Abstract
Purpose
Currently, various techniques are available to mark and selectively remove initially suspicious axillary lymph nodes (target lymph nodes, TLNs) in breast cancer patients receiving neoadjuvant chemotherapy (NACT). To date, limited data are available on whether the use of magnetic seeds (MS) is suitable for localizing TLNs. This study aimed to investigate the feasibility of MS in patients undergoing target lymph node biopsy (TLNB) or targeted axillary dissection (TAD) after NACT.
Methods
Prospective data from the ongoing multicentric AXSANA study were extracted from selected patients in whom the TLN had been marked with an MS before NACT and who were enrolled from June 2020 to June 2023. The endpoints of the analysis were the detection rate, the rate of lost markers, and the potential impairment on magnetic resonance imaging (MRI) assessment.
Results
In 187 patients from 27 study sites in seven countries, MS were placed into the TLN before NACT. In 151 of these, post-NACT surgery had been completed at the time of analysis. In 146 patients (96.0%), a TLN could successfully be detected. In three patients, the seed was removed but no lymphoid tissue was detected on histopathology. The rate of lost markers was 1.2% (2 out of 164 MS). In 15 out of 151 patients (9.9%), MRI assessment was reported to be compromised by MS placement.
Conclusion
MS show excellent applicability for TLNB/TAD when inserted before NACT with a high DR and a low rate of lost markers. Axillary MS can impair MRI assessment of the breast.
Trial registration number
NCT04373655 (date of registration May 4, 2020).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, ongoing clinical trials compare different surgical axillary staging procedures for breast cancer patients who receive neoadjuvant chemotherapy (NACT) and achieve conversion from initially clinically node-positive (cN+) to clinically node-negative disease (ycN0). Various international guidelines recommend either sentinel lymph node biopsy (SLNB) alone or targeted axillary dissection (TAD) as less radical alternatives than axillary lymph node dissection (ALND) [1], which represented the gold standard for decades. In TAD, the largest biopsy-confirmed axillary metastasis or most suspicious axillary lymph node, determined as the target lymph node (TLN), is marked before NACT and removed at post-NACT surgery along with the sentinel lymph node (SLN) [2]. This procedure reliably reduces the false negative rate (FNR) from 13% for SLNB alone to 5% [3]. To allow selective removal of the TLN after NACT, any pre-NACT marking technique must permit its reliable identification after NACT even in cases of complete clinical or radiological response in the axilla. Available techniques include visual identification of a TLN tattooed by carbon particles [4], image-guided preoperative wire insertion after pre-NACT placement of metallic clips [5, 6], or probe-based techniques using radioactive iodine seeds [7, 8], radiofrequency identification tags (RFIDs) [9], radar markers [10, 11], or magnetic seeds (MS) [12]. While the use of carbon particles is cheap and reliable, this technique may be associated with more extensive surgical dissection to visualize the TLN [4]. The placement of metallic markers and subsequent image-guided wire localization after NACT yields detection rates (DR) of only 70–78%. This technique requires high expertise for the secondary localization procedure and is less reproducible in clinical routine [5, 6]. Furthermore, it is associated with logistic challenges (localization on the same day as surgery is scheduled) and discomfort for the patient. In addition, the wire can dislocate between localization and surgery or when positioning and prepping the patient in the theater. In contrast, probe-guided techniques appear promising since the above-described additional localization procedures can be avoided and the workflow thus improved. As an example, the prospective multicenter RISAS trial tested the use of radioactive iodine seeds which were identified by a gamma probe and reported a DR of 94.1% [8]. Radioactive seeds are, however, not allowed in many countries due to radiation protection regulations [1].
In the localization of non-palpable breast lesions, the placement of MS with subsequent probe-guided tumor resection has shown non-inferiority compared to the most popular and widely used technique of stereotactic clip localization [13]. An MS is a 0.9 × 5 mm stainless steel seed and is inserted into the lymph node via an 18 Gauge needle usually under ultrasound guidance. It is transcutaneously detectable up to a penetration depth of 30 mm [12]. The necessary magnetometer probe generates an alternating magnetic field to transiently magnetize the iron-containing seed [14]. Although MS are considered magnetic resonance imaging (MRI) compatible, they cause extinction artifacts of 4–6 cm on breast MRI [12]. Since 2020, MS are approved to reside in soft tissue without a time limit, which allows their insertion into the TLN before NACT [15]. In the few available studies that assessed the feasibility of marking the TLN with an MS, high DRs up to 100% could be shown (Table 1). In most patients, however, the TLN was first marked with a metallic clip pre-NACT, and the MS was only placed after NACT to avoid wire localization [16,17,18,19,20,21,22,23,24,25,26,27]. This two-stage procedure does, however, not overcome the problem of ultrasound-guided clip detection in patients with a good response to NACT in the TLN.
The objectives of the current study were to determine the DR and the rate of lost MS after TLN marking before NACT in the largest so far investigated study cohort, as well as to investigate how often the assessment of an MRI to determine tumor response under NACT was limited by the MS in the axilla.
Patients and methods
AXSANA study
The ongoing AXSANA study (NCT04373655) is an international, multicenter, prospective registry study initiated by the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) that started recruitment in June 2020. Breast cancer patients with cN+ disease who receive at least four cycles of NACT are eligible. Axillary staging is performed according to institutional and national standards and includes ALND, SLNB, target lymph node biopsy (TLNB), or TAD (i.e., SLNB + TLNB). Co-primary endpoints are invasive disease-free survival, axillary recurrence rate, health-related quality of life, and arm morbidity for the different surgical staging procedures. The trial has high-quality standards with 100% of the datasets being monitored by breast surgeons. In patients scheduled for TLNB or TAD, any currently available techniques for marking the TLN are allowed [28].
A secondary endpoint of the trial is the performance of different marking techniques for the TLN.
Patients
The current analysis selectively included patients enrolled in the AXSANA study who had TLN marking with an MS (Magseed®, Endomagnetics Ltd, Cambridge, United Kingdom) before NACT and who had undergone axillary surgery by June 01, 2023. The number of labeled TLNs was not prescribed in the study protocol. At surgery, the TLN was identified using the SentiMag® (Endomagnetics Ltd, Cambridge, United Kingdom) handheld magnetometer probe. Clinical lymph node status before and after NACT was assessed according to institutional standards (palpation ± imaging of choice). Pathological complete response (pCR) was defined as the absence of invasive tumor cells in the breast and axilla [29]. Thus, the presence of isolated tumor cells in any axillary lymph nodes (ypN0i+) was defined as non-pCR.
Statistical analysis
For descriptive analysis, absolute frequencies and proportions were reported for categorial parameters and median values (minimum–maximum) for quantitative parameters. DR was defined as the proportion of patients with successful perioperative identification of at least one lymph node marked with an MS out of all included patients. The rate of lost markers was determined from the proportion of all unsuccessfully removed magnetic seeds out of all initially inserted MS. Statistical analysis was performed using SPSS® version 27 (IBM, Armonk, New York, USA).
Results
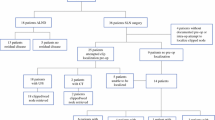
During the here studied time frame, 3859 patients from 27 countries and 286 study sites were included in the AXSANA study. In 2135 out of 3859 patients (55.3%), the TLN was labeled before NACT, and amongst these, MS was inserted in 187 cases (8.8%). All 151 out of these 187 patients (80.7%) in whom surgery had been performed and documented by June 01, 2023, were included in the current analysis (Fig. 1). These patients were recruited from 27 study sites in seven countries. Clinicopathologic characteristics are shown in Table 2.
In all patients, MS were placed before NACT and were the only markers used for TLN labeling. In 138 (91.4%) patients, only one MS was inserted, and in 13 (8.6%) patients, two were used, resulting in a total of 164 MS applied. The median time between TLN labeling and TLN removal was 168 (58–291) days. In 150 (99.3%) patients, TLN marking was performed under ultrasound guidance.
At least one MS-marked TLN could be detected in 146 out of 151 patients (DR 96.0%). The median number of histologically detected TLNs was 1 (0–6). In three out of six patients with unsuccessful TLN identification, the MS had been successfully removed, but no lymph node was histologically detectable in the surgical specimen. In one patient a probe-guided TLN detection had not been attempted since the patient underwent primary ALND; in two further patients, the MS had been detected by the probe in the ALND specimen but the TLN had not been evaluated separately by the pathologist.
A TAD was planned in 127 out of 151 patients (84.1%). The median number of TAD nodes removed was 2 (0–9). The SLN was labeled before surgery by a magnetic tracer in 51 patients (40.2%), technetium in 50 patients (39.4%), blue dye in four patients (3.1%), indocyanine green in 1 patient (0.8%), and dual marking in 21 patients (16.5%). The SLN could be detected in 116 out of 127 cases (91.3%) and corresponded to the TLN in 80 (69.0%) patients. ALND was performed in 74 patients (49.0%), in 55 patients (74.3%) within primary surgery, and in 19 patients (25.6%) as a secondary procedure.
Of the 164 initially applied seeds, 162 (98.8%) could successfully be removed after NACT. The rate of lost markers is thus 1.2%. In one patient, only one of the two initially applied MS was detectable; in the second case, it remained unclear whether the MS was still in situ because no intraoperative localization was performed. The seed was not mentioned in the pathology report. In both patients, no additional imaging was ordered to verify the in-situ retention of the seeds.
In 121 patients (80.1%), information on experience with the MS technique for TLN labeling was provided by the study site: in 43 cases (35.5%), at least 30 TLN labelings with MS had been performed at the sites to date. None of the patients with non-detection of TLN or lost marker had undergone surgery at such a high-experience site.
A breast MRI was performed in 31/151 patients after NACT but before surgery (20.5%). Radiologists described MRI artifacts caused by MS in 28/31 patients (90.3%). In 15 of these (48.4%) the radiologist described an impairment of the interpretation of the MRI due to artifacts caused by the axillary magnetic marker (Fig. 2) resulting in a rate of 9.9% (15/151 patients) for the entire study cohort.
Discussion
For patients who initially present with a clinically positive lymph node status and convert to ycN0 through NACT, the historic gold standard of ALND is increasingly abandoned in favor of less invasive techniques such as SLNB, TLNB, or the combination of both (TAD). Although long-term data on the oncologic outcome of these procedures are still scarce, most available studies, which still have insufficient statistical power, indicate no impairment of regional recurrences or disease-free survival [30,31,32]. Large prospective multicenter studies like AXSANA and MINIMAX (NCT04486495) will provide clarification.
TAD significantly lowers the FNR of SLNB alone and is currently the most popular procedure to stage the axilla in this patient cohort as could be shown in an international survey published recently by the EUBREAST network [33].
A recent meta-analysis compared the DR for different TLN marking techniques, such as iodine seeds (95.6%), metallic clips (91.7%), and carbon particles (97.1%). The identification of metallic clips, the currently most popular procedure, is associated with highly variable results (DR 70–98%) [34]. In the German SenTa study, a prospective trial using metallic clips for TLN labeling, the DR for the TLN was only 77.8% in a multicenter setting [5]. This suggests an unfavorable reproducibility of ultrasound-guided wire localization of axillary clips with a potential impact on experience, the type of clip, and whether or not intraoperative ultrasound was used.
High DRs have been described for the use of carbon particles. No comparative data are currently available from clinical trials as to whether this non-probe, visual-only detection of TLN results in more extensive dissection in the axilla or increased postoperative morbidity [4].
The DR for MS-marked TLNs reported here (96.0%) outperforms the recently published DR for radioactive seed-marked TLNs (94.1%) in the multicentric prospective RISAS study [8], which is also a probe-guided technique. In contrast to iodine seeds, MS are approved for long-term residence in the body and are not subject to legal regulations based on radioactivity.
The use of MS, as a wireless and non-radioactive procedure appears as an attractive alternative to avoid disadvantages of the established techniques. This procedure has shown excellent results for the localization of non-palpable breast lesions [35]. Since limited data are available on their use in TLNB, they were not included in the above-mentioned meta-analysis regarding different techniques for TLN marking [34]. The restricted approval of MS to remain in the body for up to 30 days was removed in 2020 [27]. Therefore, in most studies published to date, the number of patients with MS placed before NACT is very low (Table 1). Mainly, the TLN was marked before NACT using a metallic clip and the MS was placed in the TLN after NACT under ultrasound guidance [18, 19, 21, 23,24,25, 27]. Since initially clip-marked TLNs cannot be visualized by ultrasound after NACT in about 20% of patients [5], a targeted application of the seed after NACT under ultrasound guidance in these cases seems not feasible. In addition, MS appear to be significantly more likely to dislocate from the TLN into the perinodal tissue when inserted after NACT as compared to an application before NACT (27% versus 1.7%, p<0.001) [16]. To our knowledge, we present here the largest prospective cohort analyzing the performance of MS placed before neoadjuvant treatment. In this setting, any additional localization procedure before NACT can be avoided. Our data show a high DR of 96.0% for TLNs MS-marked before NACT and confirm preliminary data from smaller series [16, 17, 20, 22, 26].
The fact that all cases with unsuccessful localization procedures in our study occurred within the learning curve of the respective site suggests that even better results can be expected in experienced hands. Clip and carbon labeling studies of TLN have also described higher DR with increasing expertise of the center with each technique [5, 36]. Thus, for the SenTa study, a lower DR (69.3%) was described for sites with little experience (less than 20 cases) with clip marking of the TLN than for sites with at least 20 cases (DR 88.6%) [5]. Overall, however, the DR was significantly lower than that determined here for MS. Although two-thirds of the patients underwent surgery after MS labeling in this study at sites with limited experience (less than 30 MS applications), there was a high DR and only two lost seeds, so the procedure can be assumed to be straightforward to learn.
A potential disadvantage of magnetic markers compared to other techniques is an impairment of MRI assessment, and thus the monitoring of tumor response under NACT [35]. The current study is the first in which the impairment of breast MRI assessment by axillary-applied MS was investigated. Although the MS was located in the axilla and not in the breast, the assessability of tumor response in the breast after NACT was impaired in half of the patients in whom breast MRI was performed preoperatively. Therefore, in patients in whom breast MRI is to be performed to assess tumor response, a different marker should be considered to label the TLN.
Although the chance of unsuccessful seed removal is low (1.2% lost marker), patients should be informed about this potential risk. In particular, if MRI is indicated during follow-up.
In addition to a possible impairment of the assessability of a breast MRI, the high cost of the MS and the acquisition of the probe system, especially compared to metallic clips and carbon suspension, may limit their application in clinical practice [37].
A strength of the present analysis is the large number of patients enrolled in a prospective, multicenter study design. All datasets have been monitored by surgeons experienced in the field of breast surgery. A limitation is that no direct comparison with competing techniques is possible so far. This relates especially to innovative probe-guided procedures.
Conclusion
TLN marking using MS is a highly effective method that outperforms most competing techniques. As a wireless and non-radioactive procedure, this technique appears highly attractive in the setting of TAD. However, it should be noted with planned breast MRI that artifacts due to axillary MS may limit its assessability.
Data availability
The dataset generated and analyzed during the current study is available from the corresponding author upon reasonable request.
References
Banys-Paluchowski M, Gasparri ML, de Boniface J, Gentilini O, Stickeler E, Hartmann S, Thill M, Rubio IT, Di Micco R, Bonci EA, Niinikoski L, Kontos M, Karadeniz Cakmak G, Hauptmann M, Peintinger F, Pinto D, Matrai Z, Murawa D, Kadayaprath G, Dostalek L, Nina H, Krivorotko P, Classe JM, Schlichting E, Appelgren M, Paluchowski P, Solbach C, Blohmer JU, Kühn T, The Axsana Study Group (2021) Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA study. Cancers (Basel) 13(7):1565.https://doi.org/10.3390/cancers13071565
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF, Adrada BE, Shaitelman SF, Chavez-MacGregor M, Smith BD, Candelaria RP, Babiera GV, Dogan BE, Santiago L, Hunt KK, Kuerer HM (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078. https://doi.org/10.1200/jco.2015.64.0094
Swarnkar PK, Tayeh S, Michell MJ, Mokbel K (2021) The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) 13(7):1539. https://doi.org/10.3390/cancers13071539
Hartmann S, Stachs A, Kühn T, de Boniface J, Banys-Paluchowski M, Reimer T (2021) Targeted removal of axillary lymph nodes after carbon marking in patients with breast cancer treated with primary chemotherapy. Geburtshilfe Frauenheilkd 81(10):1121–1127. https://doi.org/10.1055/a-1471-4234
Kuemmel S, Heil J, Rueland A, Seiberling C, Harrach H, Schindowski D, Lubitz J, Hellerhoff K, Ankel C, Graßhoff ST, Deuschle P, Hanf V, Belke K, Dall P, Dorn J, Kaltenecker G, Kuehn T, Beckmann U, Potenberg J, Blohmer JU, Kostara A, Breit E, Holtschmidt J, Traut E, Reinisch M (2022) A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg 276(5):e553–e562. https://doi.org/10.1097/sla.0000000000004572
Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A (2018) Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol 44(9):1307–1311. https://doi.org/10.1016/j.ejso.2018.05.035
Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, van Tinteren H, Sonke GS, Rutgers EJ, Vrancken Peeters MJ (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261(2):378–382. https://doi.org/10.1097/sla.0000000000000558
Simons JM, van Nijnatten TJA, van der Pol CC, van Diest PJ, Jager A, van Klaveren D, Kam BLR, Lobbes MBI, de Boer M, Verhoef C, Sars PRA, Heijmans HJ, van Haaren ERM, Vles WJ, Contant CME, Menke-Pluijmers MBE, Smit LHM, Kelder W, Boskamp M, Koppert LB, Luiten EJT, Smidt ML (2022) Diagnostic accuracy of radioactive iodine seed placement in the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer. JAMA Surg 157(11):991–999. https://doi.org/10.1001/jamasurg.2022.3907
Malter W, Eichler C, Hanstein B, Mallmann P, Holtschmidt J (2020) First reported use of radiofrequency identification (RFID) technique for targeted excision of suspicious axillary lymph nodes in early stage breast cancer—evaluation of feasibility and review of current recommendations. In Vivo 34(3):1207–1213. https://doi.org/10.21873/invivo.11894
Baker JL, Haji F, Kusske AM, Fischer CP, Hoyt AC, Thompson CK, Lee MK, Attai D, DiNome ML (2022) SAVI SCOUT® localization of metastatic axillary lymph node prior to neoadjuvant chemotherapy for targeted axillary dissection: a pilot study. Breast Cancer Res Treat 191(1):107–114. https://doi.org/10.1007/s10549-021-06416-z
Gallagher KK, Iles K, Kuzmiak C, Louie R, McGuire KP, Ollila DW (2022) Prospective evaluation of radar-localized reflector-directed targeted axillary dissection in node-positive breast cancer patients after neoadjuvant systemic therapy. J Am Coll Surg 234(4):538–545. https://doi.org/10.1097/xcs.0000000000000098
Žatecký J, Kubala O, Jelínek P, Lerch M, Ihnát P, Peteja M, Brát R (2020) Magnetic marker localization in breast cancer surgery. Arch Med Sci 19(1):122–127. https://doi.org/10.5114/aoms.2020.93673
Banys-Paluchowski M, Kühn T, Masannat Y, Rubio I, de Boniface J, Ditsch N, Karadeniz Cakmak G, Karakatsanis A, Dave R, Hahn M, Potter S, Kothari A, Gentilini OD, Gulluoglu BM, Lux MP, Smidt M, Weber WP, Aktas Sezen B, Krawczyk N, Hartmann S, Di Micco R, Nietz S, Malherbe F, Cabioglu N, Canturk NZ, Gasparri ML, Murawa D, Harvey J (2023) Localization techniques for non-palpable breast lesions: current status, knowledge gaps, and rationale urope MELODY study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers (Basel) 15(4):1173. https://doi.org/10.3390/cancers15041173
Petrillo A, Di Giacomo R, Esposito E, Vallone P, Setola SV, Raso MM, Granata V, Barretta ML, Siani C, Rinaldo C, Donzelli I, Marone U, Melucci MT, Fucito A, Saponara R, Di Bonito M, Fusco R, Rinaldo M, Avino F (2022) Preoperative urope ation of nonpalpable breast lesions using magnetic markers in a tertiary cancer centre. Eur Radiol Exp 6(1):28. https://doi.org/10.1186/s41747-022-00280-2
Magseed® S. (2023) Magnetic marker approved for any soft tissue and long-term implantation across urope. https://www.sysmex-europe.com/company/news-and-events/news-listings/news-details/magseedr-magnetic-marker-approved-for-any-soft-tissue-and-long-term-implantation-across-europe.html. Accessed on 24 Apr 2023
Barry PA, Harborough K, Sinnett V, Heeney A, St John ER, Gagliardi T, Bhaludin BN, Downey K, Pope R, O’Connell RL, Tasoulis MK, MacNeill F, Rusby JE, Gui G, Micha A, Chen S, Krupa CKD (2023) Clinical utility of axillary nodal markers in breast cancer. Eur J Surg Oncol 49(4):709–715. https://doi.org/10.1016/j.ejso.2022.12.019
Martínez M, Jiménez S, Guzmán F, Fernández M, Arizaga E, Sanz C (2022) Evaluation of axillary lymph node marking with Magseed® before and after neoadjuvant systemic therapy in breast cancer patients: MAGNET Study. Breast J 2022:6111907. https://doi.org/10.1155/2022/6111907
Miller ME, Patil N, Li P, Freyvogel M, Greenwalt I, Rock L, Simpson A, Teresczuk M, Carlisle S, Peñuela M, Thompson CL, Shenk R, Dietz J (2021) Hospital system adoption of magnetic seeds for wireless breast and lymph node localization. Ann Surg Oncol 28(6):3223–3229. https://doi.org/10.1245/s10434-020-09311-x
Simons JM, Scoggins ME, Kuerer HM, Krishnamurthy S, Yang WT, Sahin AA, Shen Y, Lin H, Bedrosian I, Mittendorf EA, Thompson A, Lane DL, Hunt KK, Caudle AS (2021) Prospective registry trial assessing the use of magnetic seeds to locate clipped nodes after neoadjuvant chemotherapy for breast cancer patients. Ann Surg Oncol 28(8):4277–4283. https://doi.org/10.1245/s10434-020-09542-y
Reitsamer R, Peintinger F, Forsthuber E, Sir A (2021) The applicability of Magseed® for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy. Breast 57:113–117. https://doi.org/10.1016/j.breast.2021.03.008
Mariscal Martínez A, Vives Roselló I, Salazar Gómez A, Catanese A, Pérez Molina M, Solà Suarez M, Pascual Miguel I, Blay Aulina L, Ríos Gozálvez C, Julián Ibáñez JF, Rodríguez Martínez P, Martínez Román S, Margelí Vila M, Luna Tomás MA (2021) Advantages of preoperative localization and surgical resection of metastatic axillary lymph nodes using magnetic seeds after neoadjuvant chemotherapy in breast cancer. Surg Oncol 36:28–33. https://doi.org/10.1016/j.suronc.2020.11.013
McCamley C, Ruyssers N, To H, Tsao S, Keane H, Poliness C, Mehta K, Rose A, Baker C, Mann GB (2021) Multicentre evaluation of magnetic technology for localization of non-palpable breast lesions and targeted axillary nodes. ANZ J Surg 91(11):2411–2417. https://doi.org/10.1111/ans.17108
Žatecký J, Kubala O, Coufal O, Kepičová M, Faridová A, Rauš K, Lerch M, Peteja M, Brát R (2021) Magnetic seed (Magseed) localization in breast cancer surgery: a multicentre clinical trial. Breast Care (Basel) 16(4):383–388. https://doi.org/10.1159/000510380
Laws A, Dillon K, Kelly BN, Kantor O, Hughes KS, Gadd MA, Smith BL, Lamb LR, Specht M (2020) Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol 27(12):4819–4827. https://doi.org/10.1245/s10434-020-08902-y
Greenwood HI, Wong JM, Mukhtar RA, Alvarado MD, Price ER (2019) Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol 213(4):953–957. https://doi.org/10.2214/ajr.19.21378
García-Moreno JL, Benjumeda-Gonzalez AM, Amerigo-Góngora M, Landra-Dulanto PJ, Gonzalez-Corena Y, Gomez-Menchero J (2019) Targeted axillary dissection in breast cancer by marking lymph node metastasis with a magnetic seed before starting neoadjuvant treatment. J Surg Case Rep 2019(11):rjz344. https://doi.org/10.1093/jscr/rjz344
Rodriguez Gallo E, Vives I, Alonso I, Caparros FX, Ganau S, Bargallo X, Ubeda B, Perissinotti A, Tapias A, Vidal-Sicart S (2018) A novel dual technique combining radiotracer and magnetism for restaging axilla after neoadjuvant therapy in axillary node-positive breast cancer patients. J Nucl Med Radiat Ther 9(6):387. https://doi.org/10.4172/2155-9619.1000387
Axillary Surgery After NeoAdjuvant Treatment (2023) AXSANA. https://clinicaltrials.gov/ct2/show/NCT04373655. Accessed on 24 Apr 2023
Sahoo S, Lester SC (2009) Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med 133(4):633–642. https://doi.org/10.5858/133.4.633
Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, Vicini E, Morigi C, Corso G, Intra M, Canegallo F, Ratini S, Leonardi MC, La Rocca E, Bagnardi V, Montagna E, Colleoni M, Viale G, Bottiglieri L, Grana CM, Biasuz JV, Veronesi P, Galimberti V (2021) Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol 47(4):804–812. https://doi.org/10.1016/j.ejso.2020.10.014
Kuemmel S, Heil J, Bruzas S, Breit E, Schindowski D, Harrach H, Chiari O, Hellerhoff K, Bensmann E, Hanf V, Graßhoff ST, Deuschle P, Belke K, Polata S, Paepke S, Warm M, Meiler J, Schindlbeck C, Ruhwedel W, Beckmann U, Groh U, Dall P, Blohmer JU, Traut A, Reinisch M (2023) Safety of targeted axillary dissection after neoadjuvant therapy in patients with node-positive breast cancer. JAMA Surg. https://doi.org/10.1001/jamasurg.2023.1772
Montagna G, Mrdutt M, Botty A, Barrio AV, Sevilimedu V, Boughey JC, Hoskin TL, Rosenberger LH, Hwang ES, Ingham A, Papassotiropoulos B, Nguyen-Sträuli BD, Kurzeder C, Aybar DD, Vorburger D, Matlac DM, Ostapenko E, Riedel F, Fitzal F, Meani F, Fick F, Sagasser J, Heil J, Dedes KJ, Romics L, Banys-Paluchowski M, Del Rosario Cueva Perez M, Diaz MC, Heidinger M, Fehr MK, Reinisch M, Maggi N, Rocco N, Ditsch N, Gentilini OD, Paulinelli RR, Zarhi SS, Küemmel S, Bruzas S, Di Lascio S, Parissenti T, Güth U, Ovalle V, Tausch C, MorrowM, Kühn T, Weber WP (2022) Oncological outcomes following omission of axillary lymph node dissection in node-positive patients downstaging to node negative with neoadjuvant chemotherapy: the OPBC-04/EUBREAST-06/OMA study. Cancer Res 83(5_Supplement):GS4-02. https://doi.org/10.1158/1538-7445.SABCS22-GS4-02
Gasparri ML, de Boniface J, Poortmans P, Gentilini OD, Kaidar-Person O, Banys-Paluchowski M, Di Micco R, Niinikoski L, Murawa D, Bonci EA, Pasca A, Rubio IT, Karadeniz Cakmak G, Kontos M, Kühn T (2022) Axillary surgery after neoadjuvant therapy in initially node-positive breast cancer: international EUBREAST survey. Br J Surg 109(9):857–863. https://doi.org/10.1093/bjs/znac217
Song YX, Xu Z, Liang MX, Liu Z, Hou JC, Chen X, Xu D, Fei YJ, Tang JH (2022) Diagnostic accuracy of de-escalated surgical procedure in axilla for node-positive breast cancer patients treated with neoadjuvant systemic therapy: a systematic review and meta-analysis. Cancer Med 11(22):4085–4103. https://doi.org/10.1002/cam4.4769
Gera R, Tayeh S, Al-Reefy S, Mokbel K (2020) Evolving role of magseed in wireless localization of breast lesions: Systematic review and pooled analysis of 1,559 procedures. Anticancer Res 40(4):1809–1815. https://doi.org/10.21873/anticanres.14135
De Boniface J, Frisell J, Kühn T, Wiklander-Bråkenhielm I, Dembrower K, Nyman P, Zouzos A, Gerber B, Reimer T, Hartmann S (2022) False-negative rate in the extended prospective TATTOO trial evaluating targeted axillary dissection by carbon tattooing in clinically node-positive breast cancer patients receiving neoadjuvant systemic therapy. Breast Cancer Res Treat 193(3):589–595. https://doi.org/10.1007/s10549-022-06588-2
Murthy V, Young J, Tokumaru Y, Quinn M, Edge SB, Takabe K (2021) Options to determine pathological response of axillary lymph node metastasis after neoadjuvant chemotherapy in advanced breast cancer. Cancers (Basel) 13(16):4167. https://doi.org/10.3390/cancers13164167
Acknowledgements
The authors wish to thank Jana Shabbir, Bilge Sezen Aktas, Angelika Jursik, Markus Höing, and Marina Mangold for the coordination of the AXSANA study and all participating study sites for their valuable contribution to this trial. The AXSANA study is supported by the AGO‑B, the Claudia von Schilling Foundation for Breast Cancer Research, the Ehmann Foundation Savognin, AWOgyn, EndoMag, Merit Medical GmbH, Mammotome, German Breast Group Forschungs GmbH (GBG), and Nord-Ostdeutsche Gesellschaft für Gynäkologische Onkologie (NOGGO e.V.).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the AGO Breast Study Group (AGO-B), the Claudia von Schilling Foundation for Breast Cancer Research, the Ehmann Foundation Savognin, Arbeitsgemeinschaft für ästhetische, plastische und wiederherstellende Operationsverfahren in der Gynäkologie e.V. (AWOgyn), EndoMag, Merit Medical GmbH, and Mammotome.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study`s conception and design. Material preparation, data collection, and analysis were performed by SH, SF, and TK. The first draft of the manuscript was written by SH and TK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Steffi Hartmann, Elmar Stickeler, Jana De Boniface, Oreste Davide Gentilini, Michalis Kontos, Stephan Seitz, Gabriele Kaltenecker, Linda Holmstrand Zetterlund, and Sarah Fröhlich have no relevant financial or non-financial interests to disclose. Maggie Banys-Paluchowski receives fees for lectures and advisory activities from Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Medical, Syantra, resitu, Pierre Fabre, ExactSciences, study support from EndoMag, Mammotome, MeritMedical, Sirius Medical, Gilead, Hologic, and ExactSciences, and travel support from Eli Lilly, ExactSciences, Pierre Fabre, Pfizer, Daiichi Sankyo, and Roche. Fredrik Wärnberg has recent and ongoing research collaboration with Endomagnetics Ltd. Institutional, funding has been received by Sahlgrenska University Hospital from Endomag. Hans-Christian Kolberg has received fees from Pfizer, Seagen, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, TEVA, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, SurgVision, Onkowissen, Gilead, Daiichi Sankyo, Sysmex and MSD, travel support from Carl Zeiss meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo and Tesaro, and holds stock of Theraclion SA und Phaon Scientific GmbH. Thorsten Kühn received fees from Gilead, Pfizer, MSD, Lilly, Astra Zeneca, Exact Sciences, Endomag, Hologic, Merit Medical, and Sirius Medical.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Aachen, Germany (Date: April 28, 2020, Number: EK 013/20).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hartmann, S., Banys-Paluchowski, M., Stickeler, E. et al. Applicability of magnetic seeds for target lymph node biopsy after neoadjuvant chemotherapy in initially node-positive breast cancer patients: data from the AXSANA study. Breast Cancer Res Treat 202, 497–504 (2023). https://doi.org/10.1007/s10549-023-07100-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07100-0