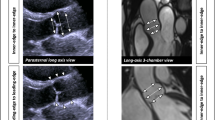
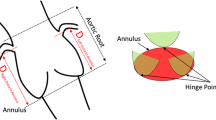
Abstract
To evaluate the accuracy, reproducibility, and transcatheter heart valve (THV) sizing efficiency of an automated 3-dimensional transesophageal echocardiographic (3D-TEE) post-processing software in the assessments of aortic roots, intra-individually compared with multidetector computed tomography (MDCT). We prospectively studied 67 patients with normal aortic roots. We measured diameters of aortic annulus (AA), sinus of Valsalva (SOV), and sino-tubular junction (STJ) by full-automated and semi-automated methods using 3D-TEE datasets, then compared them to corresponding transthoracic echocardiography and MDCT values. THV sizes were chosen based on echocardiography and MDCT measurements according to recommended criterion. Taking MDCT planimetered diameters as reference, the full-automated (r: 0.4745–0.8792) and semi-automated (r: 0.6647–0.8805) 3D-TEE measurements were linearly correlated (p < 0.0001). The average differences between semi-automated or full-automated measurements and reference were 0.3 mm or 1.3 mm for AA, − 1.9 mm or − 0.5 mm for SOV, and − 0.1 mm or 1.9 mm for STJ, respectively. The intra-class correlation coefficients of semi-automated method were 0.79–0.96 (intra-observer) and 0.75–0.92 (inter-observer). THV sizing by semi-automated measurements using echocardiographic criteria was larger than that by MDCT measurements using MDCT criteria (p < 0.0001) but equivalent (p > 0.05) if both using MDCT standards. The new automated 3D-TEE software allows modeling and quantifying aortic roots with high reproducibility. Measurements by the semi-automated method closely approximate and well correlate with the corresponding MDCT, thus THV sizing by this modeled 3D-TEE measurements should adopt recommended MDCT criteria but not echocardiographic criteria. The full-automated 3D-TEE segmentations are yet immature. (Semi-automated assessMent of Aortic Roots by Three-dimensional transEsophageal echocaRdiography [SMARTER], NCT02724709)



Similar content being viewed by others
References
Giannini F, Baldetti L, Gallone G et al (2018) Transcatheter valve replacement in Asia Pacific: current practice and perspectives. J Am Coll Cardiol 72:3189–3199
Khalique OK, Hamid NB, White JM et al (2017) Impact of methodologic differences in three-dimensional echocardiographic measurements of the aortic annulus compared with computed tomographic angiography before transcatheter aortic valve replacement. J Am Soc Echocardiogr 30:414–421
Leipsic JA, Blanke P, Hanley M et al (2017) ACR appropriateness criteria((R)) imaging for transcatheter aortic valve replacement. J Am Coll Radiol 14:S449–S455
Soon J, Pibarot P, Blanke P et al (2017) Multimodality imaging for planning and follow-up of transcatheter aortic valve replacement. Can J Cardiol 33:1110–1123
Bons LR, Duijnhouwer AL, Boccalini S et al (2019) Intermodality variation of aortic dimensions: how, where and when to measure the ascending aorta. Int J Cardiol 276:230–235
Dulgheru R, Pibarot P, Sengupta PP et al (2016) Multimodality imaging strategies for the assessment of aortic stenosis: Viewpoint of the Heart Valve Clinic International Database (HAVEC) group. Circ Cardiovasc Imaging 9:e4352
Blanke P, Weir-McCall JR, Achenbach S et al (2019) Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging 12:1–24
Pibarot P, Magne J, Leipsic J et al (2019) Imaging for predicting and assessing prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging 12:149–162
Kato N, Shibayama K, Noguchi M et al (2018) Superiority of novel automated assessment of aortic annulus by intraoperative three-dimensional transesophageal echocardiography in patients with severe aortic stenosis: comparison with conventional cross-sectional assessment. J Cardiol 72:321–327
Lang RM, Badano LP, Tsang W et al (2012) EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 25:3–46
Garcia-Martin A, Lazaro-Rivera C, Fernandez-Golfin C et al (2016) Accuracy and reproducibility of novel echocardiographic three-dimensional automated software for the assessment of the aortic root in candidates for thanscatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging 17:772–778
Yang L, Xu L, Schoepf UJ et al (2015) Prospectively ECG-triggered sequential dual-source coronary CT angiography in patients with atrial fibrillation: influence of heart rate on image quality and evaluation of diagnostic accuracy. PLoS ONE 10:e134194
Wang Y, Wang M, Song G et al (2019) Optimal pre-TAVR annulus sizing in patients with bicuspid aortic valve: area-derived perimeter by CT is the best-correlated measure with intraoperative sizing. Eur Radiol 29:259–269
Ren X, Zhang M, Liu K et al (2016) The significance of aortic valve calcification in patients with bicuspid aortic valve disease. Int J Cardiovasc Imaging 32:471–478
Mayr A, Klug G, Reinstadler SJ et al (2018) Is MRI equivalent to CT in the guidance of TAVR? A pilot study. Eur Radiol 28:4625–4634
Mahmood F, Shernan SK (2016) Perioperative transoesophageal echocardiography: current status and future directions. Heart 102:1159–1167
Khalique OK, Kodali SK, Paradis JM et al (2014) Aortic annular sizing using a novel 3-dimensional echocardiographic method: use and comparison with cardiac computed tomography. Circ Cardiovasc Imaging 7:155–163
Prihadi EA, van Rosendael PJ, Vollema EM et al (2018) Feasibility, accuracy, and reproducibility of aortic annular and root sizing for transcatheter aortic valve replacement using novel automated three-dimensional echocardiographic software: comparison with multi-detector row computed tomography. J Am Soc Echocardiogr 31:505–514
Achenbach S, Delgado V, Hausleiter J et al (2012) SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 6:366–380
Podlesnikar T, Delgado V (2016) Update: Cardiac Imaging (II). transcatheter aortic valve replacement: advantages and limitations of different cardiac imaging techniques. Rev Esp Cardiol (Engl Ed) 69:310–321
Husser O, Holzamer A, Resch M et al (2013) Prosthesis sizing for transcatheter aortic valve implantation—comparison of three dimensional transesophageal echocardiography with multislice computed tomography. Int J Cardiol 168:3431–3438
Ng AC, Delgado V, van der Kley F et al (2010) Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 3:94–102
Vaquerizo B, Spaziano M, Alali J et al (2016) Three-dimensional echocardiography vs. computed tomography for transcatheter aortic valve replacement sizing. Eur Heart J Cardiovasc Imaging 17:15–23
Tamborini G, Fusini L, Muratori M et al (2014) Feasibility and accuracy of three-dimensional transthoracic echocardiography vs. multidetector computed tomography in the evaluation of aortic valve annulus in patient candidates to transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging 15:1316–1323
Calleja A, Thavendiranathan P, Ionasec RI et al (2013) Automated quantitative 3-dimensional modeling of the aortic valve and root by 3-dimensional transesophageal echocardiography in normals, aortic regurgitation, and aortic stenosis: comparison to computed tomography in normals and clinical implications. Circ Cardiovasc Imaging 6:99–108
Zamorano J, Pardo A (2016) 3D-ECHO for TAVI: two arrows, just in case. Eur Heart J Cardiovasc Imaging 17:9–10
Hahn RT, Little SH, Monaghan MJ et al (2015) Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc Imaging 8:261–287
Liao YB, Zhao ZG, Wei X et al (2017) Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: preliminary experiences in China. Catheter Cardiovasc Interv 89:528–533
Jurencak T, Turek J, Kietselaer BL et al (2015) MDCT evaluation of aortic root and aortic valve prior to TAVI. What is the optimal imaging time point in the cardiac cycle? Eur Radiol 25:1975–1983
Sengupta PP, Adjeroh DA (2018) Will artificial intelligence replace the human echocardiographer? Circulation 138:1639–1642
Acknowledgements
The authors thank Dr. Chuangshi Wang (Medical Research and Biometrics Center, State Key Laboratory of Cardiovascular Diseases, National Center for Cardiovascular Diseases; 15 Fengcunxili, Beijing 102308, China) and Dr. Zhilan Zheng (Division of Ultrasound Application, Siemens Healthineers China; 7 Wangjing Zhonghuan South Road, Beijing 100102, China) for their helps.
Funding
This work was supported by the Peking Union Medical College Youth Fund from the Fundamental Research Funds for the Central Universities (No. 3332015013, to MZ) and partly by the National Natural Science Foundation of China (No. 81470080, to WH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 14875 kb) Dynamic videos of 3D-TEE recording (example)
Supplementary file2 (AVI 11145 kb) Dynamic videos of 3D-TEE recording (example)
10554_2019_1664_MOESM8_ESM.docx
Supplementary file8 (DOCX 189 kb) Supplementary Figure 2: Bland-Altman analysis of AA, SOV, and STJ diameters, regarding MDCT-Area as reference standard
10554_2019_1664_MOESM12_ESM.docx
Supplementary file12 (DOCX 141 kb) Supplementary Figure 6: Variability of the semi-automated and full-automated modeling measurements
10554_2019_1664_MOESM14_ESM.docx
Supplementary file14 (DOCX 18 kb) Supplementary Table 1: Manufacturer recommended sizing criterion (SAPIEN 3 & CoreValve)
10554_2019_1664_MOESM17_ESM.docx
Supplementary file17 (DOCX 81 kb) Supplementary Table 4: The average differences among various measurements of AA, SOV, and STJ diameters
Rights and permissions
About this article
Cite this article
Zhang, M., Wan, L., Liu, K. et al. Aortic roots assessment by an automated three-dimensional transesophageal echocardiography: an intra-individual comparison. Int J Cardiovasc Imaging 35, 2029–2036 (2019). https://doi.org/10.1007/s10554-019-01664-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01664-z