Abstract
The objective of this study was to evaluate the influence of iterative reconstruction on coronary calcium scores (CCS) at different heart rates for four state-of-the-art CT systems. Within an anthropomorphic chest phantom, artificial coronary arteries were translated in a water-filled compartment. The arteries contained three different calcifications with low (38 mg), medium (80 mg) and high (157 mg) mass. Linear velocities were applied, corresponding to heart rates of 0, < 60, 60–75 and > 75 bpm. Data were acquired on four state-of-the-art CT systems (CT1–CT4) with routinely used CCS protocols. Filtered back projection (FBP) and three increasing levels of iterative reconstruction (L1–L3) were used for reconstruction. CCS were quantified as Agatston score and mass score. An iterative reconstruction susceptibility (IRS) index was used to assess susceptibility of Agatston score (IRSAS) and mass score (IRSMS) to iterative reconstruction. IRS values were compared between CT systems and between calcification masses. For each heart rate, differences in CCS of iterative reconstructed images were evaluated with CCS of FBP images as reference, and indicated as small (< 5%), medium (5–10%) or large (> 10%). Statistical analysis was performed with repeated measures ANOVA tests. While subtle differences were found for Agatston scores of low mass calcification, medium and high mass calcifications showed increased CCS up to 77% with increasing heart rates. IRSAS of CT1–T4 were 17, 41, 130 and 22% higher than IRSMS. Not only were IRS significantly different between all CT systems, but also between calcification masses. Up to a fourfold increase in IRS was found for the low mass calcification in comparison with the high mass calcification. With increasing iterative reconstruction strength, maximum decreases of 21 and 13% for Agatston and mass score were found. In total, 21 large differences between Agatston scores from FBP and iterative reconstruction were found, while only five large differences were found between FBP and iterative reconstruction mass scores. Iterative reconstruction results in reduced CCS. The effect of iterative reconstruction on CCS is more prominent with low-density calcifications, high heart rates and increasing iterative reconstruction strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iterative reconstruction for computed tomography (CT) is a powerful technique which can be used to reduce CT radiation dose [1,2,3,4,5,6,7,8,9,10]. The effect of iterative reconstruction on quantitative measurements in cardiovascular imaging has been subject of recent studies [11,12,13,14,15,16,17,18]. One of the most commonly used cardiovascular risk assessment tools in CT is coronary calcium scoring (CCS). CCS can be expressed as Agatston score, which can be used to estimate the risk of future cardiovascular events [19, 20]. In addition, the coronary calcium mass score has been introduced which is known to have a better stability under the influence of multiple parameters, including varying heart rates and high image noise [21]. According to the most recent guidelines from the American Heart Association (AHA), Agatston score risk categories can guide treatment strategies for asymptomatic adults [22]. Consequently, accurate determination of CCS is important to assign individual patients to correct risk categories.
Currently, CT acquisition is synchronized to the diastole resting period, where, with appropriate electrocardiograph (ECG) triggering, it is assumed that coronary arteries are imaged at relative rest. Nevertheless, the coronary arteries are known to move at velocities of up to 30 mm/s even during the rest phase, depending on both heart rate and coronary artery [23, 24]. These velocities strongly influence the stability of CCS, together with other factors including calcification mass and density or imaging factors such as image noise and use of iterative reconstruction [21, 24,25,26,27]. However, a systematic understanding of the mutual dependence of these factors and their influence on CCS is still lacking. We hypothesize that the influence of iterative reconstruction at different heart rates will differ between different calcification densities. Therefore, the aim of this study was to evaluate the influence of iterative reconstruction on CCS for various calcifications at different heart rates using four state-of-the-art CT systems.
Materials and methods
Within an anthropomorphic chest phantom (QRM-Chest, QRM, Moehrendorf, Germany), artificial coronary arteries were moved in a water-filled compartment. The artificial coronary arteries contained hydroxyapatite (HA) calcifications of low (38.5 ± 1.7 mg), medium (80.1 ± 3.3 mg) and high (157.1 ± 6.5 mg) mass. The cylindrical calcifications were 5.0 ± 0.1 mm in diameter, with a length of 10.0 ± 0.1 mm resulting in densities of 196 ± 3, 408 ± 2 and 800 ± 2 HA/cm3 for the low, medium and high mass calcification, respectively. The chest phantom contained artificial lungs, a spine insert and a shell of soft tissue equivalent material. To mimic an averaged sized patient, an extension ring of fat equivalent material was used to increase the size of the phantom to 400 × 300 mm [13].
The artificial coronary arteries were moved with a computer-controlled lever (QRM-Sim2D, QRM, Moehrendorf, Germany) at constant linear velocities of 0, 10, 20 and 30 mm/s in a horizontal plane perpendicular to the scan direction. The linear velocities used corresponded to heart rates of 0, < 60, 60–75 and > 75 bpm [24].
Raw data were acquired with routinely used CCS protocols on four CT systems (CT1–CT4): Discovery CT 750 HD (GE Healthcare, Waukesha, Wisconsin, USA), Brilliance iCT (Philips Healthcare, Best, The Netherlands), Somatom Definition Flash (Siemens Healthcare, Forchheim, Germany) and Aquilion One Vision (Toshiba Medical Systems, Otawara, Japan), respectively (Table 1).
Acquisition was performed five times for each heart rate, with a small deviation and rotation between the scans by manual repositioning of the phantom. The ECG trigger output of the computer-controlled lever was used as an ECG trigger input of the CT scanner to ensure data acquisition during linear movement of the artificial artery. Acquisition was performed at 60% of the movement, so that turning points of the artificial coronary artery were not included.
FBP and (hybrid) iterative reconstruction were used to reconstruct raw data on each CT system (Table 1) [28]. In addition, for each CT system, the lowest (L1), an intermediate (L2) and the highest (L3) available level of iterative reconstruction were used. CT-system specific noise levels in FBP images were assessed by calculating the standard deviation in the average Hounsfield value in a uniform water region (Table 1). CCS was quantified for each reconstruction as Agatston and mass score, with a default threshold of 130 Hounsfield Units (HU). CCS was assessed using the vendor specific software (Table 1), where one observer selected each calcification. A CT-specific calibration factor was used for the mass score, which was calculated as described previously [29]. For this, a dedicated, stationary, insert (CCI, QRM, Moehrendorf, Germany) for the QRM thorax phantom with a water cylinder was used. From the five measurements, mean CCS and standard deviation were calculated per calcification mass for each combination of CT system, heart rate and reconstruction.
A root mean square measure was used to quantify the susceptibility of CCS to iterative reconstruction for each CT system. The iterative reconstruction susceptibility (IRS) index was defined as:
in which x0 is the CCS at FBP, xi is the CCS at iterative reconstruction level i and N is the total number of reconstructions. Low IRS indicates a low susceptibility of CCS to differences in reconstruction type. IRS values were compared between CT systems and between calcification masses for each CT system. For each heart rate, differences in CCS of iterative reconstructed images with respect to the reference of CCS of FBP images were calculated and indicated as small (< 5%), medium (5–10%) or large (> 10%).
Statistical analysis was performed with SPSS for Windows (IBM SPSS Statistics, Armonk, New York, USA), version 22.0, where a p value smaller than 0.05 was used to determine significance. Normal distribution of the data was analyzed with the Shapiro–Wilk test. Significant differences between CCS of iterative reconstructed images and FBP images were assessed with repeated measures ANOVA tests, as well as differences in IRS (Fig. 1).
Results
Accuracy of CCS
The routinely used CCS protocols resulted in comparable noise levels in the reference CCS images for the used CT systems (Table 1). For the low mass calcification, only minor differences were shown between reference Agatston scoresfrom different CT systems (Fig. 2, top row). In comparison with reference Agatston scores at 0 bpm, the average differences with reference Agatston scores at > 75 bpm were − 18, − 9, 5 and − 13% for CT1–CT4, respectively. These differences in reference Agatston scores between 0 and > 75 bpm were more pronounced for medium and high mass calcifications. For medium mass scores increased with 38, 3, 11 and 37% for CT1–CT4, respectively, while the high mass calcification even showed increases in reference Agatston score of 77, 49, 23 and 63%.
On all CT systems, reference mass scores generally underestimated physical mass (Fig. 2, bottom row). For the low mass calcification, and averaged over all heart rates, mean reference mass scores were 28, 24, 20 and 26 for CT1–CT4, respectively. For the medium mass calcification mean reference scores were 73, 76, 62 and 83 and for the high mass 134, 166, 131 and 178. For CT1–CT4, differences in reference mass scores between 0 and > 75 bpm were − 25, − 14, − 8 and − 18%, respectively. Relative differences in reference CCS between 0 and > 75 bpm for the medium and high mass calcification were smaller for mass scores than for Agatston scores. Medium mass calcification differences were − 8, 10, − 2 and 1% for CT1–CT4, respectively, while for the high mass calcification differences of − 18, 13, 4 and 10% were found.
Influence of reconstruction method on CCS
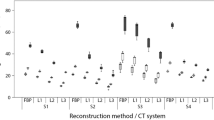
On average, Agatston scores were more susceptible to application of iterative reconstruction than mass scores, (Fig. 3). Averaged over all heart rates and calcifications, IRSAS for CT1–CT4 were 17, 41, 130 and 22% higher than IRSMS. Both IRSAS and IRSMS were found to differ significantly between CT systems (Agatston score: p = 0.001, mass score: p < 0.000). Also, for each individual CT system, significant differences in IRSAS and IRSMS between all calcification masses were found. The IRS was significantly larger for low mass calcifications compared to medium and high mass calcifications.
IRSAS (mean and SD) and IRSMS (bottom) for all CT systems at 0, < 60, 60–75 and > 75 bpm and for the low, medium and high mass calcification. Smaller IRS values indicate smaller susceptibility to differences in reconstruction type. p values below the bars indicate significant differences of IRS between calcification masses. If available, significantly different pairs of calcification masses are indicated with square brackets. A square bracket therefore points out that the influence of changes in reconstruction is significantly different for these two calcifications
For each reference Agatston score, differences between Agatston scores and their reference increased with increasing iterative reconstruction level (Table 2). These differences increased also with increasing heart rate and decreasing calcification mass. For almost all calcification masses and heart rates, significant differences were found between Agatston scores from different reconstructions. These differences resulted in large differences between iterative reconstruction and FBP Agatston scores for only the low mass calcifications. A maximum difference of − 21% was found for CT3 at > 75 bpm and L3, where the average Agatston decreased from 100.8 to 80.1. In total, 21 combinations of heart rate and calcification mass resulted in large differences in Agatston score between FBP and iterative reconstruction, while 40 medium differences were found. Large differences were not found for CT2, where only small and medium differences were given between Agatston scores from FBP and iterative reconstruction.
Similar trends were found for mass scores, although the differences were much smaller than for Agatston scores (Table 3). This resulted in a decrease in the number of large (n = 5) and medium (n = 26) differences. Despite these smaller differences, we still found large differences for low mass calcifications in combination with certain heart rates. The maximum difference of − 13% was associated with the low mass calcification on CT4 at > 75 bpm. While an increase in iterative reconstruction was typically associated with a decrease in mass score, an increase in mass score was shown for CT1 with the medium mass calcification. At > 75 bpm and L2, an increase in mass score of 11% was shown.
Discussion
In conclusion, the results of this study indicate that the effect of iterative reconstruction on CCS depends not only on CT system or calcification mass but also on heart rate. For repeated CCS measurements it was demonstrated that CT system, reconstruction type and heart rate should be similar.
The most clinically relevant finding of this study is that changes in reconstruction type as well as heart rate can cause changes in the measured amount of coronary calcium. The extent of deviation depends on the exact combination of CT system and iterative reconstruction type, as well as cardiac frequency. Our results, as stated in Tables 2 and 3, can be used to adjust CT measurements for changes in reconstruction, given a stable heart rate and CT system. Second, the impact of changes in iterative reconstruction strength on CCS was significantly different between all CT systems, as indicated by IRS analysis. In addition, the effect of iterative reconstruction on CCS was more prominent in calcification masses with lower density compared to higher density, these differences were also significant. For these lower density calcifications it was also found that the influence of iterative reconstruction is found to be comparable to the influence of heart rate. The main reason for this density-based iterative reconstruction influence is the threshold of 130 HU, which is used for CCS. With higher iterative reconstruction levels, more voxels inside the calcified region of interest fall below this threshold, because the calcification edge becomes less clear at increased heart rates, which in turn results in a decreased number of voxels above the threshold of 130 HU. The importance of adequate quantification of low density calcifications was emphasized by Criqui et al., who showed that coronary calcification density was inversely associated with coronary heart disease and cardiovascular events [30]. Third, differences in CCS between FBP and iterative reconstruction were shown to increase with increasing iterative reconstruction level, increasing heart rate and decreasing calcification mass. This resulted in significant differences in CCS between all used reconstructions per heart rate for most combinations of CT systems and calcification masses. As stated before, CCS are used for treatment strategies according to recent AHA guidelines [22]. Not only CCS at baseline is important, also progression of CCS is gaining more interest [31,32,33,34,35]. For this reason, accurate determination of CCS in follow-up studies is necessary. The use of the more stable mass score, in comparison with the Agatston, could be helpful in this context. However, due to a lack of accepted reference values, this score is not widely used yet. In light of the results found in the current study, we recommend that consecutive CCS measurements should therefore be performed with the use of the same CT systems, the same reconstruction and, preferably, at a similar heart rate. Future research is needed to develop specific acquisition protocol recommendations for CCS assessment.
Previous studies have also focused on the influence of heart rate on CCS [26, 27, 36,37,38]. However, these studies did not assess the influence of iterative reconstruction at different heart rates. Our study is, to the best of our knowledge, the first study to assess this influence. We have shown for low mass calcifications that the influence of heart rate is comparable with the influence of iterative reconstruction for CT1 (− 18 and − 19%), CT2 (− 9 and − 6%) and CT4 (− 13 and − 13%), respectively. In contrast with CT3, where the influence of heart rate (5%) was not comparable with the influence of IR (− 21%). The difference between CT3 and the other CT systems arises from the smaller temporal resolution of this system. In the current study, significant differences in CCS from different CT systems were shown. This finding is in agreement with a study from Willemink et al., who showed differences in median Agatston scores up to 43.9% between CT systems from four manufacturers [14]. However, this study assessed neither the influence of iterative reconstruction or heart rate, as it was performed on cadaveric human hearts. In addition, the underestimation of physical mass with the mass score was also demonstrated in previous studies, [39, 40]. Our study showed that the influence on CCS of changes in reconstruction is smaller for mass scores than for Agatston scores. IRSAS were up to 130% higher than IRSMS. This finding is in agreement with recent studies, which show smaller variability for mass scores [26, 38, 41, 42]. Many recent studies have shown that increasing levels of iterative reconstruction causes a reduction in CCS [12, 16, 40, 43,44,45,46,47,48]. This result was also established in our study.
Limitations of this study have to be taken into consideration. First, although the motion of the phantom used in this study was linear and perpendicular to the scan direction, and the in vivo motion of the coronary arteries exhibits a complex movement in 3D, we estimate that due to the fast rotation time and relatively short scan times, the motion in our phantom is a reliable first approximation of this complex motion. Second, for acquisition only sequential scans were performed. With new, high-pitch spiral mode scanning for coronary calcium appearing, this method could further improve temporal resolution over longer scan lengths. Third, only mild to severe coronary plaque burden was evaluated by the used calcium inserts. Therefore, our results are not directly applicable to very low to near-zero CCS. Fourth, the used calcium inserts had a relatively large size (diameter 5 mm, length 10 mm). However, quantification of the inserts still resulted in clinically relevant Agatston scores ranging from 100 to 400. Finally, acquisition protocols were not optimized for CT system comparisons.
In conclusion, the impact of iterative reconstruction on CCS is large for low mass calcifications. For medium and high mass calcifications, this impact is relatively small. The impact of iterative reconstruction on CCS further increases with increasing heart rate. In addition, IRS were significantly different between all CT systems and calcification masses. Therefore, this phantom study shows that for repeated CCS measurements, reconstruction type should be kept constant, especially for low mass calcifications. Also, heart rate and CT system should be kept constant for follow-up studies. These findings underscore that repeated CCS measurements should be acquired on the same CT system using the same reconstruction type and, ideally, at a similar heart rate. If the same setup is not available for repeated CCS measurements, the possible change in CCS as a result of a change in setup should be taken into account when interpreting the results.
Abbreviations
- AHA:
-
American Heart Association
- BPM:
-
Beats per minute
- CCS:
-
Coronary calcium score
- CT:
-
Computed tomography
- ECG:
-
Electrocardiograph
- FBP:
-
Filtered back projection
- HA:
-
Hydroxyapatite
- HU:
-
Hounsfield units
- IRS:
-
Iterative reconstruction susceptibility
- IRSAS :
-
Susceptibility of Agatston score on iterative reconstruction
- IRSMS :
-
Susceptibility of mass score on iterative reconstruction
References
Sato J, Akahane M, Inano S et al (2012) Effect of radiation dose and adaptive statistical iterative reconstruction on image quality of pulmonary computed tomography. Jpn J Radiol 30:146–153. https://doi.org/10.1007/s11604-011-0026-7
Moscariello A, Takx R, Schoepf U (2011) Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique—comparison with traditional filtered back projection. Eur Radiol 21:2130–2138. https://doi.org/10.1007/s00330-011-2164-9
Funama Y, Taguchi K, Utsunomiya D et al (2012) Combination of a low-tube-voltage technique with hybrid iterative reconstruction (iDose) algorithm at coronary computed tomographic angiography. J Comput Assist Tomogr 35:480–485. https://doi.org/10.1097/RCT.0b013e31821fee94
Gervaise A, Osemont B, Lecocq S et al (2012) CT image quality improvement using adaptive iterative dose reduction with wide-volume acquisition on 320-detector CT. Eur Radiol 22:295–301. https://doi.org/10.1007/s00330-011-2271-7
Korn A, Fenchel M, Bender B et al (2012) Iterative reconstruction in head CT: image quality of routine and low-dose protocols in comparison with standard filtered back-projection. Am J Neuroradiol 33:218–224. https://doi.org/10.3174/ajnr.A2749
Prakash P, Kalra MK, Digumarthy SR et al (2010) Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr 34:40–45. https://doi.org/10.1097/RCT.0b013e3181b26c67
Winklehner A, Karlo C, Puippe G et al (2011) Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol 21:2521–2526. https://doi.org/10.1007/s00330-011-2227-y
Leipsic J, LaBounty TM, Heilbron B et al (2010) Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. Am J Roentgenol 195:655–660. https://doi.org/10.2214/AJR.10.4288
Singh S, Kalra MKM, Gilman MMD et al (2011) Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology 259:565–573. https://doi.org/10.1148/radiol.11101450
Nelson RC, Feuerlein S, Boll DT (2011) New iterative reconstruction techniques for cardiovascular computed tomography: how do they work, and what are the advantages and disadvantages? J Cardiovasc Comput Tomogr 5:286–292. https://doi.org/10.1016/j.jcct.2011.07.001
Schindler A, Vliegenthart R, Schoepf UJ et al (2014) Iterative image reconstruction techniques for CT coronary artery calcium quantification: comparison with traditional filtered back projection in vitro and in vivo. Radiology 270:387–393. https://doi.org/10.1148/radiol.13130233
Van Osch JAC, Mouden M, Van Dalen JA et al (2014) Influence of iterative image reconstruction on CT-based calcium score measurements. Int J Cardiovasc Imaging 30:961–967. https://doi.org/10.1007/s10554-014-0409-9
Willemink MJ, Abramiuc B, den Harder AM et al (2015) Coronary calcium scores are systematically underestimated at a large chest size: a multivendor phantom study. J Cardiovasc Comput Tomogr 9:415–421. https://doi.org/10.1016/j.jcct.2015.03.010
Willemink MJ, Vliegenthart R, Takx R P, et al (2014) Coronary artery calcification scoring with state-of-the-art CT scanners from different vendors has substantial effect on risk classification. Radiology 273:695–702. https://doi.org/10.1148/radiol.14140066
Murazaki H, Funama Y, Hatemura M et al (2011) Quantitative evaluation of calcium (content) in the coronary artery using hybrid iterative reconstruction (iDose) algorithm on low-dose 64-detector CT: comparison of iDose and filtered back projection. Nihon Hoshasen Gijutsu Gakkai Zasshi 67:360–366. https://doi.org/10.6009/jjrt.67.360
Kurata A, Dharampal A, Dedic A et al (2013) Impact of iterative reconstruction on CT coronary calcium quantification. Eur Radiol 23:3246–3252. https://doi.org/10.1007/s00330-013-3022-8
Gebhard C, Fiechter M, Fuchs TA et al (2013) Coronary artery calcium scoring: Influence of adaptive statistical iterative reconstruction using 64-MDCT. Int J Cardiol 167:2932–2937. https://doi.org/10.1016/j.ijcard.2012.08.003
Szilveszter B, Elzomor H, Károlyi M et al (2016) The effect of iterative model reconstruction on coronary artery calcium quantification. Int J Cardiovasc Imaging 32:153–160. https://doi.org/10.1007/s10554-015-0740-9
Agatston AS, Janowitz WR, Hildner FJ et al (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832 pii]
Wilson PW, D’Agostino RB, Levy D et al (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. https://doi.org/10.1161/01.CIR.97.18.1837
Greuter MJW, Groen JM, Nicolai LJ et al (2009) A model for quantitative correction of coronary calcium scores on multidetector, dual source, and electron beam computed tomography for influences of linear motion, calcification density, and temporal resolution: a cardiac phantom study. Med Phys 36:5079–5088. https://doi.org/10.1118/1.3213536
Goff DC, Lloyd-Jones DM, Bennett G et al (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 129:49–76. https://doi.org/10.1161/01.cir.0000437741.48606.98
Achenbach S, Ropers D, Holle J et al (2000) In-plane coronary arterial motion velocity: measurement with electron-beam CT1. Radiology 216:457–463. https://doi.org/10.1148/radiology.216.2.r00au19457
Husmann L, Leschka S, Desbiolles L et al (2007) Coronary artery motion and cardiac phases: dependency on heart rate -- implications for CT image reconstruction. Radiology 245:567–576. https://doi.org/10.1148/radiol.2451061791
Brodoefel H, Burgstahler C, Tsiflikas I et al (2008) Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology 247:346–355. https://doi.org/10.1148/radiol.2472070906
Groen JM, Greuter MJW, Vliegenthart R et al (2008) Calcium scoring using 64-slice MDCT, dual source CT and EBT: a comparative phantom study. Int J Cardiovasc Imaging 24:547–556. https://doi.org/10.1007/s10554-007-9282-0
Tigges S, Arepalli CD, Tridandapani S et al (2012) A phantom study of the effect of heart rate, coronary artery displacement and vessel trajectory on coronary artery calcium score: potential for risk misclassification. J Cardiovasc Comput Tomogr 6:260–267. https://doi.org/10.1016/j.jcct.2012.01.005
Willemink MJ, De Jong PA, Leiner T et al (2013) Iterative reconstruction techniques for computed tomography part 1: technical principles. Eur Radiol 23:1623–1631. https://doi.org/10.1007/s00330-012-2765-y
McCollough CH, Ulzheimer S, Halliburton SS et al (2007) Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology 243:527–538. https://doi.org/10.1148/radiol.2432050808
Criqui MH, Denenberg JO, Ix JH et al (2014) Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 311:271–278. https://doi.org/10.1001/jama.2013.282535
Youssef G, Kalia N, Darabian S, Budoff MJ (2014) Coronary calcium: new insights, recent data, and clinical role. Curr Cardiol Rep 15:1–13. https://doi.org/10.1007/s11886-012-0325-3.Coronary
Budoff MJ, Hokanson JE, Nasir K et al (2010) Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 3:1229–1236. https://doi.org/10.1016/j.jcmg.2010.08.018
Raggi P, Shaw LJ, Berman DS, Callister TQ (2004) Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 43:1663–1669. https://doi.org/10.1016/j.jacc.2003.09.068
Raggi P, Cooil B, Shaw LJ et al (2003) Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol 92:827–829
Gopal A, Nasir K, Liu ST et al (2007) Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol 117:227–231. https://doi.org/10.1016/j.ijcard.2006.04.081
Funabashi N, Irie R, Namihira Y et al (2015) Influence of tube voltage and heart rate on the Agatston calcium score using an in vitro, novel ECG-gated dual energy reconstruction 320 slice CT technique. Int J Cardiol 180:218–220. https://doi.org/10.1016/j.ijcard.2014.11.164
Hong C, Bae KT, Pilgram TK, Zhu F (2003) Coronary artery calcium quantification at multi-detector row CT: influence of heart rate and measurement methods on interacquisition variability initial experience. Radiology 228:95–100. https://doi.org/10.1148/radiol.2281020685
Groen JM, Greuter MJ, Schmidt B et al (2007) The influence of heart rate, slice thickness, and calcification density on calcium scores using 64-slice multidetector computed tomography: a systematic phantom study. Invest Radiol 42:848–855. https://doi.org/10.1097/RLI.0b013e318154c549
Groen JM, Kofoed KF, Zacho M et al (2013) Calcium score of small coronary calcifications on multidetector computed tomography: results from a static phantom study. Eur J Radiol 82:e58–e63. https://doi.org/10.1016/j.ejrad.2012.09.018
van der Werf NR, Willemink MJ, Willems TP et al (2017) Influence of dose reduction and iterative reconstruction on CT calcium scores: a multi-manufacturer dynamic phantom study. Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-017-1061-y
Fuchs A, Groen JM, Arnold BA et al (2017) Assessment of coronary calcification using calibrated mass score with two different multidetector computed tomography scanners in the Copenhagen General Population Study. Eur J Radiol 88:21–25. https://doi.org/10.1016/j.ejrad.2016.12.033
Hoffmann U, Siebert U, Bull-Stewart A et al (2006) Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort–consequences for progression studies. Eur J Radiol 57:396–402. https://doi.org/10.1016/j.ejrad.2005.12.027
Rodrigues MA, Williams MC, Fitzgerald T et al (2016) Iterative reconstruction can permit the use of lower X-ray tube current in CT coronary artery calcium scoring. Br J Radiol. https://doi.org/10.1259/bjr.20150780
Matsuura N, Urashima M, Fukumoto W et al (2014) Radiation dose reduction at coronary artery calcium scoring by using a low tube current technique and hybrid iterative reconstruction. J Comput Assist Tomogr 0:1–6. https://doi.org/10.1097/RCT.0000000000000168
Li Q, Liu S, Myers KJ et al (2016) Impact of reconstruction algorithms and gender-associated anatomy on coronary calcium scoring with CT: an anthropomorphic phantom study. Acad Radiol 23:1470–1479. https://doi.org/10.1016/j.acra.2016.08.014
Oda S, Utsunomiya D, Nakaura T et al (2017) The influence of iterative reconstruction on coronary artery calcium scoring: phantom and clinical studies. Acad Radiol 24:295–301. https://doi.org/10.1016/j.acra.2016.11.003
Takahashi M, Kimura F, Umezawa T et al (2016) Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques in quantifying coronary calcium. J Cardiovasc Comput Tomogr 10:61–68. https://doi.org/10.1016/j.jcct.2015.07.012
Ode S, Kobayashi Y, Nozu Y et al (2016) The impact of iterative reconstruction on reducing the radiation dose for coronary calcium scoring: an investigation using pulsating calcified coronary phantom. J St Marian Univ 7:95–103. https://doi.org/10.17264/stmarieng.7.95
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Radiology Department of the University Medical Center Utrecht received institutional grants from Philips Healthcare. Martin Willemink received personal fees for lectures from Philips Healthcare. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Werf, N.R., Willemink, M.J., Willems, T.P. et al. Influence of iterative reconstruction on coronary calcium scores at multiple heart rates: a multivendor phantom study on state-of-the-art CT systems. Int J Cardiovasc Imaging 34, 947–957 (2018). https://doi.org/10.1007/s10554-017-1292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1292-y