Abstract
Inflammation characterizes all stages of atherothrombosis and provides a critical pathophysiological link between plaque formation and its acute rupture, leading to coronary occlusion and heart attack. In the last 20 years the possibility of quantifying the degree of inflammation of atherosclerotic plaques and, therefore, also of vascular inflammation aroused much interest. 18Fluoro-deoxy-glucose photon-emissions-tomography (18F-FDG-PET) is widely used in oncology for staging and searching metastases; in cardiology, the absorption of 18F-FDG into the arterial wall was observed for the first time incidentally in the aorta of patients undergoing PET imaging for cancer staging. PET/CT imaging with 18F-FDG and 18F-sodium fluoride (18F-NaF) has been shown to assess atherosclerotic disease in its molecular phase, when the process may still be reversible. This approach has several limitations in the clinical practice, due to lack of prospective data to justify their use routinely, but it’s desirable to develop further scientific evidence to confirm this technique to detect high-risk patients for cardiovascular events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current evidence has shown a central role for inflammation in all stages of the atherosclerotic process.
Numerous studies have identified how inflammation is widely involved in early atherogenesis, in progression of lesions and in thrombotic complications of the disease and how circulating markers of inflammation and the propensity to develop ischemic events are strongly related [1].
Inflammation characterizes all phases of atherothrombosis and provides a critical pathophysiological link between plaque formation and its acute rupture, which leads to coronary occlusion and heart attack. High levels of cholesterol and triglycerides in the blood cause the small particles of lipoproteins to bind to proteoglycans and accumulate in the intima layer; the lipoprotein particles bound to proteoglycans have a greater susceptibility to oxidation (Fig. 1). Oxidative stress, including products present in modified lipoproteins, induces the expression of cytokines and chemokines which, locally, recall leukocytes.
In response to chemotactic cytokines (e.g., Monocyte Chemotactic Protein-1 [MCP-1]), monocytes cross the artery wall and amplify the release of growth factors and stimulants for formation of macrophages. As the process progresses, foam cells develop, which are macrophages filled with oxidized lipoproteins and fats. The cascade of local chemokines continues until it attracts smooth muscle cells, which begin to process the extracellular matrix and the “fatty strip” evolves into a fibro-adipose lesion. In the later stages calcification can occur (Fig. 2), first as microcalcification of the vessels, then as macrocalcification last to a formation of a core [2,3,4,5].
To date, therefore, it is well known that vascular inflammation is a central component of the atherosclerotic process as supported by several experimental studies, observational data and by demonstration of potential beneficial effects of anti-inflammatory therapies in advanced atherosclerotic disease [6].
Often cardiovascular clinical events, such as myocardial infarction, result from sudden rupture of atherosclerotic plaques and inflammation and plaque erosion are the main factors [7].
Evolution of an atherosclerotic plaque
Positron Emission Tomography (PET) and cardiovascular diseases
The PET method has a relatively low spatial resolution [3,4,4 mm], which requires the use of simultaneous structural imaging (CT or MRI) to guide the localization of the 18F-fluorodeoxyglucose (18F-FDG) signal.
Widely used in oncology, for staging and searching metastases, in cardiology, 18F-FDG-PET is commonly used to estimate the consumption of glucose in the myocardium and therefore evaluate the residual vitality and possible benefit to myocardial revascularization.
Since several years, 18F-FDG-PET is widely used in case of suspicious of endocarditis or with suspected cardiac implantable electronic device (CIED) infection or LVAD (Left Ventricular Assistance Devices) infections. The sensitivity and specificity of [18F]FDG PET/CT in prosthetic valve endocarditis are 73–100% and 71–100%, respectively. [18F]FDG PET/CT also improved the sensitivity of the modified Duke criteria from 52–70% to 91–97% (8).
In native valve endocarditis, [18F]-FDG PET/CT has a relatively limited role, but it has a potential usefulness to identify extracardiac manifestations (ie, embolic stroke or septic embolization) [9].
[18F]FDG PET/CT shows very high diagnostic accuracy in detecting pocket/generator infection (sensitivity = 93%, specificity = 98%) and in cases of lead-related IE, [18F]-FDG PET/CT is highly specific (88%) with low sensitivity (65%), due to small vegetation(s) characterized by low-metabolic activity [10, 11].
Moreover, FDG-PET has emerged as the most commonly used technique to assess the extension of systemic sarcoidosis and also to assess extent and activity of myocardial involvement. In addition, FDG-PET in conjunction with MPI are recommended radionuclide method for evaluation of cardiac sarcoidosis, as well as to identify perfusion defects as important prognostic factor [12,13,14].
Furthermore, FDG PET/CT emerged as a useful modality for the diagnosis of large-vessel vasculitis, include giant cell arteritis and Takayasu arteritis, and evidence suggests that it provides independent prognostic information, as well as it can be used to monitor and modulate therapy [15, 16].
In recent years, as part of the expansion of imaging towards the identification of vascular inflammation, PET has been used to assessment of atherosclerotic disease at an early stage. In this field, 18F-FDG and 18F-sodium fluoride (18F-NaF) are the most commonly PET tracers used. 18F-FDG is absorbed by macrophages activated in plaques, highly sensitive for the metabolically active processes using glucose, while 18F-NaF is deposited in the microcalcification sites by chemical exchange of the 18F-ion with the hydroxyl group in the hydroxyapatite. PET/CT imaging with 18F-FDG and 18F-NaF has the ability to assess atherosclerotic disease in its molecular stage when the process may still be reversible [17,18,19,20].
The role of imaging in the atherosclerosis disease
In the last twenty years, the possibility of quantifying the degree of inflammation of atherosclerotic plaques and vascular inflammation raised a lot of interest. Traditional imaging modalities, such as ultrasound, computed tomography (CT), and magnetic resonance angiography (MRI), are all widely used in clinical practice to identify symptomatic macroscopic plaques, but have significant limitations to finding early stages of atherosclerosis when plaques are more biologically active [21, 22].
These evidences have led to expand imaging beyond the traditional anatomical and physiological domains, exploiting the metabolic processes underlying cardiovascular diseases [23], and to identify diagnostic methods capable to detecting atherosclerosis before the incidence of cardiovascular disease and in early stages of the disease [24, 25].
18 F-FDG and PET
During inflammatory processes, in a molecular point of view, cells must use exogenous glucose as fuel and since 18F-FDG is an analogue of glucose, it absorbed by macrophages resident in plaque and making them detectable by PET imaging [26, 27].
As it illustrated before, atherosclerotic plaques are rich in macrophages and other inflammatory cells and Tawakol et al. were able to demonstrate that the most inflamed areas of plaque accumulate almost 20 times more 18F-FDG than the control arteries. They confirmed that the metabolic signal measured in the plaques is mainly due to inflammatory activity and to the presence of macrophages [28, 29].
In addition to the macrophage content, there are also other circulating inflammatory biomarkers that determine a relationship between arterial 18F-FDG signal and inflammation, such as for example C-reactive protein (PCR), interleukin-6 (IL-6), selectin -P-soluble, selectin-E-soluble [30, 31] and these data confirm relationship between inflammatory process and progression of atherosclerosis disease.
18 F-NaF and PET
Over the past decade, interest in 18F-NaF has been revived by its ability to detect molecular calcifications in plaques by being absorbed only at sites of active calcification/ossification and in no other organ or pathological process [32].
Furthermore, 18F-NaF is rapidly cleared from the bloodstream (60–90 min) reaching a high contrast between the calcification sites and background activity [33, 34].
The uptake of 18F-NaF from the arterial wall is more consistently related to cardiovascular risk factors than the accumulation of 18F-FDG [35,36,36].
In fact, it has been shown that 18F-NaF deposits in the arterial level are more consistent in patients with angina than in healthy control subjects [37, 38]. Furthermore, coronary uptake of 18F-NaF has been shown to precede gross calcification visible on intravascular or CT ultrasound and 18F-NaF does not accumulate in the myocardium, contrary to high physiological myocardial uptake of 18F-FDG [39, 40].
Prognostic role of PET imaging
In a rabbit atherosclerosis model, authors observed when plaque rupture is promoted by venom injection, only aortic plaques with the highest pre-rupture 18F-FDG levels progress and undergo rupture and thrombosis [41].
These finds led to speculation a possible predictive role of 18F-FDG imaging for plaque rupture events and, therefore, major cardiovascular events (e.g., heart attack).
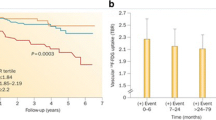
In vivo, some evidences correlated arterial absorption of 18F-FDG with subsequent risk of both plaque rupture and clinical events. Patients with the highest uptake of 18F-FDG were more likely to have previously had a vascular event or to have experienced one during the 6 months following PET imaging [42].
Moreover, Arauz et al. presented prognostic data in patients with symptomatic carotid artery disease; 85% of subjects had high absorption levels of 18F-FDG and they had worse outcomes over following 6 months (recurrent stroke, stent artery death or restenosis) than those with initially lower 18F-FDG levels [43].
More recently, McCabe et al. provided that in individuals with recent ischemic stroke/TIA and ipsilateral carotid stenosis, carotid plaque inflammation-related 18FDG uptake on PET/CT angiography was associated with 5-year recurrent ipsilateral stroke [44].
In last years, numerous studies were performed using 18F-FDG-PET to assess vascular inflammation in different subset of patients. In a large cohort of cancer patients (932 patients), asymptomatic for cardiovascular diseases, it was demonstrated that arterial uptake of 18F-FDG and calcifications in large arteries is related to the highest risk for a future vascular event (ischemic stroke, myocardial infarction, need for revascularization) [45].
In other smaller studies were found a significant relationship between the absorption of 18F-FDG in the arterial wall and the degree of inflammation and atherosclerosis plaques, respectively in end-stage renal disease (ESRD) patients and in chronic obstructive pulmonary disease (COPD) [46,47,48]. They confirmed how well-known conditions (COPD and ESRD) characterized by systemic inflammation are related to a higher grade of vascular inflammation and subclinical atherosclerosis and so an augmented risk for cardiovascular events.
Joshi AA et al. reached to the same conclusion demonstrating how the presence of aortic vascular inflammation detected by PET/CT with 18F-FDG is associated with the presence of coronary artery disease assessed by coronary CT (CCT) [49].
In a cross-sectional cohort study (215 patients) affected by psoriasis, authors quantified aortic vascular inflammation using 18F-FDG-PET/CT and at the same time they assessed the degree of coronary artery disease with CCT. They showed that extension of CAD (as higher prevalence of luminal stenosis, more severe luminal stenosis and higher prevalence of high-risk plaque) is greater in subjects with high aortic vascular inflammation and, regardless of cardiovascular risk factors, a strong association between aortic vascular inflammation and CAD was found.
This helped to demonstrate how aortic vascular inflammation assessed by 18F-FDG-PET/CT can be a potential surrogate for the assessment of early CAD.
PET imaging to evaluate anti-inflammatory therapies response
In last two decades, the grade of inflammation by PET/CT imaging as a surrogate marker of inflammatory activity in atherosclerosis has also been used for observing and evaluating the response to therapies [50, 51].
CANTOS [52] and COLCOT [53] trials demonstrated respectively that canakinumab (monoclonal antibody targeting interleukin-1β) and colchicine led to a significantly lower rate and lower risk of cardiovascular events. They proved that therapies targeting inflammation or immune-system pathway can improve cardiovascular outcomes.
According with these evidences, a single-center open-label study for first [54] and few years later a multicenter trial [55] observed a significant dose response in the reduction in FDG uptake between the high- and low-dose statin groups and showed that an incremental benefit of statin intensification was related to a lower plaque inflammation measured using FDG-PET imaging. In line with observations from large-scale trials of low-versus high-dose statins [56, 57], these data support the hypothesis that the cardiovascular benefit from statin therapy may be due to a rapid reduction in arterial inflammation.
In this context, PET imaging can provide a direct measure of inflammation arising in the vascular wall and it will also support early assessment of treatment effect of anti-inflammatory drugs [58, 59], as well as it will useful to evaluate the benefit of non-immunomodulator drugs in the inflammation processes [60].
18 F-FDG or 18F-NaF in coronary heart disease
Several technical hurdles must be overcome before 18F-FDG PET imaging can be applied to measure inflammation at the coronary plaque level. The significant uptake of 18F-FDG into the myocardium, tissue with physiological increased glucose metabolism, has prevented the use of this technology to detect atherosclerosis in coronary arteries, a major cause of population morbidity and mortality in the Western world [61].
Cardiac motion also leads to blurring of coronary plaque uptake of 18F-FDG, especially in the distal coronary arteries. Routine PET acquisitions are typically gated and obtained in 15 min, therefore, there may be spatial discordance between PET and CT images. The small coronary plaque size also requires a relatively high degree of focal 18F-FDG accumulation before a detectable signal can be measured due to modest resolution of PET. The main challenge seems to relate to the combined effects of heart and respiratory movements during data acquisition over a long period of time, as well as the difficulty in identifying small plaques (1–2 mm) using the tracers [62].
Furthermore, mechanisms other than inflammation can generate the 18F-FDG signal associated with atheromas. An example, hypoxia can lead to an increase in the use of glucose by macrophages; in fact, hypoxia stimulates glucose intake by the cells and, like inflammation, can cause an increased absorption of 18F-FDG in the cells present in atheromas [63].
Despite these challenges and limitations, several working groups have investigated the accumulation of 18F-FDG on coronary vessels [64, 65].
Particularly, comparing uptake of 18F-FDG at the level of coronary arteries with culprit lesions in patients with acute coronary syndrome (ACS) and stable angina controls, Rogers et al.they observed a significantly higher uptake of 18F-FDG from the coronary artery, higher in plaques of ACS patients. This finding is consistent with the notion that SCA is associated with increased arterial inflammation [66].
Although 18F-FDG PET has been shown to be useful for quantifying inflammation within atherosclerosis, Joshi et al. [67] were among the first to demonstrate the superiority of 18F-NaF PET-CT as the first non-invasive imaging method to identify and locate ruptured or high-risk coronary plaque for rupture. In their prospective clinical study, patients with myocardial infarction (n = 40) and stable angina (n = 40) who underwent PET-CT with 18F-NaF and 18F-FDG and coronary angiography were enrolled. It has been shown that intense absorption of 18F-NaF is localized at the level of the plaque undergoing recent rupture in patients with acute myocardial infarction. The fact that 18F-NaF can also be used to detect plaque in coronary arteries is a big plus for this tracer [68, 69].
In recent years, a major trial (CAMONA trial: Cardiovascular Molecular Calcification Assessed by 18F-NaF PET/CT) has been conducted to compare the performance of 18F-FDG- and 18F-NaF-PET in atherosclerosis.
Healthy volunteers and patients with a previous episode of chest pain was recruited to study the relationship between CVD risk, estimated by the Framingham Risk Score (FRS) and arterial inflammation. The study found that the increased risk of CVD is associated with marked increases in vascular calcification, assessed by PET imaging with 18F-NaF, and by vascular calcium load, assessed by imaging CT scan, but not associated with the degree of arterial inflammation, evaluated by 18F-FDG PET imaging [70, 71].
Outlook: new nuclear probes for vascular inflammation and atherosclerosis
Alternative molecular targets and PET tracers for imaging vascular inflammation are being studied and, according pathophysiology of the inflammation, their target is not directly linked to inflammation but it’s also direct to plaques microcalcifications, hypoxia or apoptosis process. Immune and inflammation cells are associated with cytokine and receptors expression, resulting in a numerous potential imaging targets for new PET tracers (Table 1).
Moreover, thanks to development of nanotechnologies, nanoparticles are emerging to detect and quantify macrophages and atherosclerotic plaques. They have the great advantage to be easily tunable and often they present longer half-lives than others PET radionuclides [72,73,74,75], but more data and prospective study in human are needed.
A great review about PET tracers in vascular inflammation, with a focused update on recent radiopharmaceuticals research related to nuclear imaging and an overview of ongoing research in this field, it’s been recently done by Prigent et Vigne [76].
It desirable that the development of PET probes will implement nuclear traces able to explore new molecular targets and PET imaging allows for direct visualization of metabolic processes, including other inflammation mechanisms unknown yet.
Future prospects for PET imaging
As emerged from this review, PET imaging could either be used to further stratify groups of high-risk patients and evaluate therapies response.
However, although a link between vascular inflammation and future cardiovascular risk identified by 18F-FDG PET can be implied through its association with clinical risk factors, inflammatory biomarkers, high-risk plaque, stroke recurrence and major adverse clinical events in retrospective analyses of large PET imaging datasets, definitive prospective clinical outcome data is needed. In the near future 18F-NaF could be play a key role to detect atherosclerosis, but considering the limited data available, it is certainly desirable to develop further scientific evidence to confirm this technique and new PET tracers as a good weapon to intercept high-risk patient and prevent major cardiovascular events.
References
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874. https://doi.org/10.1038/nature01323
Ley K, Miller YI, Hedrick CC (2011) Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 31:1506. https://doi.org/10.1161/ATVBAHA.110.221127
Galkina E, Ley K (2007) Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27:2292. https://doi.org/10.1161/ATVBAHA.107.149179
Woollard KJ et al (2010) Monocytes in atherosclerosis: Subsets and functions. Nat Rev Cardiol 7(2):77–86. https://doi.org/10.1038/nrcardio.2009.228
Hutcheson JD, Goettsch C, Bertazzo S et al (2016) Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater 15:335–343. https://doi.org/10.1038/nmat4519
Ridker PM, Everett BM, Thuren T et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. NEJM 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Libby P (2013) Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368:2004–2013. https://doi.org/10.1056/NEJMra1216063
Gomes A, Glaudemans A, Touw DJ et al (2017) Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 17(1):e1–e14. https://doi.org/10.1016/S1473-3099(16)30141-4
Casali M, Lauri C, Altini C et al (2021) State of the art of 18F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging 9(4):299–339. https://doi.org/10.1007/s40336-021-00445-w
Juneau D, Golfam M, Hazra S et al (2017) Positron emission tomography and single-photon emission computed tomography imaging in the diagnosis of cardiac implantable electronic device infection: a systematic review and meta-analysis. Circ Cardiovasc Imaging 10(4):e005772. https://doi.org/10.1161/CIRCIMAGING.116.005772
Ploux S, Riviere A, Amraoui S et al (2011) Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 8(9):1478–1481. https://doi.org/10.1016/j.hrthm.2011.03.062
Treglia G, Annunziata S, Sobic-Saranovic D et al (2014) The role of 18F-FDG-PET and PET/CT in patients with sarcoidosis: an updated evidence-based review. Acad Radiol 21:675–684
Ahmadian A, Brogan A, Berman J et al (2014) Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol 21:925–939
Blankstein R, Osborne M, Naya M et al (2014) Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 63:329–336
Pelletier-Galarneau M, Ruddy TD (2019) PET/CT for diagnosis and management of large-vessel vasculitis. Curr Cardiol Rep 21(5):34. https://doi.org/10.1007/s11886-019-1122-z
Soussan M, Nicolas P, Schramm C et al (2015) Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore) 94(14):e622. https://doi.org/10.1097/MD.0000000000000622
Le Guludec D, Lautamäki R, Knuuti J et al (2008) Present and future of clinical cardiovascular PET imaging in Europe—a position statement by the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 35(9):1709–1724. https://doi.org/10.1007/s00259-008-0859-1
Skagen K, Johnsrud K, Evensen K et al (2015) Carotid plaque inflammation assessed with (18)F-18F-FDG PET/CT is higher in symptomatic compared with asymptomatic patients. Int J Stroke 10(5):730–736. https://doi.org/10.1111/ijs.12430
Rudd JHF, Narula J, William Strauss H et al (2010) Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol 55(23):2527–2535. https://doi.org/10.1016/j.jacc.2009.12.061
Gaemperli O, Boyle JJ, Rimoldi OE et al (2010) Molecular imaging of vascular inflammation. Eur J Nucl Med Mol Imaging 37:1236. https://doi.org/10.1007/s00259-009-1371-y
Syed MB, Fletcher AJ, Forsythe RO et al (2019) Emerging techniques in atherosclerosis imaging. Br J Radiol 92(1103):20180309. https://doi.org/10.1259/bjr.20180309
Takx RA, Partovi S, Ghoshhajra BB (2016) Imaging of atherosclerosis. Int J Cardiovasc Imaging 32:5–12. https://doi.org/10.1007/s10554-015-0730-y
Sanz J, Fayad ZA (2008) Imaging of atherosclerotic cardiovascular disease. Nature 451(7181):953–957. https://doi.org/10.1038/nature06803
Grammaticos PC (2014) Diagnosing atherosclerosis makes nuclear medicine a tissue imaging modality. Hell J Nucl Med 17(1):12
Buscombe JR (2015) Exploring the nature of atheroma and cardiovascular inflammation in vivo using positron emission tomography (PET). Br J Radiol 88(1053):20140648. https://doi.org/10.1259/bjr.20140648
Ogawa M, Ishino S, Mukai T et al (2004) (18)F-18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 45(7):1245–1250
Leppänen O, Björnheden T, Evaldsson M et al (2006) ATP depletion in macrophages in the core of advanced rabbit atherosclerotic plaques in vivo. Atherosclerosis 188(2):323–330. https://doi.org/10.1016/j.atherosclerosis.2005.11.017
Tawakol A, Migrino RQ, Hoffmann U et al (2005) Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 12(3):294–301. https://doi.org/10.1016/j.nuclcard.2005.03.002
Tawakol A et al (2006) In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 48(9):1818–1824. https://doi.org/10.1016/j.jacc.2006.05.076
Duivenvoorden R, Mani V, Woodward M et al (2013) Relationship of serum inflammatory biomarkers with plaque inflammation assessed by 18F-FDG PET/CT: the dal-PLAQUE study. JACC Cardiovasc Imaging 6(10):1087–1094. https://doi.org/10.1016/j.jcmg.2013.03.009
Tahara N, Kai H, Yamagishi S et al (2007) Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol 49(14):1533–1539. https://doi.org/10.1016/j.jacc.2006.11.046
Al-Zaghal A, Raynor W, Khosravi M et al (2018) Applications of PET imaging in the evaluation of musculoskeletal diseases among the geriatric population. Semin Nucl Med 48(6):525–534. https://doi.org/10.1053/j.semnuclmed.2018.07.002
Paydary K, Revheim M-E, Emamzadehfard S et al (2020) Quantitative thoracic aorta calcification assessment by (18)F-18F-NaF PET/CT and its correlation with atherosclerotic cardiovascular disorders and increasing age. Eur Radiol 31(2):785–794. https://doi.org/10.1007/s00330-020-07133-9
Raynor WY, Borja J, Rojulpote C et al (2021) 18-sodium fluoride: an emerging tracer to assess active vascular microcalcification. J Nucl Cardiol 28(6):2706–2711. https://doi.org/10.1007/s12350-020-02138-9
Fujimoto K, NorikanE T, YamamotO Y et al (2019) Association between carotid (18)F-18F-NaF and (18)F-18F-FDG uptake on PET/CT with ischemic vascular brain disease on MRI in patients with carotid artery disease. Ann Nucl Med 33(12):907–915. https://doi.org/10.1007/s12149-019-01403-3
McKenney-Drake ML, Moghbel MC, PaydarY K et al (2018) (18)F-18F-NaF and (18)F-18F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging 45(12):2190–2200. https://doi.org/10.1007/s00259-018-4078-0
Alavi A, Werner TJ, Raynor W et al (2021) Critical review of PET imaging for detection and characterization of the atherosclerotic plaques with emphasis on limitations of 18F-FDG-PET compared to 18F-NaF-PET in this setting. Am J Nucl Med Mol Imaging 15(11):337–351
Piri R, Gauher Lici G, Riyahimanesh P et al (2021) Two-year change in 18F-sodium fluoride uptake in major arteries of healthy subjects and angina pectoris patients. Int J Cardiovasc Imaging 37(10):3115–3126. https://doi.org/10.1007/s10554-021-02263-7
Høilund-Carlsen PF, Piri R, Constantinescu C et al (2020) Atherosclerosis Imaging with 18F-Sodium Fluoride PET. Diagnostics 10(10):852. https://doi.org/10.3390/diagnostics10100852
Arani LS, Gharavi MH, Zadeh MZ et al (2019) Association between age, uptake of 18F-fluorodeoxyglucose and of 18F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med 22(1):14–19. https://doi.org/10.1967/s002449910954
Sorci O, Batzdorf AS, Mayeret M et al (2020) 18F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur J Nucl Med Mol Imaging 47(7):1678–1687. https://doi.org/10.1007/s00259-019-04590-3
Aziz K, Berger K, Claycombe K et al (2008) Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation 117(16):2061–2070. https://doi.org/10.1161/CIRCULATIONAHA.106.652313
Arauz A, Hoyos L, Zenteno M et al (2007) Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography. Pilot study. Clin Neurol Neurosurg 109(5):409–412. https://doi.org/10.1016/j.clineuro.2007.02.012
McCabe JJ, Pol C-R, Giannotti N et al (2021) Carotid plaque inflammation imaged by PET and prediction of recurrent stroke at 5 years. Neurology 97(23):e2282–e2291. https://doi.org/10.1212/WNL.0000000000012909
Rominger A, Saam T, Wolpers S et al (2009) 18F–18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 50(10):1611–1620. https://doi.org/10.2967/jnumed.109.065151
van der Valk FM, Verweij SL, Zwinderman KAH et al (2016) Thresholds for arterial wall inflammation quantified by 18F-FDG PET imaging: implications for vascular interventional studies. JACC: Cardiovasc Imaging 9(10):1198–1207. https://doi.org/10.1016/j.jcmg.2016.04.007
Bural GG, Torigian DA, Sözmen M et al (2018) Comparison of atherosclerotic inflammation and calcification in subjects with end stage renal disease (ESRD) on hemodialysis to normal controls utilizing (18)F-18F-FDG PET/CT. Hell J Nucl Med 21(3):169–174. https://doi.org/10.1967/s002449910901
Vanfleteren LE, van Meerendonk AMG, Franssen FM et al (2014) A possible link between increased metabolic activity of fat tissue and aortic wall inflammation in subjects with COPD. A retrospective 18F–18F-FDG-PET/CT pilot study. Respir Med 108:883–890. https://doi.org/10.1016/j.rmed.2014.04.001
Joshi AA, Lerman JB, Dey AK et al (2018) Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol 3(10):949–956. https://doi.org/10.1001/jamacardio.2018.2769
Watanabe T, Kawasaki M, Tanaka R et al (2015) Anti-inflammatory and morphologic effects of pitavastatin on carotid arteries and thoracic aorta evaluated by integrated backscatter trans-esophageal ultrasound and PET/CT: a prospective randomized comparative study with pravastatin (EPICENTRE study). Cardiovasc Ultrasound 2(13):17. https://doi.org/10.1186/s12947-015-0012-9
Mizoguchi M, Tahara N, Tahara A et al (2011) Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial 18F-FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging 4(10):1110–1118. https://doi.org/10.1016/j.jcmg.2011.08.007
Ridker PM, Thuren T, Zalewski A et al (2011) Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 162:597–605
Tardif JC, Kouz S, Waters DD et al (2019) Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 381(26):2497–2505. https://doi.org/10.1056/NEJMoa1912388
Tahara N, Kai H, Ishibashi M et al (2006) Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 48(9):1825–1831. https://doi.org/10.1016/j.jacc.2006.03.069
Tawakol A, Fayad ZA, Mogg R et al (2013) Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Feasibility Study. J Am Coll Cardiol 62(10):909–917. https://doi.org/10.1016/j.jacc.2013.04.066
Cannon CP, Braunwald E, McCabe CH et al (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350:1495–1504
Ray KK, Cannon CP, McCabe CH et al (2005) for the PROVE IT-TIMI 22 investigators early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 46:1405–1410
Thuy Trang DAMT, Okamura K, Suto T et al (2021) Do biologic therapies reduce aortic inflammation in rheumatoid arthritis patients? Arthritis Res Ther 23(1):206. https://doi.org/10.1186/s13075-021-02585-w
Kaijasilta J-P, Kerola AM, Tuompo R et al (2022) Adalimumab and sulfasalazine in alleviating sacroiliac and aortic inflammation detected in PET/CT in patients with axial spondyloarthritis: PETSPA. Immun Inflamm Dis 10(2):155–162. https://doi.org/10.1002/iid3.552
Jensen JK, Binderup T, Grandjean CE et al (2022) Semaglutide reduces vascular inflammation investigated by PET in a rabbit model of advanced atherosclerosis. Atherosclerosis 352:88–95. https://doi.org/10.1016/j.atherosclerosis.2022.03.032
Camici PG, Rimoldi QE, Gaemperli O, Libby P et al (2012) Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J 33(11):1309–1317. https://doi.org/10.1093/eurheartj/ehs067
Alavi A, Werner TJ, Raynor W et al (2021) Critical review of PET imaging for detection and characterization of the atherosclerotic plaques with emphasis on limitations of 18F-FDG-PET compared to 18F-NaF-PET in this setting. Am J Nucl Med Mol Imaging 11(5):337–351
Folco EJ, Sheikine Y, Rocha VZ et al (2011) Hypoxia but not inflammation augments glucose uptake in human macrophages. Implications for imaging atherosclerosis with 18F-FDG-PET. J Am Coll Cardiol 58(6):603–614. https://doi.org/10.1016/j.jacc.2011.03.044
Wykrzykowska J, Lehman S, Williams G et al (2009) Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 50(4):563–568. https://doi.org/10.2967/jnumed.108.055616
Dunphy MP, Freiman A, Larson SM et al (2005) Association of vascular 18F-18F-FDG uptake with vascular calcification. J Nucl Med 46(8):1278–1284
Rogers IS, Nasir K, Figueroa AL et al (2010) Feasibility of 18F-FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 3(4):388–397. https://doi.org/10.1016/j.jcmg.2010.01.004
Joshi NV, Vesey AT, Williams MC et al (2014) 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383(9918):705–713. https://doi.org/10.1016/S0140-6736(13)61754-7
Dweck MR, Chow MWL, Joshi NV et al (2012) Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 59(17):1539–1548. https://doi.org/10.1016/j.jacc.2011.12.037
Nakahara T, Dweck MR, Narula N et al (2017) Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging 10(5):582–593
Blomberg BA, Thomassen A, Takx RAP et al (2014) Delayed 18F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol 21(3):588–597. https://doi.org/10.1007/s12350-014-9884-6
Blomberg BA, de Jong PA, Thomassen A et al (2017) Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging 44(2):249–258. https://doi.org/10.1007/s00259-016-3552-9
Senders M, Hernot S, Carlucci G et al (2019) Nanobody-facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc Imaging 12:2015–2026. https://doi.org/10.1016/j.jcmg.2018.07.027
Gaemperli O, Shalhoub J, Owen D et al (2012) Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 33:1902–1910. https://doi.org/10.1093/eurheartj/ehr367
Luehmann H, Detering L, Gropler RJ et al (2017) C–C chemokine receptor type 2 (CCR2) targeted PET imaging of early atherosclerosis. Circ Am Heart Assoc 136:A20674
Woodard PK, Liu Y, Pressly ED et al (2016) Design and modular construction of a polymeric nanoparticle for targeted atherosclerosis positron emission tomography imaging: a story of 25% (64)Cu-CANF-Comb. Pharm Res 33:2400–2410. https://doi.org/10.1007/s11095-016-1963-8
Prigentl K, Vigne J (2021) Advances in radiopharmaceutical sciences for vascular inflammation imaging: focus on clinical applications. Molecules 26(23):7111. https://doi.org/10.3390/molecules26237111
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
AMS, RF, AD: wrote the manuscript, FD, PB, FB and EV: review the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sammartino, A.M., Falco, R., Drera, A. et al. “Vascular inflammation and cardiovascular disease: review about the role of PET imaging”. Int J Cardiovasc Imaging 39, 433–440 (2023). https://doi.org/10.1007/s10554-022-02730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02730-9