Abstract
To evaluate the effect of percutaneous coronary intervention (PCI) of coronary chronic total occlusions (CTOs) on left ventricular (LV) strain assessed using cardiac magnetic resonance (CMR) tissue tracking. In 150 patients with a CTO, longitudinal (LS), radial (RS) and circumferential shortening (CS) were determined using CMR tissue tracking before and 3 months after successful PCI. In patients with impaired LV strain at baseline, global LS (10.9 ± 2.4% vs 11.6 ± 2.8%; P = 0.006), CS (11.3 ± 2.9% vs 12.0 ± 3.5%; P = 0.002) and RS (15.8 ± 4.9% vs 17.4 ± 6.6%; P = 0.001) improved after revascularization of the CTO, albeit to a small, clinically irrelevant, extent. Strain improvement was inversely related to the extent of scar, even after correcting for baseline strain (B = − 0.05; P = 0.008 for GLS, B = − 0.06; P = 0.016 for GCS, B = − 0.13; P = 0.017 for GRS). In the vascular territory of the CTO, dysfunctional segments showed minor improvement in both CS (10.8 [6.9 to 13.3] % vs 11.9 [8.1 to 15.0] %; P < 0.001) and RS (14.2 [8.4 to 18.7] % vs 16.0 [9.9 to 21.8] %; P < 0.001) after PCI. Percutaneous revascularization of CTOs does not lead to a clinically relevant improvement of LV function, even in the subgroup of patients and segments most likely to benefit from revascularization (i.e. LV dysfunction at baseline and no or limited myocardial scar).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary chronic total occlusions (CTOs) are present in approximately 1 in 4 patients with obstructive coronary artery disease (CAD) on invasive coronary angiography [1]. The presence of a CTO confers unfavorable prognosis, with higher rate of major adverse cardiovascular events including death [2]. Contemporary guidelines consider the treatment of CTO lesions analogous to that of non-CTO lesions, indicating that revascularization is recommended for relieving symptoms in patients with refractory angina refractory and for improving prognosis in patients with a large area of viable myocardium at risk [3]. Percutaneous coronary intervention (PCI) of CTO lesions however comes at the expense of higher contrast use, longer fluoroscopy time and increased complication rates in comparison with non-CTO lesions [4]. In addition, CTO-PCI did not improve outcome compared with conservative treatment in 3 recent randomized trials [5,6,7]. The benefit of CTO-PCI is therefore controversial and careful selection of patients is required before attempting revascularization. Functional recovery of hibernating, viable myocardium is one of the potential benefits of CTO-PCI. Cardiac magnetic resonance imaging (CMR) is considered the gold standard for quantifying cardiac function and has been extensively used to study functional recovery after CTO-PCI, with conflicting results [8,9,10,11,12,13,14,15,16,17,18,19]. Furthermore, no differences in regional function in the CTO territory between patients treated with CTO-PCI versus patients receiving optimal medical therapy only was observed [11]. Importantly, prior studies used left ventricular (LV) ejection fraction and analysis of wall thickening to measure global and regional function, respectively. Assessment of myocardial strain using CMR tissue tracking has been proposed as a more sensitive method to measure LV dysfunction [20]. Myocardial strain provides prognostic information incremental to LV ejection fraction and is superior to wall thickening in quantifying regional myocardial function [20, 21]. Therefore, the aim of the present study was to evaluate the effect of CTO-PCI on global and regional myocardial strain assessed using CMR tissue tracking.
Materials and methods
Study population and design
Consecutive patients with a CTO of a native coronary artery referred to the Amsterdam University Medical Center were prospectively enrolled between 2013 and 2018. CTO was defined as a vessel with Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 or 1 with an estimated duration of ≥ 3 months. Baseline CMR was performed prior to CTO-PCI in all patients and follow-up CMR was scheduled 3 months after successful percutaneous revascularization. Exclusion criteria were recent myocardial infarction, concomitant non-ischemic cardiomyopathy and non-diagnostic baseline or follow-up CMR cine images. Since myocardial strain values are dependent on acquisition method and post-processing software, 100 healthy volunteers without a history of cardiovascular disease underwent CMR to calculate normal values. The study was approved by the Institutional Review Board of the Amsterdam University Medical Center, location VUmc. All subjects provided written informed consent.
CMR image acquisition
CMR was performed in all subjects on a 1.5-T clinical scanner (Magnetom Avanto, Siemens Healthineers) using identical imaging parameters. Cine images were obtained using a balanced steady-state free-precession sequence in the 2-, 3- and 4-chamber long-axis views and multiple short-axis views covering the entire LV from base to apex. Typical imaging parameters were: echo time, 1.5 ms; repetition time, 3.2 ms; α, 60 to 80°; spatial resolution, 1.6 × 1.6 mm; slice thickness, 5 mm; gap, 5 mm; temporal resolution, 30 to 50 ms. For assessment of myocardial scar, late gadolinium enhancement (LGE) images were obtained 10 to 15 min after administration of 0.2 mmol/kg of a gadolinium-based contrast agent (Dotarem®, Guerbet) using a segmented inversion recovery gradient-echo pulse sequence. Slice positions of the LGE images were identical to those of the cine images. Typical imaging parameters were: echo time, 4.4 ms; repetition time, 9.6 ms; α, 25°; spatial resolution, 1.6 × 1.6 mm; slice thickness, 5 mm; inversion time, 250 to 350 ms.
CMR image analysis
CMR analysis was performed by a single observer (H.E.) blinded to all clinical data, angiographic data and timing of imaging. Conventional analysis of cine and LGE images was performed using QMass software (version 7.6, Medis Medical Imaging Systems). LV ejection fraction, mass and volumes were calculated from the short-axis cine images. Infarct size was determined from the LGE images using the full-width-at-half maximum method, followed by manual correction [22]. Analysis of myocardial strain was performed using CMR42 software (version 5.11, Circle Cardiovascular Imaging Inc.). Endo- and epicardial contours were manually drawn on the end-diastolic and end-systolic phases and reference points for segmentation were manually defined at the anterior- and inferior RV insertion points. In line with prior reports, longitudinal and circumferential strain were expressed as absolute, positive values and the term shortening instead of strain was used [23]. Global longitudinal shortening (GLS) was calculated from the 3 long-axis cine views. Global circumferential and radial shortening (GCS and GRS, respectively) were calculated from the short-axis cine views. For regional analysis, the LV was divided into 16 segments (true apex not included) according to the segmentation model of the American Heart Association (AHA) [24]. A single observer (S.S) assessed coronary dominance using the invasive coronary angiogram. Segments were subsequently assigned to coronary arteries by H.E. accounting for dominance. In a right dominant system, the standard segmentation model of the AHA was used [24]. In a co-dominant system, segments 4 and 10 were transferred from the RCA to the Cx. In a left dominant system, segments 3, 4, 9, 10 and 15 were all transferred from the RCA tot the Cx as the RCA does not supply the left ventricle. Remote myocardium was defined as the myocardial segments opposite to the vascular territory of the CTO. Remote myocardium was only included in analysis if the myocardial segments showed no hyperenhancement and the supplying coronary vessel had < 50% diameter stenosis. Circumferential and radial shortening (CS and RS, respectively) as well as percentage of hyperenhancement were calculated for each segment. Segments with > 50% hyperenhancement were considered non-viable. Cut-off values for global strain were determined by calculating the fifth percentile of GLS, GCS and GRS in the cohort of healthy volunteers. Given that strain values are independent of initial segment length, impaired regional strain was defined using the same cut-off parameters as used for global strain. The percentage of LV that was dysfunctional but viable was calculated for every patient by dividing the number of segments with ≤ 50% LGE and impaired strain by 16 and multiplying this value with 100. CMR strain analysis was repeated by the first observer (H.E) as well as a second observer (M.B.) in 50 randomly selected scans to assess intra- and interobserver variability, respectively.
Statistical analysis
Pearson’s and spearman’s correlations were used to quantify association between normally and non-normally distributed continuous data, respectively. Reproducibility of CMR strain measurements was evaluated by intraclass correlation coefficients (ICCs) and Bland–Altman analysis. ICCs for absolute agreement of single measures were estimated using a two-way random effects model. Paired samples T-tests were used to compare global strain and scar size before and after CTO-PCI. Association between extent of scar and global strain improvement was tested using analysis of covariance with global strain improvement as dependent variable and correcting for global strain at baseline. On a segmental level, means of strain before and after CTO-PCI were compared using a linear mixed model with a fixed effect for imaging timing and random effects for patient and segment nested within patient. All statistical tests were two tailed and a p value of < 0.05 was considered statistically significant. Statistical analysis was done with SPSS (version 26 for Windows, IBM).
Results
Characteristics of the study population
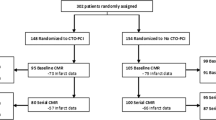
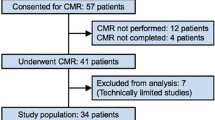
Figure 1 displays the flowchart of the study population. A total of 275 patients underwent CMR, CTO-PCI was attempted in 237 (86%) patients and paired CMR images were available in 158 (57%) patients. Eight patients were excluded post-hoc (reasons presented in Fig. 1) resulting in a final sample size of 150 patients. Table 1 lists the baseline characteristics of the study cohort and Table 2 lists angiographic characteristics. Median time between PCI and follow-up CMR was 101 [94 to 117] days.
CMR assessment of myocardial scar
Although only 30 (20%) patients had a documented prior myocardial infarction related to the CTO vessel, CMR revealed hyperenhancement in the vascular territory of the CTO in 112 (75%) patients.
Median scar size was 4.4 [1.0 to 10.4] % before revascularization and increased to 5.0 [2.1 to 14.4] % after (P < 0.001).
Reproducibility of strain measurements
Global and regional strain measurements had excellent intra- and interobserver reproducibility (Table 3). Bland–Altman plots demonstrated no significant bias and narrow limits of agreement (Supplementary Figs. 1 and 2).
Strain in healthy volunteers
In the cohort of healthy volunteers, GLS ranged from 13.3% to 21.7% with a mean 17.6 ± 1.8%, GCS from 13.6% to 23.3% with a mean of 18.5 ± 2.2% and GRS from 20.8% to 46.1% with a mean of 31.3 ± 6.1% (data presented in Supplementary Table 1). Impaired global and radial strain were defined as LS < 14.4%, CS < 15.0% and RS < 22.1%.
Global LV function
GLS, GCS and GRS correlated strongly with LV ejection fraction (r = 0.86; P < 0.001 for GLS, r = 0.93; P < 0.001 for GCS, r = 0.90; P < 0.001 for GRS) and inversely with the extent of myocardial scar (ρ = − 0.59; P < 0.001 for GLS, ρ = − 0.64; P < 0.001 for GCS, ρ = − 0.63; P < 0.001 for GRS) (Supplementary Fig. 3). GLS was impaired in 62 (41%) patients, GCS in 60 (40%) patients and GRS in 58 (39%) patients. A total of 51 (34%) patients had impaired LV strain, defined as the combination of impaired GLS, GCS and GRS. Table 4 presents global LV function before and after PCI. In the overall population, CTO-PCI resulted in a small but significant improvement in LV ejection fraction and a reduction in LV volumes. In contrast, GLS, GCS and GRS did not improve after CTO-PCI. The subgroup of patients with impaired strain at baseline however demonstrated an increase in GLS, GCS and GRS. Figure 2 depicts a case example of a patient with impaired LV function at baseline in whom strain markedly improved after CTO-PCI. Notably, GLS decreased in patients with preserved strain at baseline while no significant changes in GCS, GRS or LV ejection fraction were observed. Figure 3 shows the relationship between global strain improvement and the extent of viability (i.e. the percentage of the LV that was dysfunctional but viable). Strain improvement correlated significantly with the extent of viability, defined by either CS or RS. In addition, the improvements in strain after CTO-PCI were inversely related to the extent of scar, even after correcting for baseline strain (B = − 0.05; P = 0.008 for GLS, B = − 0.06; P = 0.016 for GCS, B = − 0.13; P = 0.017 for GRS).
Case example of a patient with a CTO of the RCA. The RCA is proximally occluded and the distal coronary segments are filled through collaterals (top row, left). CMR demonstrates subendocardial hyperenhancement (top row, right, arrows) and regional wall motion abnormalities (middle row, left and center) of the inferior wall. Global longitudinal, circumferential and radial strain are all impaired (middle row, right). Successful restoration of coronary patency (top, middle) results in marked improvements of global myocardial shortening (bottom row). CMR cardiac magnetic resonance imaging, CTO coronary chronic total occlusion, ED end-diastole, ES end-systole, GCS global circumferential shortening, GLS global longitudinal shortening, GRS global radial shortening, PCI percutaneous coronary intervention, RCA right coronary artery
Relationship between global strain improvement and extent of viability. Scatterplots demonstrating the relationship of improvement in GLS (top), GCS (bottom, left) and GRS (bottom, right) with the percentage of LV that was dysfunctional but viable. CS circumferential shortening, RS radial shortening; other abbreviations as in Fig. 2
Regional LV function
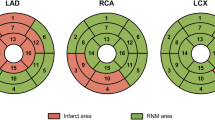
Segmental CS and RS correlated inversely with the percentage of scar (ρ = − 0.48; P < 0.001 for CS, ρ = − 0.47; P < 0.001 for RS, Supplementary Fig. 4). Table 5 presents regional strain before and after PCI-CTO stratified according to baseline strain and extent of hyperenhancement. Analysis of all segments in the vascular territory of the CTO revealed a significant increase in CS, but not in RS. In segments with impaired strain at baseline, both CS and RS increased after CTO-PCI. Conversely, CS and RS decreased in segments with preserved strain at baseline. Revascularization had no effect on strain in remote myocardium. Surprisingly, dysfunctional segments with > 50% hyperenhancement demonstrated an increase in CS and RS at follow-up. Although baseline strain was significantly lower in dysfunctional segments with > 50% hyperenhancement compared with dysfunctional segments with ≤ 50% hyperenhancement, the improvement in CS (1.3 [− 0.2 to 5.2] % vs 1.8 [− 0.4 to 4.2] %; P = 0.37) and RS (1.5 [− 0.1 to 6.6] % vs 2.8 [− 0.8 to 6.7] %; P = 0.97) was similar (Fig. 4).
Discussion
The present study is the largest thus far to investigate functional recovery after CTO-PCI using CMR, which is considered the gold standard for assessment of myocardial function. The main findings of our study can be summarized as follows: (1) CTO-PCI did not improve global longitudinal, circumferential and radial shortening; (2) CTO-PCI resulted in a small but significant improvement of global strain in patients with impaired LV function; (3) This improvement was primarily driven by strain recovery in dysfunctional myocardium in the vascular territory of the CTO.
Previous CMR studies investigating functional improvement after CTO-PCI have shown conflicting results. While some studies reported an increase in LV ejection fraction after CTO-PCI, others found no improvement in LV function [8,9,10,11,12,13,14,15,16,17,18,19]. Interestingly, mean LV ejection fraction prior to revascularization was well preserved (i.e. > 60%) in studies that failed to demonstrate improvement [8,9,10,11]. Conversely, mean baseline LV ejection fraction was impaired in most studies showing an increase in global function [12,13,14,15,16,17,18,19]. Logically, the presence of LV dysfunction appears to be a prerequisite for functional improvement. On a regional level, wall thickening in the vascular territory of the CTO also improves predominantly in segments with hypo- or akinesia at baseline [11]. In addition to LV dysfunction, the presence of viable myocardium has been described as a second prerequisite for functional recovery. Dysfunctional segments with limited or no scar have a high likelihood of improvement, whereas LV function is unlikely to recover in myocardium with transmural infarction [25]. It is therefore imperative to perform both cine and LGE imaging when using CMR to select patients who may benefit from revascularization in terms of functional improvement. Several studies in patients with a CTO reported that LGE imaging aids in predicting functional recovery after PCI [8, 15, 16]. However, Stuijfzand et al. and Fiocchi et al. found no relationship between improvement of regional wall thickening and extent of infarction [13, 17].
In the present study, functional recovery after CTO-PCI was investigated using myocardial strain indices rather than LV ejection fraction and analysis of wall thickening. LV ejection fraction is the cornerstone in the assessment of cardiac function, but is confounded by loading conditions and geometric factors such as LV wall thickness and cavity dimensions [26]. Although myocardial strain is also load dependent, it provides a more accurate assessment of LV function as strain parameters directly quantify myocardial fiber shortening [27]. Myocardial strain imaging is also an auspicious tool to quantify regional function, as conventional analysis of wall thickening suffers from relatively high observer variability due to systolic trabecular infolding [21]. Despite these advantages, myocardial strain imaging using CMR tissue tracking also has shortcomings. Various vendors offer software packages that allow to quantify strain from cine images. In the absence of an industry standard, these software packages use different algorithms with various agreement between the calculated results. In addition, acquisition parameters such as flip angle, spatial and temporal resolution but also the administration of contrast before obtaining the cine images may all influence the calculated strain values to an unknown degree. As a consequence, strain values obtained at one center are not transferable to another center if these factors are not taken into account. In order to usher CMR feature tracking into clinical practice, acquisition protocols and post-processing methods will have to be standardized and harmonized.
Similar to CMR tissue tracking, myocardial strain can also be assessed with echocardiography using a technique referred to as speckle tracking. Speckle tracking echocardiography is more widely available than CMR and has a higher spatial resolution. On the other hand, it has a lower signal-to-noise ratio and may be hampered by poor acoustic windows. Several studies have investigated the effects of CTO-PCI on LV function using speckle tracking echocardiography [28,29,30,31,32,33]. Although follow-up in these studies ranged from 1 month to 2 years, the majority documented significant improvements in echocardiography derived strain parameters after CTO-PCI. Contrary to these findings, no improvement in LV strain was observed in the overall cohort of present study. Small but statistically significant improvements in global LV function were however noted among patients with LV dysfunction prior to PCI. Conversely, patients with preserved LV function at baseline demonstrated a slight decrease in GLS. These patients have little to gain from PCI in terms of functional recovery, but may still experience loss of contracting cardiomyocytes due to periprocedural injury. This hypothesis is supported by the observation that infarct size increased after CTO-PCI. GCS and GRS remained unaltered in these patients, which is not surprising given that GLS has been documented as being more sensitive to subtle changes in contractility [27]. On a regional level, CS improved in the vascular territory of the CTO but remained unchanged in remote myocardium. Functional recovery after CTO-PCI is consequently a direct result of increased contractility in the vascular territory of the CTO. Functional improvement was most prominent in segments with dysfunction at baseline and was associated with the extent of scar. Surprisingly, strain in dysfunctional segments with > 50% hyperenhancement improved after CTO-PCI similar to the improvement observed in dysfunctional segments with ≤ 50% hyperenhancement.
In summary, LV function did not improve after CTO-PCI. Even in patients with LV dysfunction and myocardial viability at baseline, who are most likely to benefit from revascularization, the functional recovery that could be gained through CTO-PCI was only minor and not clinically relevant. It is therefore unlikely that CTO-PCI will reduce the incidence of heart failure or otherwise significantly improve prognosis for the individual patient through vast improvement of LV function. This should be taken into consideration when scheduling a patient for CTO-PCI, especially given the higher complication rate of CTO-PCI in comparison with regular PCI [4]. CTO-PCI may nevertheless be beneficial to selected patients with borderline LV function who might avoid implantable cardioverter defibrillator and/or cardiac resynchronization therapy through revascularization. In addition, CTO-PCI may halt negative LV remodeling and progression of heart failure, can profoundly reduce ischemic burden and has been shown to improve quality of life [6, 7, 12, 13]. Furthermore, previous studies have demonstrated that LV function may continue to improve up to 3 years after revascularization [10]. To date, randomized trials have consistently failed to show a reduction in hard clinical endpoints, although these trials lacked power and suffered from cross-over [5,6,7]. Future large-scale studies are therefore warranted to answer the question whether PCI improves cardiovascular outcome in patients with a CTO and these trials should include serial imaging for an extended period.
Study limitations
The follow-up period of 3 months after successful PCI was arbitrary and longer follow-up may have increased the observed improvement in LV function. Moreover, CTO-PCI was not performed in patients without evidence of viability on baseline CMR. Although such a diagnostic work-up is advocated by current guidelines, the exclusion of patients with predominantly transmural scar in the CTO territory may explain the modest relationship between strain improvement and myocardial scar observed in the present study [3]. Finally, the use of a different contrast agent may have improved the delineation between infarcted myocardium, blood pool and non-infarcted myocardium, and increased slice thickness may have resulted in more optimal cine images.
Conclusions
Percutaneous revascularization of CTOs does not improve global longitudinal, circumferential or radial shortening. Even in the subgroup of patients and segments most likely to benefit from revascularization (i.e. LV dysfunction at baseline and no or limited myocardial scar), CTO-PCI failed to show a clinically relevant improvement in myocardial contractility. It is therefore unlikely that CTO-PCI will favorably affect patient outcomes through recovery of LV function.
Data availability
Data will be made available upon reasonable request.
Code availability
Yes.
Abbreviations
- CAD:
-
Coronary artery disease
- CMR:
-
Cardiac magnetic resonance imaging
- CTO:
-
Coronary chronic total cocclusion
- (G)CS:
-
(Global) circumferential shortening
- GLS:
-
Global longitudinal shortening
- (G)RS:
-
(Global) radial shortening
- ICC:
-
Intraclass correlation coefficients
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- PCI:
-
Percutaneous coronary intervention
- TIMI:
-
Thrombolysis in myocardial infarction
References
Tsai TT, Stanislawski MA, Shunk KA, Armstrong EJ, Grunwald GK, Schob AH, Valle JA, Alfonso CE, Nallamothu BK, Ho PM, Rumsfeld JS, Brilakis ES (2017) Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the VA CART program. JACC Cardiovasc Interv 10(9):866–875. https://doi.org/10.1016/j.jcin.2017.02.044
Werner GS, Gitt AK, Zeymer U, Juenger C, Towae F, Wienbergen H, Senges J (2009) Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol 98(7):435–441. https://doi.org/10.1007/s00392-009-0013-5
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Group ESCSD (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40(2):87–165. https://doi.org/10.1093/eurheartj/ehy394
Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA (2015) Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 8(2):245–253. https://doi.org/10.1016/j.jcin.2014.08.014
Henriques JP, Hoebers LP, Ramunddal T, Laanmets P, Eriksen E, Bax M, Ioanes D, Suttorp MJ, Strauss BH, Barbato E, Nijveldt R, van Rossum AC, Marques KM, Elias J, van Dongen IM, Claessen BE, Tijssen JG, van der Schaaf RJ, Investigators ET (2016) Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol 68(15):1622–1632. https://doi.org/10.1016/j.jacc.2016.07.744
Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, di Mario C, Hovasse T, Teruel L, Bufe A, Lauer B, Bogaerts K, Goicolea J, Spratt JC, Gershlick AH, Galassi AR, Louvard Y (2018) A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 39(26):2484–2493. https://doi.org/10.1093/eurheartj/ehy220
Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, Kang H, Kang SJ, Kim YH, Lee CW, Park SW, Hur SH, Rha SW, Her SH, Choi SW, Lee BK, Lee NH, Lee JY, Cheong SS, Kim MH, Ahn YK, Lim SW, Lee SG, Hiremath S, Santoso T, Udayachalerm W, Cheng JJ, Cohen DJ, Muramatsu T, Tsuchikane E, Asakura Y, Park SJ (2019) Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation 139(14):1674–1683. https://doi.org/10.1161/CIRCULATIONAHA.118.031313
Baks T, van Geuns RJ, Duncker DJ, Cademartiri F, Mollet NR, Krestin GP, Serruys PW, de Feyter PJ (2006) Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol 47(4):721–725. https://doi.org/10.1016/j.jacc.2005.10.042
Cheng AS, Selvanayagam JB, Jerosch-Herold M, van Gaal WJ, Karamitsos TD, Neubauer S, Banning AP (2008) Percutaneous treatment of chronic total coronary occlusions improves regional hyperemic myocardial blood flow and contractility: insights from quantitative cardiovascular magnetic resonance imaging. JACC Cardiovasc Interv 1(1):44–53. https://doi.org/10.1016/j.jcin.2007.11.003
Kirschbaum SW, Baks T, van den Ent M, Sianos G, Krestin GP, Serruys PW, de Feyter PJ, van Geuns RJ (2008) Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol 101(2):179–185. https://doi.org/10.1016/j.amjcard.2007.07.060
Mashayekhi K, Nuhrenberg TG, Toma A, Gick M, Ferenc M, Hochholzer W, Comberg T, Rothe J, Valina CM, Loffelhardt N, Ayoub M, Zhao M, Bremicker J, Jander N, Minners J, Ruile P, Behnes M, Akin I, Schaufele T, Neumann FJ, Buttner HJ (2018) A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion: the REVASC trial. JACC Cardiovasc Interv 11(19):1982–1991. https://doi.org/10.1016/j.jcin.2018.05.041
Bucciarelli-Ducci C, Auger D, Di Mario C, Locca D, Petryka J, O’Hanlon R, Grasso A, Wright C, Symmonds K, Wage R, Asimacopoulos E, Del Furia F, Lyne JC, Gatehouse PD, Fox KM, Pennell DJ (2016) CMR guidance for recanalization of coronary chronic total occlusion. JACC Cardiovasc Imaging 9(5):547–556. https://doi.org/10.1016/j.jcmg.2015.10.025
Stuijfzand WJ, Biesbroek PS, Raijmakers PG, Driessen RS, Schumacher SP, van Diemen P, van den Berg J, Nijveldt R, Lammertsma AA, Walsh SJ, Hanratty CG, Spratt JC, van Rossum AC, Nap A, van Royen N, Knaapen P (2017) Effects of successful percutaneous coronary intervention of chronic total occlusions on myocardial perfusion and left ventricular function. EuroIntervention 13(3):345–354. https://doi.org/10.4244/EIJ-D-16-01014
Cardona M, Martin V, Prat-Gonzalez S, Ortiz JT, Perea RJ, de Caralt TM, Masotti M, Perez-Villa F, Sabate M (2016) Benefits of chronic total coronary occlusion percutaneous intervention in patients with heart failure and reduced ejection fraction: insights from a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 18(1):78. https://doi.org/10.1186/s12968-016-0287-5
Chadid P, Markovic S, Bernhardt P, Hombach V, Rottbauer W, Wohrle J (2015) Improvement of regional and global left ventricular function in magnetic resonance imaging after recanalization of true coronary chronic total occlusions. Cardiovasc Revasc Med 16(4):228–232. https://doi.org/10.1016/j.carrev.2015.03.003
Kirschbaum SW, Rossi A, Boersma E, Springeling T, van de Ent M, Krestin GP, Serruys PW, Duncker DJ, de Feyter PJ, van Geuns RJ (2012) Combining magnetic resonance viability variables better predicts improvement of myocardial function prior to percutaneous coronary intervention. Int J Cardiol 159(3):192–197. https://doi.org/10.1016/j.ijcard.2011.02.048
Fiocchi F, Sgura F, Di Girolamo A, Ligabue G, Ferraresi S, Rossi R, D’Amico R, Modena MG, Torricelli P (2009) Chronic total coronary occlusion in patients with intermediate viability: value of low-dose dobutamine and contrast-enhanced 3-T MRI in predicting functional recovery in patients undergoing percutaneous revascularisation with drug-eluting stent. Radiol Med 114(5):692–704. https://doi.org/10.1007/s11547-009-0426-2
Pujadas S, Martin V, Rossello X, Carreras F, Barros A, Leta R, Alomar X, Cinca J, Sabate M, Pons-Llado G (2013) Improvement of myocardial function and perfusion after successful percutaneous revascularization in patients with chronic total coronary occlusion. Int J Cardiol 169(2):147–152. https://doi.org/10.1016/j.ijcard.2013.08.017
Roifman I, Paul GA, Zia MI, Williams LK, Watkins S, Wijeysundera HC, Crean AM, Strauss BH, Dick AJ, Wright GA, Connelly KA (2013) The effect of percutaneous coronary intervention of chronically totally occluded coronary arteries on left ventricular global and regional systolic function. Can J Cardiol 29(11):1436–1442. https://doi.org/10.1016/j.cjca.2013.06.007
Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, Shah DJ, Jue J, White BE, Indorkar R, Shenoy C, Farzaneh-Far A (2018) Feature-tracking global longitudinal strain predicts death in a multicenter population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to ejection fraction and late gadolinium enhancement. JACC Cardiovasc Imaging 11(10):1419–1429. https://doi.org/10.1016/j.jcmg.2017.10.024
Everaars H, Robbers L, Gotte M, Croisille P, Hirsch A, Teunissen PFA, van de Ven PM, van Royen N, Zijlstra F, Piek JJ, van Rossum AC, Nijveldt R (2018) Strain analysis is superior to wall thickening in discriminating between infarcted myocardium with and without microvascular obstruction. Eur Radiol 28(12):5171–5181. https://doi.org/10.1007/s00330-018-5493-0
Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC (2011) Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 4(2):150–156
Flachskampf FA, Blankstein R, Grayburn PA, Kramer CM, Kwong RYK, Marwick TH, Nagel E, Sengupta PP, Zoghbi WA, Chandrashekhar Y (2019) Global longitudinal shortening: a positive step towards reducing confusion surrounding global longitudinal strain. JACC Cardiovasc Imaging 12(8 Pt 1):1566–1567. https://doi.org/10.1016/j.jcmg.2019.03.032
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542. https://doi.org/10.1161/hc0402.102975
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343(20):1445–1453. https://doi.org/10.1056/NEJM200011163432003
Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW (2017) Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 70(8):942–954. https://doi.org/10.1016/j.jacc.2017.06.046
Tops LF, Delgado V, Marsan NA, Bax JJ (2017) Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail 19(3):307–313. https://doi.org/10.1002/ejhf.694
Chimura M, Yamada S, Yasaka Y, Kawai H (2019) Improvement of left ventricular function assessment by global longitudinal strain after successful percutaneous coronary intervention for chronic total occlusion. PLoS ONE 14(6):e0217092. https://doi.org/10.1371/journal.pone.0217092
Sotomi Y, Okamura A, Iwakura K, Date M, Nagai H, Yamasaki T, Koyama Y, Inoue K, Sakata Y, Fujii K (2017) Impact of revascularization of coronary chronic total occlusion on left ventricular function and electrical stability: analysis by speckle tracking echocardiography and signal-averaged electrocardiogram. Int J Cardiovasc Imaging 33(6):815–823. https://doi.org/10.1007/s10554-017-1064-8
Kholeif AE, El Sharkawy E, Loutfi M, ElGowelly M (2019) Evaluation of the impact of percutaneous coronary intervention of chronic total occlusion on regional myocardial function using strain echocardiography. Egypt Heart J 71(1):8. https://doi.org/10.1186/s43044-019-0007-1
Erdogan E, Akkaya M, Bacaksiz A, Tasal A, Sonmez O, Elbey MA, Kul S, Vatankulu MA, Turfan M, Goktekin O (2013) Early assessment of percutaneous coronary interventions for chronic total occlusions analyzed by novel echocardiographic techniques. Clinics 68(10):1333–1337. https://doi.org/10.6061/clinics/2013(10)07
Wang P, Liu Y, Ren L (2019) Evaluation of left ventricular function after percutaneous recanalization of chronic coronary occlusions: the role of two-dimensional speckle tracking echocardiography. Herz 44(2):170–174. https://doi.org/10.1007/s00059-017-4663-1
Meng S, Qiu L, Wu J, Huang R, Wang H (2021) Two-year left ventricular systolic function of percutaneous coronary intervention vs optimal medical therapy for patients with single coronary chronic total occlusion. Echocardiography 38(2):368–373. https://doi.org/10.1111/echo.14976
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all individual participants in the study. The authors affirm that the human research participant provided informed consent for publication of the images in Fig. 2.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Everaars, H., Schumacher, S.P., Stuijfzand, W.J. et al. Functional recovery after percutaneous revascularization of coronary chronic total occlusions: insights from cardiac magnetic resonance tissue tracking. Int J Cardiovasc Imaging 37, 3057–3068 (2021). https://doi.org/10.1007/s10554-021-02355-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02355-4