingerprinting using deep learning-based tissue quantification. Magn Reson Med 84:579–591
Cruz G, Jaubert O, Qi H, Bustin A, Milotta G, Schneider T, Koken P, Doneva M, Botnar RM, Prieto C (2020) 3D free-breathing cardiac magnetic resonance fingerprinting. NMR Biomed 33:e4370
Liu Y, Hamilton J, Rajagopalan S, Seiberlich N (2018) Cardiac magnetic resonance fingerprinting: technical overview and initial results. JACC Cardiovasc Imaging 11:1837–1853
Nielles-Vallespin S, Scott A, Ferreira P, Khalique Z, Pennell D, Firmin D (2020) Cardiac diffusion: technique and practical applications. J Magn Reson Imaging 52:348–368
Leiner T, Rueckert D, Suinesiaputra A, Baeßler B, Nezafat R, Išgum I, Young AA (2019) Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson 21:61
Bernard O, Lalande A, Zotti C, Cervenansky F, Yang X, Heng PA, Cetin I, Lekadir K, Camara O, Gonzalez Ballester MA, Sanroma G, Napel S, Petersen S, Tziritas G, Grinias E, Khened M, Kollerathu VA, Krishnamurthi G, Rohe MM, Pennec X, Sermesant M, Isensee F, Jager P, Maier-Hein KH, Full PM, Wolf I, Engelhardt S, Baumgartner CF, Koch LM, Wolterink JM, Isgum I, Jang Y, Hong Y, Patravali J, Jain S, Humbert O, Jodoin PM (2018) Deep Learning Techniques for Automatic MRI Cardiac Multi-Structures Segmentation and diagnosis: is the Problem Solved? IEEE Trans Med Imaging 37:2514–2525
Hamilton JI, Currey D, Rajagopalan S, Seiberlich N (2021) Deep learning reconstruction for cardiac magnetic resonance fingerprinting T1 and T2 mapping. Magn Reson Med 85:2127–2135
Fahmy AS, Rowin EJ, Chan RH, Manning WJ, Maron MS, Nezafat R (2021) Improved quantification of myocardium scar in late gadolinium enhancement images: Deep Learning Based Image Fusion Approach. J Magn Reson Imaging 54:303–312
Ferdian E, Suinesiaputra A, Fung K, Aung N, Lukaschuk E, Barutcu A, Maclean E, Paiva J, Piechnik SK, Neubauer S, Petersen SE, Young AA (2020) Fully automated myocardial strain estimation from Cardiovascular MRI-tagged images using a Deep Learning Framework in the UK Biobank. Radiol Cardiothorac Imaging 2:e190032
Baessler B, Treutlein M, Schaarschmidt F, Stehning C, Schnackenburg B, Michels G, Maintz D, Bunck AC (2017) A novel multiparametric imaging approach to acute myocarditis using T2-mapping and CMR feature tracking. J Cardiovasc Magn Reson 19:71
Baessler B, Luecke C, Lurz J, Klingel K, von Roeder M, de Waha S, Besler C, Maintz D, Gutberlet M, Thiele H, Lurz P (2018) Cardiac MRI texture analysis of T1 and T2 maps in patients with Infarctlike Acute Myocarditis. Radiology 289:357–365
Régis C, Martineau P, Harel F, Pelletier-Galarneau M (2020) Personalized Cardiac Imaging with New PET Radiotracers. Curr Cardiovasc Imaging Rep ;13
Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, Bauersachs J, Wollert KC, Bengel FM (2018) Myocardial inflammation predicts remodeling and Neuroinflammation after myocardial infarction. J Am Coll Cardiol 71:263–275
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caobelli, F., Cabrero, J.B., Galea, N. et al. Cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) imaging in the diagnosis and follow-up of patients with acute myocarditis and chronic inflammatory cardiomyopathy. Int J Cardiovasc Imaging 39, 2221–2235 (2023). https://doi.org/10.1007/s10554-023-02927-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02927-6