Abstract
Background
COVID-19 has caused a global pandemic unprecedented in a century. Though primarily a respiratory illness, cardiovascular risk factors predict adverse outcomes. We aimed to investigate the role of baseline echocardiographic abnormalities in further refining risk in addition to clinical risk factors.
Methods
Adults with COVID-19 positive RT-PCR test across St Luke’s University Health Network between March 1st 2020-October 31st 2020 were identified. Those with trans-thoracic echocardiography (TTE) within 15–180 days preceding COVID-19 positivity were selected, excluding severe valvular disease, acute cardiac event between TTE and COVID-19, or asymptomatic patients positive on screening. Demographic, clinical, and echocardiographic variables were manually extracted from patients’ EHR and compared between groups stratified by disease severity. Logistic regression was used to identify independent predictors of hospitalization.
Results
192 patients met inclusion criteria. 87 (45.3%) required hospitalization, 34 (17.7%) suffered severe disease (need for ICU care/mechanical ventilation/in-hospital death). Age, co-morbidities, and several echocardiographic abnormalities were more prevalent in those with moderate-severe disease than in mild disease, with notable exceptions of systolic/diastolic dysfunction. On multivariate analysis, age (OR 1.039, 95% CI 1.011–1.067), coronary artery disease (OR 4.184, 95% CI 1.451–12.063), COPD (OR 6.886, 95% CI 1.396–33.959) and left atrial diameter ≥ 4.0 cm (OR 2.379, 95% CI 1.031–5.493) predicted need for hospitalization. Model showed excellent discrimination (ROC AUC 0.809, 95% CI 0.746–0.873).
Conclusions
Baseline left atrial enlargement is an independent risk factor for risk of hospitalization among patients with COVID-19. When available, baseline LA enlargement may identify patients for (1) closer outpatient follow up, and (2) counseling vaccine-hesitancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As of January 2022, coronavirus disease 2019 (COVID-19) has caused over 296 million infections and over 5.4 million fatalities worldwide, representing by far the largest global infectious disease pandemic in over a century [1]. Several large cohorts have reported certain baseline demographic and clinical features being consistently associated with disease severity and mortality among patients with COVID-19 [2,3,4,5]. In particular, cardiovascular (CV) risk factors such as age, diabetes, obesity, and hypertension have been associated with adverse outcomes among patients with COVID-19, including risk of severe disease, need for mechanical ventilation, and mortality [3,4,5]. Moreover, although primarily a respiratory disease, multi-organ involvement is common in COVID-19 and contributes significantly to adverse outcomes [6, 7].
However, patients with these risk factors themselves represent a fairly heterogenous group, both based on number of such co-morbidities, their severity (for example uncontrolled versus mild or well-controlled diabetes/hypertension), and duration of co-morbidities. Obviously, structural and functional myocardial abnormalities, as assessed using trans-thoracic echocardiography (TTE), are a consequence of long-standing or advanced phenotypes of the above cardiovascular risk factors. Indeed, extant literature spanning several decades overwhelmingly shows that presence of echocardiographic abnormalities in the general population as well as in those with cardiovascular risk factors has incremental prognostic value to clinical factors alone in predicting risk of stroke, sudden cardiac death and cardiovascular morbidity and mortality [8,9,10,11]. Along those lines, we hypothesized that pre-existent echocardiographic abnormalities may perhaps be able to identify a higher risk stratum of patients within a clinically high-risk, albeit heterogenous, group of patients. If so, TTE abnormalities could prove additive to clinical risk-stratification among COVID-19 patients. At the very least, the identification of such associations should add to the overall body of knowledge regarding a novel infection. Ultimately, given the enormous and unprecedented burden of COVID-19 on health-care systems across the world it is clearly imperative to continue to refine triage of patients, for more efficient planning and use of resources. Moreover, TTE is currently one of the most commonly performed diagnostic test, especially in those above 60–65 years old, an age group that is particularly vulnerable to severe COVID-19. Hence, presence of baseline TTE features as predictors of outcomes in COVID-19 may aid risk-stratification in a large target population.
Methods
Patient population
Electronic health records (EHR) were searched for all adult patients testing positive for COVID-19 due to suspicious symptoms across the St. Luke’s University Health Network (SLUHN) between March 1st and October 31st, 2020. Patients with a baseline echocardiogram within 15–180 days preceding the COVID-19 infection were selected. Each patient’s EHR was manually searched for baseline demographic information (age, gender, race), clinical co-morbidities, and transthoracic echocardiographic (TTE) variables. Patients were excluded if, (1) suffering severe aortic or mitral valvular stenosis or regurgitation, (2) any acute cardiovascular event (acute myocardial infarction, new onset congestive heart failure, or stroke) between the dates of TTE and COVID-19 positivity, (3) testing positive on a screening test for COVID-19 (for example for employment or placement in nursing home), but without any symptoms of COVID-19. All variables were extracted to a pre-designed spreadsheet. Major outcomes of interest were need for hospitalization, and among those hospitalized, the need for ICU care, mechanical ventilation, and in-hospital mortality. The study protocol was approved by the SLUHN Institutional Review Board (IRB).
Outcome definition and data synthesis
Patients were stratified into mild, moderate and severe COVID-19. Those not requiring hospital admission were categorized as having mild disease, surviving hospitalization without a need for ICU admission or mechanical ventilation as having moderate disease, and those requiring ICU admission/mechanical ventilation or died during the hospital stay as severe. In order to simplify statistical analysis, and for greater clinical applicability, important echocardiographic variables were transformed into binary variables based on established cut-offs of normality per the American Society of Echocardiography (ASE) guidelines. Left ventricular hypertrophy (LVH) was defined as indexed left ventricular mass (LVMi) ≥ 95 g/m2 in females and 115 g/m2 for males, relative wall thickness (RWT) as increased when > 0.42, left atrial (LA) enlargement as LA diameter ≥ 4 cm. Severity of pulmonary hypertension was reported as per TTE interpretation, since mean or systolic pulmonary artery pressures were seldom quantified in patients with no evidence of elevated pressures or obviously in those without meaningful tricuspid regurgitation, and ultimately classified as moderate or higher pulmonary artery hypertension (PAH). Finally left ventricular systolic dysfunction was defined as left ventricular ejection fraction (LVEF) < 0.50, for simplicity of analysis.
Statistical analysis
Patients were compared across groups stratified by disease severity, i.e. mild, moderate, or severe, as described above. Continuous variables were compared across the three groups using one-way analysis of variance (ANOVA). Post-hoc comparisons between groups were performed using Tukey’s honestly significant difference test (Tukey’s HSD) in case of homogeneity of variance (Levene’s test p > 0.05), and the Games-Howell test when not. Categorical variables were compared using the χ2-test. Given our findings (noted below) on post-hoc testing, need for hospitalization (indicating moderate or severe disease, in other words, non-mild disease) was chosen as the primary outcome for regression analysis. Univariate analysis was performed to identify significant predictors for the multivariate model at p < 0.10. Multivariate backward stepwise logistic regression was performed to identify independent predictors of hospitalization. Echocardiographic variables with potential significant interactions (such as left ventricular linear dimensions used in calculation of LVMi and LVH) were not entered into the multivariate regression despite their correlation to outcomes on a univariate analysis basis. All statistical analyses were performed using SPSS version 26 (IBM Corp, Armonk, USA).
Results
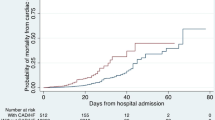
A total of 192 patients met inclusion criteria, of which 87 (45.3%) required hospitalization, and 34 (17.7% of total, 39.1% of those hospitalized) suffered severe disease (needed ICU admission/mechanical ventilation or died). 30 patients died (15.6% of total, 32.1% of those hospitalized). Mean time interval between TTE and COVID test in the entire population was 86 days (range 18–178 days). Baseline clinical and TTE characteristics, stratified by disease severity as described above, are summarized in Table 1. Patients requiring hospitalization were significantly older and more often suffered medical co-morbidities including diabetes, hypertension, and renal cardiac and chronic pulmonary disease. However, among hospitalized patients, none of these were more prevalent between moderate versus severe disease. In other words, once hospitalized, pre-existing clinical and TTE variables were not significantly predictive of risk of need for ICU care, mechanical ventilation, or death. Indeed, the major point of difference, in prevalence of clinical co-morbidities as well as TTE abnormalities was at the point of hospitalization i.e., mild versus non-mild disease. Baseline characteristics stratified by mortality (survivors vs. non-survivors) and by severe versus non-severe disease are summarized in Tables S1 and S2, respectively, in the Supplementary appendix Fig. 1.
Receiver operating characteristics curve for regression model in Table 1. AUC: 0.809 (95% CI 0.746–0.873, p < 0.001)
Several left ventricular (LV) structural parameters, including septal and posterior wall thickness, left ventricular mass (LVM), presence of LVH, and left atrial enlargement were significantly different between mild versus non-mild disease. Interestingly, left ventricular systolic (left ventricular ejection fraction) or diastolic functional parameters (mitral valve E/A ratio) did not differ between groups. Finally, peak and mean pulmonary artery pressure (PAP) were higher, and hence, moderate or worse pulmonary hypertension (PAH) was more prevalent among those with moderate-severe disease versus mild disease. Univariate analysis for predictors of need for hospitalization is summarized in Table 2. As expected from above findings, LV diastolic inflow pattern (E/A ratio) or LVEF failed to predict risk of hospitalization. On the other hand, several LV structural parameters were predictive of risk of moderate-severe disease, including septal thickness (IVSd), posterior wall thickness (LVPWd), LV mass index (LVMi), and relative wall thickness (RWT). Peak PAP, mean PAP, and presence of at least moderate PAH were also associated with moderate-severe disease.
For multivariate analysis, in addition to age and medical co-morbidities listed in Table 1, we chose TTE parameters that were most clinically relevant, especially given the high degree of expected collinearity. For the final model, we chose RWT > 0.42, presence of LVH (LVMi ≥ 95 g/m2 in females or ≥ 115 g/m2 in males), LA enlargement (LA diameter ≥ 4 cm), and moderate or worse PAH, in addition to age and clinical co-morbidities. Table 3 summarizes independent predictors of risk of hospitalization on multivariate analysis with effect sizes. LA enlargement emerged as the only echocardiographic parameter independently predicting moderate-severe COVID-19 infection, in addition to age and clinical co-morbidities. The overall model had excellent discrimination (c-statistic = 0.809; 95% confidence interval 0.746–0.873, p < 0.001).
Discussion
We present, to our knowledge, the first cohort of COVID-19 patients with baseline, pre-infection echocardiographic characteristics and their relation to disease severity. Though there have been many investigations reporting TTE findings among COVID-19 patients, [12,13,14,15] these have largely been aimed at investigating the impact of COVID-19 on the heart vis-à-vis echocardiographic abnormalities among patients hospitalized with COVID-19. Although important by way of adding to our knowledge of the full gamut of organ involvement by this relatively novel virus, and hence aiding description of the full signature of COVID-19, our study was aimed at investigating a more “upstream” role of TTE in COVID-19 patients. In other words, instead of investigating the impact of COVID-19 on echocardiographic parameters, we aimed to investigate the converse, i.e., the impact of echocardiographic parameters/abnormalities on COVID-19 patients. The most important findings from our cohort include, (1) both clinical and TTE abnormalities were substantially more common in hospitalized versus non-hospitalized patients, i.e. in those with more than mild disease, (2) in general, LV structural abnormalities and pulmonary hypertension predicted risk of non-mild disease, while LV functional (systolic or diastolic) parameters did not, and most importantly, (3) after adjusting for medical co-morbidities and other echocardiographic abnormalities, LA enlargement was independently associated with worse than mild disease.
Moreover, we found that in general, clinical co-morbidities as well as most TTE parameters were not significantly different between patients with moderate versus severe disease. In other words, it could be said that beyond a certain threshold of disease severity, clinical co-morbidities or TTE abnormalities did not seem to discriminate those with uneventful hospital course from those requiring ICU stay, mechanical ventilatory support or death from COVID-19. Admittedly, this remains speculative, since our cohort likely represents a somewhat higher risk cohort than the general population, due to the fact that patients who undergo TTE are generally older and more likely to have CV risk factors. Indeed, the prevalence of these risk factors was somewhat higher than reported in previous reports including all-comers with COVID-19 [3, 16, 17]. Hence, the uniformly high and somewhat similar prevalence of these co-morbidities among hospitalized patients across groups stratified by severe vs. non-severe disease possibly masked differences that may be evident in a larger sample. It is worth noting here that previous reports have generally reported a higher attributable risk to multi-organ injury, degree of hypoxemia, presence of respiratory distress syndrome, and/or inflammatory markers compared to medical co-morbidities among hospitalized patients, somewhat in support of our findings [3, 17,18,19]. Admittedly, this may well be due to the fact that majority of these reports pertain to hospitalized patients in whom such markers are available on admission or during the course of the hospital stay. Hence, these markers tend to be measured in those who have already crossed a certain “threshold” of disease severity, by the mere fact that they are hospitalized. Hence, when these markers are added to clinical co-morbidities in predictive models, it is somewhat expected that the predictive ability of the latter declines. It remains well-established, on the other hand, that when all COVID-19 patients (hospitalized and non-hospitalized) are considered, medical co-morbidities do indeed predict severe disease, including mortality. Regardless, among the hospitalized patients in our cohort, we did not find a significant difference in prevalence of clinical co-morbidities or TTE abnormalities among patients who survived versus those who needed ICU care/mechanical ventilation or died, likely due to a small sample and aforementioned population characteristics.
On the other hand, when considering the entire cohort, taking mortality as an outcome as noted in table S1, generally similar trends were noted as for risk of hospitalization. Hence patients who died were significantly older, had higher prevalence of co-morbidities, and generally had similar differences in TTE abnormalities. Most notably, LA enlargement and presence of pulmonary hypertension were much more prevalent among non-survivors. Interestingly, BMI and prevalence of obesity remained lower among those who died or suffered severe disease. This may again be due to our sample population. The mean age of our entire cohort was 64 ± 18 years, among those hospitalized was 73 ± 18 years, and among those who died 80 ± 10 years. It has been shown that the association between obesity and COVID-19 severity declines with age and is generally lost at > 80 years age, likely due to BMI being a generally poor indicator of fat excess in the elderly [20]. Moreover, a lower BMI in the elderly may be a worse prognostic feature by being a marker of frailty, sarcopenia, etc.
In addition to the findings of the present data, there has been an abundance of literature evaluating the role of echocardiography in refining risk of cardiovascular events and mortality [21]. Among various echocardiographic parameters, LA enlargement, a marker of long-standing or severe LV volume and/or pressure overload, and a risk factor for atrial fibrillation, thrombus formation and cardioembolic disease, has been associated with adverse cardiovascular outcomes [22,23,24,25]. LA enlargement has been found to be an independent predictor of fatal and non-fatal cardiovascular events regardless of the presence of LVH [26]. Finally, LA diameter has been shown to independently predict all-cause mortality in both men and women [27]. Thus, it is not surprising that we found LA enlargement to be an echocardiographic parameter predicting risk of non-mild COVID-19, even when adjusted for LVH, and indeed when adjusted for clinical risk factors. To reiterate, we did not find a statistically significant difference in prevalence of LA enlargement among hospitalized patients suffering severe disease, though the prevalence was numerically higher in the latter (Tables 1 and 55.9% vs. 47.2%). This was likely due to the relatively small sample size, and certainly worth investigating in larger cohorts. Conversely, it remains somewhat impressive that even in a modest-sized cohort such as ours, LA diameter proved additive to traditional risk factors in predicting risk of hospitalization. Admittedly we were restricted to using LA diameter as the parameter to define LA enlargement, rather than the currently recommended LA volume, largely because the latter was not routinely reported in majority of TTEs in our cohort. This is obviously a major limitation, especially since LA enlargement (as defined by LA diameter) emerged as the only echocardiographic predictor of hospitalization. However, ample evidence exists regarding the prognostic value of LA diameter, and though now superseded by LA volume, remains a premier predictor of outcomes [21, 23, 28]. We believe that even given this limitation, our findings still strongly support the fundamental hypothesis that LA enlargement predicts risk of hospitalization among COVID-19 patients.
There are some other important limitations to our findings. Firstly, our study is retrospective. Secondly, we had a relatively modest sample size. Thirdly, we did not have the time/resources to have all TTEs re-interpreted using a strictly standardized methodology. In other words, all TTEs parameters were extracted as originally interpreted at the time of the TTE, obviously raising the possibility of non-uniform methodology in performing and interpreting the TTE. However, all TTEs were performed in the same lab, and any heterogeneity that may exist in technique/interpretation should be randomly distributed across patient groups. To our way of thinking, this should in fact make our findings closer to the “real-world”. Moreover, our strategy to define mild versus moderate disease severity based on need for hospitalization may be criticized since different physicians may have different thresholds to admit patients with COVID-19. However, we at SLUHN had a very uniform set of criteria to guide admission with COVID-19 given the sudden surge of patients early during the pandemic in neighboring New York city. Hence, we believe that inter-physician heterogeneity in deciding to admit patients was minimal. Finally, given that patients who have TTE at baseline are generally older and more likely to have suspected or known cardiovascular disease, our findings may not be fully applicable to the general population. Regardless, we found that in patients who do have a baseline TTE, the presence of LA enlargement should raise concern about the patient’s risk of hospitalization, and perhaps warrant closer outpatient follow up and/or monitoring. At the very least our findings are novel and add to the available data on a novel and unprecedented disease. Moreover, quantifying risk is invaluable in stratifying common illnesses with a heavy disease burden, such as the current context. Our findings suggest that in patients in whom a recent TTE is available, the presence of LA enlargement may help refine triage.
Conclusions
To conclude, pending confirmation in larger cohorts, echocardiographic LA enlargement is an independent predictor of risk of hospitalization among patients with COVID-19. Major clinical implications of our findings include, (1) using this information when counseling patients who may be resistant to vaccination against COVID-19, and (2) closer outpatient follow up in patients infected with COVID-19 and having LA enlargement.
References
World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
Harmouch F, Shah K, Hippen JT, Kumar A, Goel H (2021) Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J Med Virol 93(2):973–982. doi:https://doi.org/10.1002/jmv.26347
Tian W, Jiang W, Yao J et al (2020) Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol 92(10):1875–1883. doi:https://doi.org/10.1002/jmv.26050
Mehraeen E, Karimi A, Barzegary A et al (2020) Predictors of mortality in patients with COVID-19–a systematic review. Eur J Integr Med 40. https://doi.org/10.1016/j.eujim.2020.101226
Du RH, Liang LR, Yang CQ et al (2020) Predictors of mortality for patients with COVID-19 pneumonia caused by SARSCoV- 2: A prospective cohort study. Eur Respir J 55(5). doi:https://doi.org/10.1183/13993003.00524-2020
Zou Y, Zhao X, Hou YY et al (2017) Meta-Analysis of Effects of Voluntary Slow Breathing Exercises for Control of Heart Rate and Blood Pressure in Patients With Cardiovascular Diseases. Am J Cardiol 120(1):148–153. doi:https://doi.org/10.1016/j.amjcard.2017.03.247
Cao Y, Liu X, Xiong L, Cai K (2020) Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol March1–11. doi:https://doi.org/10.1002/jmv.25822
Konety SH, Koene RJ, Norby FL et al (2016) Echocardiographic predictors of sudden cardiac death. Circ Cardiovasc Imaging 9(8). doi:https://doi.org/10.1161/CIRCIMAGING.115.004431
Lundorff I, Modin D, Mogelvang R et al (August 2020) Echocardiographic predictors of cardiovascular morbidity and mortality in women from the general population. Eur Hear J - Cardiovasc Imaging. doi:https://doi.org/10.1093/ehjci/jeaa167
Modin D, Biering-Sørensen SR, Møgelvang R, Alhakak AS, Jensen JS, Biering-Sørensen T (2019) Prognostic value of left atrial strain in predicting cardiovascular morbidity and mortality in the general population. Eur Heart J Cardiovasc Imaging 20(7):804–815. doi:https://doi.org/10.1093/ehjci/jey181
Xu Y, Zhao L, Zhang L, Han Y, Wang P, Yu S (2020) Left Atrial Enlargement and the Risk of Stroke: A Meta-Analysis of Prospective Cohort Studies. Front Neurol 11:26. doi:https://doi.org/10.3389/fneur.2020.00026
Giustino G, Croft LB, Stefanini GG et al (2020) Characterization of Myocardial Injury in Patients With COVID-19. J Am Coll Cardiol 76(18):2043–2055. doi:https://doi.org/10.1016/j.jacc.2020.08.069
Shafiabadi Hassani N, Shojaee A, Khodaprast Z, Sepahvandi R, Shahrestanaki E, Rastad H (2021) Echocardiographic Features of Cardiac Injury Related to COVID-19 and Their Prognostic Value: A Systematic Review. J Intensive Care Med 36(4):500–508. doi:https://doi.org/10.1177/0885066620981015
Szekely Y, Lichter Y, Taieb P et al (2020) Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 142(4):342–353. doi:https://doi.org/10.1161/CIRCULATIONAHA.120.047971
Ng ACT, Delgado V, Bax JJ (2020) An international, multicentre survey of echocardiographic abnormalities in COVID-19 patients. Eur Heart J Cardiovasc Imaging 21(9):959–960. doi:https://doi.org/10.1093/ehjci/jeaa218
Petrilli CM, Jones SA, Yang J et al (2020) Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 369. doi:https://doi.org/10.1136/bmj.m1966
Singer AJ, Morley EJ, Meyers K et al (2020) Cohort of Four Thousand Four Hundred Four Persons Under Investigation for COVID-19 in a New York Hospital and Predictors of ICU Care and Ventilation. Ann Emerg Med 76(4):394–404. doi:https://doi.org/10.1016/j.annemergmed.2020.05.011
Knight SR, Ho A, Pius R et al (2020) Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4 C Mortality Score. BMJ 370(September):1–13. doi:https://doi.org/10.1136/bmj.m3339
Chilimuri S, Sun H, Alemam A et al (2020) Predictors of mortality in adults admitted with COVID-19: Retrospective cohort study from New York City. West J Emerg Med 21(4):779–784. doi:https://doi.org/10.5811/westjem.2020.6.47919
Gao M, Piernas C, Astbury NM et al (2021) Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol 9(6):350–359. doi:https://doi.org/10.1016/S2213-8587(21)00089-9
Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT (2005) Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med 165(15):1788–1793. doi:https://doi.org/10.1001/archinte.165.15.1788
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA (2015) Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Hear Fail 8(2):295–303. doi:https://doi.org/10.1161/CIRCHEARTFAILURE.114.001667
Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D (1995) Left atrial size and the risk of stroke and death: The Framingham Heart Study. Circulation 92(4):835–841. doi:https://doi.org/10.1161/01.CIR.92.4.835
Patel DA, Lavie CJ, Gilliland YE, Shah SB, Dinshaw HK, Milani RV (2015) Prediction of all-cause mortality by the left atrial volume index in patients with normal left ventricular filling pressure and preserved ejection fraction. Mayo Clin Proc. ;90(11):1499–1505. doi:https://doi.org/10.1016/j.mayocp.2015.07.021
Kizer JR, Bella JN, Palmieri V et al (2006) Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am Heart J 151(2):412–418. doi:https://doi.org/10.1016/j.ahj.2005.04.031
Bombelli M, Facchetti R, Cuspidi C et al (2014) Prognostic significance of left atrial enlargement in a general population results of the PAMELA study. Hypertension 64(6):1205–1211. doi:https://doi.org/10.1161/HYPERTENSIONAHA.114.03975
Bouzas-Mosquera A, Broullón FJ, Álvarez-García N et al (2011) Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ 183(10). doi:https://doi.org/10.1503/cmaj.091688
Bouzas-Mosquera A, Broullon FJ, Alvarez-ardcia N et al (2011) Left atrial size and risk for all-cause mortality and ischemic stroke. Can Med Assoc J 183(10):E657–E664. doi:https://doi.org/10.1503/cmaj.110617
Acknowledgements
The authors would like to acknowledge the diligence and hard work of Bruce Kemmerer, Director, Clinical Analytics, and Monica Elliott, clinical Analytics analyst, St. Luke’s University Health Network in EHR search.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to data collection, and manuscript preparation. HG and JS performed statistical analysis.
Corresponding author
Ethics declarations
Disclosures
The authors have no conflicts of interest to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goel, H., Shah, K., Kothari, J. et al. Premorbid echocardiography and risk of hospitalization in COVID-19. Int J Cardiovasc Imaging 38, 1733–1739 (2022). https://doi.org/10.1007/s10554-022-02565-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02565-4