Abstract
Multi-detector computed tomography (MDCT) is the gold standard non-invasive tool for evaluating aortic root dimensions. We assessed the agreement between 4D TEE and MDCT-derived aortic valve annular dimensions, coronary ostia height, and minor dimensions of sinuses of Valsalva (SoV) and sinotubular junction (STJ). In this prospective analytical study, we measured the annular area, annular perimeter, area-derived diameter, area-derived perimeter, left and right coronary ostial heights, and minor diameters of the SoV and the STJ using ECG-gated MDCT and 4D TEE. TEE measurements were calculated semi-automatically by the eSie valve software. We enrolled 43 adult patients (27 males, median age: 46 years). We found strong correlations and good agreement between the two modalities in annular dimensions (area, perimeter, area-derived diameter, and perimeter-derived diameter), left coronary ostial height, minimum STJ diameter, and minimum SoV diameters. Moderate correlations, and agreement, with relatively large differences between the 95% LOA, were demonstrated for the right coronary artery ostial height. 4D TEE correlates well with MDCT in measuring aortic annular dimensions, coronary ostial height, SoV minor diameter, and sinotubular junction minor diameter. Whether this can affect clinical outcomes is unknown. It could replace MDCT if the latter is unavailable or contraindicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of the development in transcatheter aortic valve replacement procedures and the refinement of aortic root surgeries, non-invasive aortic root evaluation has become an increasingly essential part of the clinical practice of cardiologists, cardiac surgeons, and radiologists. The availability of a correct and reliable non-invasive technology substantially impacts clinical decisions and outcomes in aortic root pathologies and aortic valve illnesses. Multidetector computed tomography (MDCT) is currently the method of choice for measuring aortic root diameters [1]. Although MDCT has excellent temporal and spatial resolutions, it carries the risk of radiation and iodinated contrast [2]. It also has special implications in renal disease and iodinated compound allergy. These drawbacks, combined with the improvements in transesophageal echocardiography (TEE) technology, led to the increasing use of four-dimensional TEE (4D-TEE) in measuring the annulus size and aortic root dimensions. In this study, we investigated the agreement between semi-automated 4D-TEE and MDCT in measuring the aortic root dimensions and the coronary ostial heights.
Materials and methods
This was a single-center, cross-sectional observational study done in Al Nas Hospital between June 2020 and February 2022. We included 43 patients who already did an MDCT exam, either as a workup for transcatheter aortic valve replacement (TAVR), as a pre-operative coronary evaluation before non-coronary cardiac surgery, or as a part of an evaluation of the cardiac disease. We did 4D-TEE exam for all patients (either before TAVR or intraoperatively). We excluded patients under 18 years of age, those with atrial fibrillation, bicuspid aortic valves, contraindications to CT angiography (renal impairment history of allergy to ionic contrast, pregnancy), or contraindications to TEE (esophageal stricture or recent hematemesis).
4D-TEE
Electrocardiogram (ECG)-gated 2D and 4D TEE were performed according to the guidelines [3] using a commercially available TEE transducer (Z6M transducer; Siemens AG, Munich, Germany). The best mid-esophageal view was used to acquire the 4D TEE images [4].
We analyzed the data sets using the eSie valve software on the same machine (Siemens Acuson SC2000 prime ultrasound system; Siemens AG, Munich, Germany). 3D data sets were manually adjusted to include the whole aortic root, and the maximum valve opening phase identified the systole.
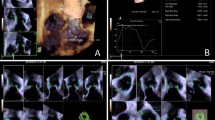
The eSie valve software determines the aortic root measurements automatically. Based on the obtained 3D dataset, the software generates a virtual model by recognizing anatomical landmarks such as valve commissures, leaflet hinges, and coronary ostia. This technology is based on a vast image database used to train artificial intelligence algorithms for image identification, allowing for robust virtual 3D modeling. Then, using automated tracking techniques, these algorithms fit the patient-specific data to a valve surface model that connects the identified landmarks [4] (Fig. 1).
Then, the aortic annular area, aortic annular perimeter, area-derived annular diameter, aortic annulus major and minor diameters, sino-tubular junction (STJ) maximum and minimum dimensions, SoV maximum and minimum diameters, and coronary ostial heights are automatically exported to a software-generated report and an excel sheet (Fig. 1).
MDCT
All examinations were carried out on a Siemens SOMATOM drive 128-slice CT scanner (Siemens AG, Munich, Germany) with the following standard technical parameters: gantry rotation time—0.33 ms; axial coverage—0.5 mm [128 × 0.6 mm]; tube voltage [weight-adjusted]—0–120 kV; milliampere intensity with Care Dose 4D modulation; and temporal resolution—70 ms. The ECG gated the images, which were then acquired during a breath hold. Contrast enhancement was achieved using 60–80 mL of iopromide (Ultravist 370 mg/mL). We used a bolus tracking method in the ascending aorta for optimal synchronization. An additional dose of oral propranolol (20–40 mg) was administered to lower the heart rates that were > 70 bpm at the time of the study, and the examination was not continued at such high heart rates. The thickness of reconstructed images was 0.5 mm [5].
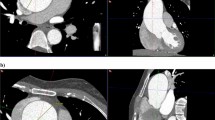
Aortic annulus measurements were done in multiplanar reconstruction images (MPR) using a dedicated software during the best systolic phase (35–45%), which was chosen after examining the acquired phases in the axial cuts [5,6,7]. The aortic annulus is the virtual ring located just below the basal attachments of all three valvular cusps. MPR images were oriented manually to show the aortic annulus at basal attachment points. Two orthogonal planes, bisecting the aortic valve in sagittal and coronal planes, were manually set. The third orthogonal plane (double-oblique transverse view) was set to bisect the aortic annulus at the most caudal attachment points of all three native cusps, orientating/positioning the virtual basal ring optimally [5]. The aortic annulus’s outer border was manually traced. A specific software calculated the annular area, perimeter, area-derived diameter, and minimum and maximum diameters based on this tracing [5]. The heights of the left and right coronary arteries (LCA and RCA, respectively) were measured in relation to the previously defined annular plane. The MPR was also used to identify the SoV and STJ planes, and the maximum and minimum diameters of both were measured (Fig. 2).
Aortic annular measurements, definitions, and geometric analysis
Measurements derived from 4D-TEE and MDCT data sets were as follows: Aortic annular area (mm2), Annular perimeter (mm). Area-derived annular diameter and perimeter-derived annular diameter were calculated as follows [8]:
An experienced echocardiographer performed the TEE and acquired the 4D TEE data, while an experienced radiologist did the MDCT analysis: both were blinded to the measurements from the other modality.
Statistical analysis
Data analysis was applied using Jamovi software version 2.2.5. The normal distribution of data was checked using the Shapiro–Wilk test. Descriptive data were expressed as median and interquartile range (IQR) if the data were skewed and mean and standard deviation (SD) if normally distributed. Categorical data were expressed as numbers and percentages. The correlation between TEE and MDCT-derived dimensions was assessed using the Pearson correlation test and calculating the concordance correlation coefficient. Bland–Altman analysis and plot were used to evaluate the agreement between TEE and MDCT in measuring different aortic parameters [9].
Results
Among 218 adult patients (183 undergoing open heart surgery and 35 undergoing elective TEE on an outpatient basis), 43 met the inclusion criteria and completed the study protocol.
The median age of the patients was 46 years. The most common indication for an MDCT scan was a pre-operative evaluation of coronary arteries before non-coronary cardiac surgery (n = 32). Eleven patients had severe aortic stenosis (AS): 3 degenerative and 8 rheumatic (Table 1).
The annular area, annular perimeter, area-derived annular diameter, perimeter-derived annular diameter, STJ, and SoV dimensions derived from 4D TEE were smaller than those of the MDCT. These differences were statistically significant, except for maximum STJ junction and maximum SoV dimensions. However, the 4D TEE-derived RCA average ostial height was greater than that of the MDCT, while there was minimal difference in the LCA ostial height (− 0.2 mm). But these differences did not reach statistical significance (Table 2).
Correlations and agreement
The 4D-TEE and MDCT-derived annular area measurements had very strong and significant positive linear correlation and showed good agreement (r = 0.987, p ≤ 0.01, CCC = 0.972, and ICCC = 0.974). The 95% limits of agreement (LOA) for Bland–Altman analysis were − 81.4 mm2 and 24.2 mm2, with a bias of − 28.6 mm2. Most data points were within the 95% LOA. The 4D-TEE and MDCT-derived annular perimeter measurements showed similar findings (r = 0.984, p ≤ 0.01, CCC = 0.954, and ICCC = 0.95). The 95% LOA for Bland–Altman analysis were − 8.02 mm and 1.56 mm, with a bias of − 3.2 mm (Fig. 3A, B).
A Scatter plot (left) and Bland–Altman plot (right) of annular area measurements. B Scatter plot (left) and Bland–Altman plot (right) of annular perimeter measurements. C Scatter plot (left) and Bland–Altman plot (right) of right coronary artery ostial height D Scatter plot (left) and Bland–Altman plot (right) of left coronary artery height. CT computed tomography, Ann annulus, TEE trans-esophageal echocardiography, Peri perimeter, RCH right coronary height, LCH left coronary height
The area-derived and perimeter-derived annular diameter measurements by 4D-TEE and MDCT also showed similar findings (r = 0.982, p = 0.01, and r = 0.983, p = 0.001, respectively). The area-derived diameter had 95% LOA between − 2.29 and 0.67 mm with a bias of − 0.81 mm, and the perimeter-derived diameter had 95% LOA between − 2.55 and 0.49 mm with a bias of − 1.02 mm (Fig. 3).
As for RCA and LCA ostial height measurements, the 4D TEE and MDCT had a moderate positive linear correlation (r = 0.68, p = 0.01, and r = 0.841, p = 0.01, respectively): the 95% LOA was between − 3.25 and 4.15 mm (with a bias of 0.448 mm) for the former and between − 2.7 and 2.59 mm (with a bias of − 0.05 mm) for the latter (Fig. 3C, D).
For the SoV minimum diameter, there was a statistically significant strong positive linear correlation and moderate agreement in terms of SoV minimum diameter (r = 0.779, p ≤ 0.01, CCC = 0.687, and ICCC = 0.692). Bland–Altman analysis showed 95% LOA between − 10.2 and 6.64 mm, with a bias of − 1.8 mm. Similarly, there was a significantly strong positive linear correlation and good agreement in terms of STJ minimum diameter (r = 0.793, p < 0.01, CCC = 0.723, and ICCC = 0.728). The 95% LOA for the Bland–Altman analysis was between − 9.2 and 7.49 mm, with a bias of − 0.86 mm (Fig. 4A, B).
Discussion
In this study, we discovered very strong correlations with good agreement between 4D TEE and MDCT-derived annular dimensions: annular area, annular perimeter, and area-derived diameter. Moreover, there were strong correlations and good agreement for LCA height, minimum STJ diameter, and minimum SoV diameters. However, we only detected moderate correlations and agreement with relatively large differences between the 95% LOA for RCA ostial height, maximum STJ diameter, and maximum SoV diameters.
Aortic annular dimensions
In this study, there were very strong correlations and agreement between 4D TEE and MDCT in the measured annular areas, perimeters, and their respective derived annular diameters. In general, the 4D TEE-derived annular dimensions were smaller than MDCT-derived diameters—it underestimated the annular area by 28.6 mm2 (5.7%), the annular perimeter by 3.2 mm (4%), the area-derived annular diameter by 0.8 mm (3.2%), and the perimeter-derived annular diameter by 1.0 mm (3.5%).
There were several comparisons between 4D TEE and MDCT in evaluating aortic root dimensions. Granata et al. was a prospective study that used the same 4D TEE software but on a smaller number of severe symptomatic AS patients (26 patients). They found a statistically significant strong correlation between the two methods in annular areas (r = 0.89, p < 0.0001), annular perimeters (r = 0.89, p < 0.0001), maximum annular diameters (r = 0.79, p < 0.001) and minimum annular diameters (r = 0.81, p < 0.001). The 4D TEE also underestimated MDCT measurements of the annular area by 65.3 mm2 (13.6%), annular perimeter by 4 mm (5.2%), maximum annular diameter by 1.2 mm (4.5%), and minimum annular diameter by 2.6 mm (11.3%) [10].
Four previous studies used older software versions. Calleja et al. was a study on 20 normal participants. It showed no significant difference between the two modalities in coronal, sagittal, and average annular dimensions, and the Bland–Altman plot showed good visual agreement [11]. Another retrospective analysis of 47 severe AS patients found significant correlations and good agreement in the area-derived annular diameters (r = 0.88, p < 0.001 and 95% LOA − 4.24 and 1.71 mm) and perimeter-derived annular diameters (r = 0.9, p < 0.001 and 95% LOA between − 3.94 and 1.73 mm) [4]. Similarly, using the same software, Kato et al. retrospectively analyzed the data of 43 severe AS patients undergoing TAVI. They demonstrated strong correlation and good agreement with narrow differences in the annular area (difference = − 6 mm2 (1.7%), r = 0.94, p < 0.001, 95% LOA between − 48.3 and 60.2 mm2) and the annular perimeter (difference = + 0.1 mm, r = 0.90, p < 0.001, 95% LOA between − 6.5 and 6.3 mm) [12]. Choi et al. found that the 4D TEE underestimated the annular area but with a significant correlation MDCT (difference = 34 mm2 (12%), r = 0.98, p = 0.018) [13].
Prihadi et al. used a different software to compare the two modalities in 150 patients with severe AS: the 4D TEE underestimated MDCT-annular dimensions, but both had strong correlation and good agreement in the annular area (difference = − 10.1 mm2 (2.2%), r = 0.91, p < 0.001, 95% LOA between − 78.5 and 58.4 mm2) and annular perimeter (difference = − 0.3 mm (0.4%), r = 0.83, p < 0.001, 95% LOA between − 8.5 and 8.2 mm) [7]. Khalique et al. reported similar findings with manual analysis of the 4D TEE datasets using a different machine: annular area difference = − 7.9 mm2 (2%) (r = 0.94, p < 0.001, 95% LOA between − 65 and 49.1 mm2), area-derived annular diameter difference = − 0.22 mm (1%), r = 0.94, p < 0.01) and annular perimeter difference = − 1 mm (1.3%), r = 0.93, p < 0.001, 95% LOA between − 6.0 and 4.0) [14].
Coronary ostial heights, STJ, and SoV dimensions
In our study, we found good correlations and good agreements with a relatively small difference for the LCA ostial height (r = 0.84, p < 0.01, CCC = 0.83, 95% LOA between − 2.29 and 0.67 mm), minimum STJ diameter (r = 0.793, p < 0.01, CCC = 0.723, 95% LOA between − 9.2 and 7.49 mm) and minimum SoV diameters (r = 0.779, p < 0.01, CCC = 0.687, 95% LOA between − 10.2 and 6.6 mm). But the correlations and agreement were moderate with relatively large differences for RCA ostial height (r = 0.68, p < 0.01, CCC = 0.67, 95% LOA between − 3.25 and 4.15 mm), maximum STJ diameter (r = 0.658, p < 0.01, CCC = 0.594, 95% LOA between − 12.02 and 12 mm) and maximum SoV diameters (r = 0.667, p < 0.01, CCC = 0.537, 95% LOA between − 14.7 and 14.5 mm). We could not come up with a plausible explanation for the moderate correlation in the RCA height versus the good correlation in LCA height, and none of the previous studies reported results on this point: they only published data on the LCA or no data on coronary ostial heights at all.
Fewer studies investigated the correlation between 4D TEE-derived and MDCT-derived coronary arteries’ ostial heights, SoV, and STJ dimensions.
Granata et al. also found a statistically significant moderate positive correlation for RCA ostial height (r = 0.53, p = 0.007) but a weak non-significant correlation for LCA ostial height (r = 0.33, p = 0.1) [10]. Tamborini et al. also reported a strong correlation (r = 0.83, p = 0.01), and a good agreement was good in the LCA height, and the difference was small and non-significant (0.4 mm) [15].
In Prihadi et al. study, 4D TEE underestimated the mean STJ diameter (− 1.4 mm) with good correlation and agreement (r = 0.73, p < 0.001, 95% LOA between − 4.8 and 4.0 mm), and the mean SoV diameter (− 0.7 mm) with good correlation and agreement (r = 0.87, p < 0.001, 95% LOA between − 4.2 and 2.8 mm) [7]. Similarly, Choi et al. study showed that 4D TEE underestimated both maximum STJ diameter (2.69 ± 0.26 vs. 3.19 ± 0.21 cm, r = 0.775, p = 0.042) and maximum SoV diameter (3.16 ± 0.32 vs. 3.92 ± 0.46 cm, r = 0.993, p = 0.007) with significant good correlation [13].
In contrast, Calleja et al. revealed a significant difference in coronary ostial heights measured by 4D TEE and MDCT. The 4D TEE measurements were smaller for the left (11.3 vs. 12.9 mm, p = 0.03) and right (11.6 vs. 13.1 mm, p = 0.001) coronary ostial heights. The mean 4D TEE-derived STJ and SoV diameters were also smaller by 1.4 mm (p < 0.01) and 2.8 mm (p < 0.01), respectively. However, these findings should be interpreted cautiously because the 4D TEE population differed from the MDCT population in this study [11].
Automated analysis of both 4D TEE and CT datasets and their validation is currently an area of active interest in medical research: due to the increased availability of transcatheter structural interventions, especially TAVR, and the increased complexity of aortic root surgeries. Currently, MDCT evaluation of the aortic root is the gold standard despite the drawbacks of ionizing radiation and the use of iodinated contrast, which can be troublesome in patients with renal impairment [1]. The improvements in hardware and software, especially artificial intelligence, made 4D TEE a theoretically viable alternative to MDCT.
To our knowledge, this study is the first to compare 4D TEE and MDCT-derived aortic root dimensions in various aortic root and valve pathologies, including normal root and valve, severe AS, and dilated aortic root. To date, only a few studies have compared semi-automated 4D TEE with MDCT in evaluating aortic root dimensions—they all focused on aortic annular dimensions and were only done in patients with severe AS in preparation for TAVR procedures.
Study limitations
This study has some limitations. First, it is a single-center study, and its results should be interpreted with caution and further validated in larger multicenter trials. Also, this study did not measure clinical outcomes based on decisions using data obtained from 4D TEE or MDCT. This question can be investigated further through clinical outcome studies. Finally, the limited ability of TEE to detect and quantify calcification can impede assessment in severely calcific aortic roots and valves.
Conclusion
4D TEE correlates well with MDCT in measuring aortic annular dimensions, coronary ostial height, SoV minor diameter, and sinotubular junction minor diameter. It could replace MDCT if the latter is unavailable or contraindicated. Whether or not this can affect clinical outcomes is yet to be determined.
References
Binder RK, Webb JG, Willson AB et al (2013) The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol 62:431–438. https://doi.org/10.1016/j.jacc.2013.04.036
Sodickson A, Baeyens PF, Andriole KP et al (2009) Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 251:175–184. https://doi.org/10.1148/radiol.2511081296
Hahn RT, Abraham T, Adams MS et al (2013) Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the american society of echocardiography and the society of cardiovascular anesthesiologists. J Am Soc Echocardiogr 26:921–964. https://doi.org/10.1016/j.echo.2013.07.009
Thalappillil R, Datta P, Datta S et al (2020) Artificial intelligence for the measurement of the aortic valve annulus. J Cardiothorac Vasc Anesth 34:65–71. https://doi.org/10.1053/j.jvca.2019.06.017
Blanke P, Weir-McCall JR, Achenbach S et al (2019) Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). JACC Cardiovasc Imaging 12:1–24. https://doi.org/10.1016/j.jcmg.2018.12.003
Delgado V, Ng ACT, van de Veire NR et al (2010) Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 31:1114–1123. https://doi.org/10.1093/eurheartj/ehq018
Prihadi EA, van Rosendael PJ, Vollema EM et al (2018) Feasibility, accuracy, and reproducibility of aortic annular and root sizing for transcatheter aortic valve replacement using novel automated three-dimensional echocardiographic software: comparison with multi-detector row computed tomography. J Am Soc Echocardiogr 31:505–514e3. https://doi.org/10.1016/j.echo.2017.10.003
von Aspern K, Foldyna B, Etz CD et al (2015) Effective diameter of the aortic annulus prior to transcatheter aortic valve implantation: influence of area-based versus perimeter-based calculation. Int J Cardiovasc Imaging 31:163–169. https://doi.org/10.1007/s10554-014-0527-4
Liu J, Tang W, Chen G et al (2016) Correlation and agreement: overview and clarification of competing concepts and measures. Shanghai Arch psychiatr 28:115–120. https://doi.org/10.11919/j.issn.1002-0829.216045
Granata G, Veltri A, Iuliano S et al (2020) P218 accuracy and reproducibility of aortic root assessment by eSie valves in patients candidate to transcatheter aortic valve implantation: a comparative study with computed tomography. Eur Hear J Cardiovasc Imaging 21:jez319084. https://doi.org/10.1093/ehjci/jez319.084
Calleja A, Thavendiranathan P, Ionasec RI, et al (2013) Automated Quantitative 3-Dimensional Modeling of the Aortic Valve and Root by 3-Dimensional Transesophageal Echocardiography in Normals, Aortic Regurgitation, and Aortic Stenosis. Circ Cardiovasc Imaging 6:99–108. https://doi.org/10.1161/CIRCIMAGING.112.976993
Kato N, Shibayama K, Noguchi M et al (2018) Superiority of novel automated assessment of aortic annulus by intraoperative three-dimensional transesophageal echocardiography in patients with severe aortic stenosis: comparison with conventional cross-sectional assessment. J Cardiol 72:321–327. https://doi.org/10.1016/j.jjcc.2018.02.017
Choi JH, Georgescu B, Ionasec R et al (2008) Abstract 5793: novel semi-automatic quantitative assessment of the aortic valve and aortic root from volumetric 3D echocardiography: comparison to volumetric cardiac computed tomography (CT). Circulation 118(18):S103–S104. https://doi.org/10.1161/circ.118.suppl_18.S_103-c
Khalique OK, Kodali SK, Paradis J-M et al (2014) Aortic annular sizing using a novel 3-dimensional echocardiographic method. Circ Cardiovasc Imaging 7:155–163. https://doi.org/10.1161/CIRCIMAGING.113.001153
Tamborini G, Fusini L, Gripari P et al (2012) Feasibility and accuracy of 3DTEE versus CT for the evaluation of aortic valve annulus to left main ostium distance before transcatheter aortic valve implantation. JACC Cardiovasc Imaging 5:579–588. https://doi.org/10.1016/j.jcmg.2012.02.012
Acknowledgements
The authors would like to thank Siemens for their collaboration in this research. They provided technical support for the use of the software in MDCT and 4D TEE machines.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by MMA. MMA and AAS analyzed the images. WAW reviewed and verified the image analysis and measurements. AAE and MMA did the statistical analysis. MH wrote the first draft of the manuscript and AAE adjusted the subsequent versions. YB supervised the research and reviewed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Cairo University (Date 27-07-2020 /No: MD-192-2020).
Consent to participate
Written informed consent was obtained from all patients.
Consent to publish
The authors affirm that human research participants proved informed consent for publication of the images in Fig 1a, b and 2a–d.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, M., Abdrabou, M.M., Wahba, W.A. et al. Agreement between 4D transesophageal echocardiography and multi-detector computed tomography in measuring aortic root dimensions and coronary ostia heights. Int J Cardiovasc Imaging 39, 1561–1569 (2023). https://doi.org/10.1007/s10554-023-02873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02873-3