Abstract
Purpose
Mammography screening has increased the detection of subcentimeter breast cancers. The prognosis for estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2)-negative T1a/bN0M0 breast cancers is excellent; however, the necessity of adjuvant endocrine therapy (ET) is uncertain.
Methods
We evaluated the effectiveness of adjuvant ET in patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer who underwent surgery from 2008 to 2012. Standard ET was administrated after surgery. The primary endpoint was the cumulative incidence of distant metastasis. All statistical tests were 2-sided.
Results
Adjuvant ET was administered to 3991 (83%) of the 4758 eligible patients (1202 T1a [25.3%] and 3556 T1b [74.7%], diseases). The median follow-up period was 9.2 years. The 9-year cumulative incidence of distant metastasis was 1.5% with ET and 2.6% without ET (adjusted subdistribution hazard ratio [sHR], 0.54; 95% CI, 0.32–0.93). In multivariate analysis, the independent risk factors for distant metastasis were no history of ET, mastectomy, high-grade, and lymphatic invasion. The 9-year overall survival was 97.0% and 94.4% with and without ET, respectively (adjusted HR, 0.57; 95% CI, 0.39–0.83). In addition, adjuvant ET reduced the incidence of ipsilateral and contralateral breast cancer (9-year rates; 1.1% vs. 6.9%; sHR, 0.17, and 1.9% vs. 5.2%; sHR, 0.33).
Conclusions
The prognosis was favorable in patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer. Furthermore, adjuvant ET reduced the incidence of distant metastasis with minimal absolute risk difference. These findings support considering the omission of adjuvant ET, especially for patients with low-grade and no lymphatic invasion disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer worldwide, affecting 2.3 million people annually and accounting for approximately a quarter of all female cancers [1, 2]. Approximately 70% of patients have cancers that are estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2)-negative [3]. Notably, screening mammography has increased the detection of small breast cancers, with an approximately 5-fold increase in the incidence of subcentimeter breast cancers (from 12.8 to 66.0 per 100,000 women) [4]. In Japan, approximately 19,000 patients with T1a/b breast cancer annually undergo surgery [5]. Patients with T1a/b breast cancer, almost N0, have an excellent prognosis, with a 10-year breast cancer mortality rate of 3.2–4% [4, 6] and a 10-year recurrent-free survival of over 90% [7]. These clinical outcomes were comparable to those of distant metastasis-free intervals (DMFIs) in particularly low-risk cases using multi-gene assays, such as the 9-year DMFI 96.8% in cases with a recurrence score of ≤ 10 using a 21-gene assay and the 8-year DMFI 97.0% in ultralow-risk cases using the 70-gene signature [8, 9].
Adjuvant endocrine therapy (ET) is a global standard treatment for ER-positive breast cancer, including T1a/b disease [10,11,12,13]. The national comprehensive cancer network (NCCN) guideline classifies adjuvant ET for T1aN0 breast cancer as category 2B, meaning that “based upon lower-level evidence, the NCCN consensus prescribes that the intervention is appropriate.” The national surgical adjuvant breast and bowel project (NSABP)-B21 trial for T1a/bN0 breast cancer demonstrated that the distant metastasis rates were 3.3% in the radiotherapy + placebo group and 1.6% in the radiotherapy + tamoxifen group (P = .28) [14]. However, a previous cohort study of T1 breast cancer reported that the 7-year cumulative incidence of metastasis was 2%, and ET was not a predictor [15]. Therefore, the necessity of adjuvant ET for T1a/bN0 breast cancer remains uncertain.
Several patients discontinue adjuvant ET despite the recommendations of clinical guidelines. For instance, 31–73% of patients discontinued ET at five years of treatment, with a mean adherence rate of 66.2% [16, 17]. Notably, side effects of ET, such as hot flashes, arthralgia, fatigue, mood disturbance, and vaginal bleeding, cause long-term patient distress [18]. Additionally, ET is reported to persistently deteriorate the quality of life (QOL) [19]. Therefore, the selection of patients for adjuvant ET is necessary.
Furthermore, the patient involvement committee of the Japan clinical oncology group (JCOG) Breast Cancer Study Group discussed treatment preferences with patients, and one of the requests from patient advocators was to omit adjuvant ET if the clinical effect was minimal. In Japan, 86.3% of patients with ER-positive and HER2-negative stage I breast cancer underwent adjuvant ET in 2018 [5]. Therefore, we conducted a multicenter cohort study to investigate the long-term prognosis and effect of adjuvant ET in ER-positive and HER2-negative T1a/bN0M0 breast cancer.
Materials and methods
Study population
We retrospectively collected the medical data of patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer who underwent radical surgery from January 2008–December 2012 in 42 JCOG Breast Cancer Study Group institutes. Tumor size and negative nodes were pathologically defined, and microinvasive disease (T1mi) was included in T1a. ER positivity was defined as ≥ 1% staining by immunohistochemistry [20]. We excluded patients who received neoadjuvant systemic therapy and those with confirmed pathogenic BRCA variants.
Statistical analysis
Baseline characteristics are described as numbers and percentages. Fisher’s exact test was used to compare the frequencies of the categorical variables. The primary endpoint was the cumulative incidence of distant metastasis. The secondary endpoints were distant disease-free survival (DDFS), disease-free survival (DFS), overall survival (OS), ipsilateral breast tumor recurrence (IBTR), and contralateral breast cancer. The effect of adjuvant ET was analyzed using Gray’s test model for cumulative incidence and log-rank test for survival. Predictive factors were assessed using the Fine-Gray model and Cox proportional hazards model. Hazard ratios (HRs) were adjusted by age, tumor size, nuclear grade, Ki-67 labeling index, lymphovascular invasion, and treatment. Statistical significance was defined as a two-sided P value < 0.05. All statistical analyses were performed using the SAS statistical software version 9.4 (SAS Institute).
The consensus of the investigators was that adjuvant ET would be the standard of care if the absolute difference in distant metastasis was 3% or greater, and no ET would be the standard of care if the absolute difference was 1% or less. If the difference was between 1 and 3%, a prospective trial would be planned or considered for shared decision-making.
Results
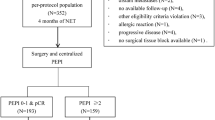
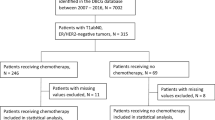
Among the 4914 patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer who underwent surgery from January 2008–December 2012, 4758 were eligible for inclusion in this study (supplementary figure S1). Patient characteristics are presented in Table 1. Half were over 55 years old, 1202 (25.3%) had T1a tumors, and 3450 (72.5%) had undergone breast-conserving surgery. Adjuvant ET was administered to 3991 (83.9%) patients, and luteinizing hormone-releasing hormone (LH-RH) analog to 577 (34.7%) of 1661 premenopausal and 7 (13.0%) of 54 perimenopausal women. Absence of ET history was related to T1a tumor, low Ki-67 labeling index, mastectomy, and absence of radiation therapy history. The reasons for not performing ET were institutional policy and physician’s decision 395 (51.5%), patient preference 200 (26.1%), and co-morbidity 19 (2.5%). The median duration of ET was five years (ranging from 0 to 15). The rate of sufficient adherence, defined as the completion of 4.5 years of ET, was 83.9%. The median follow-up period was 9.2 years.
Cumulative incidence of distant metastasis, DDFS, and OS
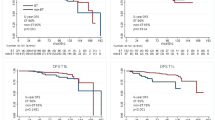
Overall, 84 patients developed distant recurrence. The 9-year cumulative incidence of distant metastasis was 1.5% (95% confidence interval [CI], 1.1–1.9%) in the ET group and 2.6% (95% CI, 1.5–4.1%) in the non-ET group (adjusted subdistribution hazard ratio [sHR], 0.54; 95% CI, 0.32–0.93, P = .027) (Fig. 1A). The 9-year DDFSs were 96.2% (95% CI, 95.5–96.8%) and 92.9% (95% CI, 90.5–94.6%) in the ET and non-ET groups, respectively (adjusted HR, 0.51; 95% CI, 0.36–0.71, P < .001) (Fig. 1B). The 9-year DFSs were 93.6% (95% CI, 92.6–94.4%) and 83.5% (95% CI, 80.3–86.2%) in the ET and non-ET groups, respectively (adjusted HR, 0.39; 95% CI, 0.31–0.50, P < .001) (Fig. 1C). The 9-year OSs were 97.0% (95% CI, 96.3–97.5%) and 94.4% (95% CI, 92.2–96.0%), respectively (adjusted HR, 0.57; 95% CI, 0.39–0.83, P = .004 (Fig. 1D).
Risk of distant metastasis and OS
In the multivariate analysis, risk factors for distant metastasis were high-grade, lymphatic invasion, mastectomy, and absence of ET history (Table 2). In contrast, the negative predictors of OS were older age, lymphatic invasion, and absence of ET history (supplementary table S1).
Distant metastasis according to risk factors
The 9-year cumulative incidences of distant metastasis were 1.5% (95% CI, 1.1–1.9%) and 4.1% (95% CI, 2.1–7.2%) in cases of nuclear grade 1–2 and 3 (Gray’s test P < .001), and 1.2% (95% CI, 0.9–1.6%) and 3.7% (95% CI, 2.2–5.9%) in lymphatic invasion negative and positive cases (Gray’s test P < .001), respectively (Fig. 2A, B). Distant metastatic events were stratified by nuclear grade and lymphatic invasion (Gray’s test P < .001, Fig. 2C). The cumulative incidence of distant metastasis and the 9-year rates with and without ET for each risk group are described in supplementary figure S2 and table S2. The effect of ET was expected, even in the low-risk group. No difference in the 9-year cumulative incidence of distant metastasis according to tumor size was observed (T1a: 1.7% and 2.7%; T1b: 1.4% and 2.5%, in the ET and non-ET groups, respectively) (supplementary figure S3).
IBTR and contralateral breast cancer
IBTR was assessed only in patients who underwent breast-conserving surgery and included local recurrence and new primary tumors. The 9-year IBTRs were 1.1% (95% CI, 0.7–1.6%) in the ET group and 6.9% (95% CI, 4.6–9.7%) in the non-ET group (sHR, 0.17; 95% CI, 0.11–0.28, P < .001) (Fig. 3A). The risk factors for IBTR were younger age, high grade, vascular invasion, absence of ET history, and absence of radiation therapy history (supplementary table S3). The 9-year incidences of contralateral breast cancer were 1.4% (95% CI, 1.0–1.8%) and 5.2% (95% CI, 3.6–7.2%), respectively (sHR, 0.33; 95% CI, 0.22–0.49, P < .001) (Fig. 3B).
Discussion
Among the 4758 patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer, distant metastasis occurred in 84 patients. Adjuvant ET significantly reduced the cumulative incidence of distant metastasis by an absolute difference of 1.1% at 9 years after surgery. Pathologically, the nuclear grade and lymphatic invasion were independent risk factors for distant recurrence.
The prognosis of T1a/bN0M0 breast cancer was previously investigated; however, most studies included all subtypes [6, 7, 15, 21]. These findings consistently indicate that hormone receptor-negative and HER2-positive status are poor prognostic factors. Furthermore, the NSABP-B21 trial did not require ER status for registration, and approximately 80% of the known cases were ER-positive (HER2 status was not reported) [14]. At a median follow-up of 8 years, adding tamoxifen to radiation therapy after lumpectomy reduced distant recurrence by 1.8% in absolute value, without statistical significance. Moreover, the integrated analysis for ER-positive T1a/bN0M0 breast cancer from three randomized studies (NSABP-B06, B-14, and B-20) revealed that adjuvant tamoxifen improved recurrence-free survival than surgery alone (HR, 0.55; 95% CI, 0.35–0.88) [22]. Furthermore, in a previous cohort study, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) reported that 5-year adjuvant tamoxifen and aromatase inhibitors reduced recurrence risk for ER-positive breast cancer, including T1 disease (tamoxifen vs. control: rate ratio [RR], 0.60 and aromatase inhibitors vs. tamoxifen: RR 0.76) [23,24,25]. In addition, a previous cohort study of ER-positive and HER2-negative T1N0M0 breast cancer from a Japanese institute reported that adjuvant ET did not improve DFS, DDFS, or OS for T1a/b disease, unlike T1c [26]. However, the median follow-up period in this study was 60 months, which was relatively short. Distant recurrence of ER-positive breast cancer persisted beyond 5 years [27]. Here, the cumulative incidence of distant metastasis was 0.7% and 1.1% at 5 years, and 1.5% and 2.6% at 9 years in the ET and non-ET groups, respectively. The recurrence prevention effect of ET is maintained after the treatment period, and the long-term outcomes should be highlighted for ER-positive breast cancer [24, 28].
Furthermore, because the absolute risk reduction of distant metastasis in all cohorts was minimal, stratification by risk factors might be helpful for treatment decisions. Our findings and previous reports indicated tumor grade and lymphatic invasion as poor prognostic factors [6, 7, 15, 29]. The 9-year cumulative incidence of distant metastasis increased by approximately 2% for each additional risk factor. Unfortunately, almost all patients with two risk factors, especially high nuclear grade, underwent ET, and it was impossible to estimate the effect of ET for each risk factor.
One of the disadvantages of ET is its side effects, which include the following: hot flashes, arthralgia, fatigue, mood disturbance, and vaginal bleeding [18]. These symptoms afflict patients and worsen their QOL in the long term during the ET period [19, 30, 31]. Furthermore, several integrative therapies and medications have been examined, including meditation, relaxation, yoga, massage, and music therapy for mood disturbance; acupuncture, yoga, and duloxetine for aromatase inhibitor-related arthralgia; and meditation, yoga, and exercise for QOL [32,33,34,35,36]. However, the effects of these supportive therapies have not been satisfactory, and approximately half of the patients discontinue ET at 5 years of treatment [16, 17, 37,38,39,40]. Recently, extended ET was offered even to patients with stage I breast cancer [41]. However, long-term ET raises concerns about further adverse events, including the incidence of endometrial cancer and pulmonary embolism with tamoxifen [42], osteoporosis, bone fracture, arthralgia, myalgia, and cardiovascular events with aromatase inhibitors [43, 44].
Interestingly, adjuvant ET also reduced IBTR (including local recurrence and second cancer) and contralateral breast cancer [14, 25, 45]. In addition, extended ET further reduced contralateral breast cancer [42, 46,47,48], equivalent to preventing the incidence of breast cancer [49]. Moreover, IBTR and contralateral breast cancer were more frequent than distant metastasis (6.9%, 5.2%, and 2.6% at 9 years after surgery). Therefore, clinicians should inform patients of the risk of developing a second breast cancer.
The patient involvement committee in JCOG was established in 2018. The committee requested the omission of excessive ET for breast cancer with an excellent prognosis because patients suffer from the side effects of long-term ET. This study determined the incidence of distant metastasis based on the risk of recurrence and the effectiveness of adjuvant ET but failed to conclude on a recommendation of adjuvant ET based on the predefined consensus. After several discussions in the committee, patients’ expected outcomes were diverse, with distant metastasis, survival, IBTR, and contralateral breast cancer all being important and prioritized by each individual. Therefore, the committee recommended that our findings should be used for shared decision-making for treatment selection rather than conducting a further prospective study.
Limitations and strengths
The strengths of this study include its large patient cohort with long-term follow-up and reliable data based on individual medical records. However, this study suffered from biases associated with retrospective studies, such as inherent selection bias. In addition, mastectomy was one of the potential risk factors for distant metastasis. Previous large, randomized trials have demonstrated equal survival for mastectomy and breast-conserving therapy in early breast cancer [50,51,52]. However, a population-based study reported mastectomy as a poor prognostic factor because of the lack of an additional value of radiation therapy [53]. This study did not identify the cause of this finding. Therefore, the analysis was designed to measure associations between patient variables, treatments, and outcomes.
Conclusions
The prognosis for patients with ER-positive and HER2-negative T1a/bN0M0 breast cancer was as favorable as that for genetically low-risk breast cancer and was stratified by clinical risks. Adjuvant ET improved the incidence of distant metastasis and overall survival with a small absolute risk difference. These findings support shared decision-making that considers the implementation of adjuvant ET by weighing the prevention of recurrence and second cancer, side effect, and QOL.
Data Availability
The data underlying this article cannot be directly shared due to the privacy of individuals that participated in the study. Access to these data can be provided to researchers under certain circumstances, pending approval by the Institutional Review Board of Nagoya City University and agreement with the JCOG Breast Cancer Study Group.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P (2020) Incidence Trends of breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US from 2010 to 2016. JAMA Netw Open 3:e2013226. https://doi.org/10.1001/jamanetworkopen.2020.13226
Welch HG, Prorok PC, O’Malley AJ, Kramer BS (2016) Breast-Cancer tumor size, overdiagnosis, and Mammography Screening effectiveness. N Engl J Med 375:1438–1447. https://doi.org/10.1056/NEJMoa1600249
Japanese Breast Cancer Society. National Breast Cancer Patient Registry Survey (2018) https://memberpage.jbcs.gr.jp/uploads/ckfinder/files/nenjitouroku/2018kakutei.pdf. Accessed October 17, 2022
Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V (2007) Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol 25:4952–4960. https://doi.org/10.1200/jco.2006.08.0499
Hanrahan EO, Valero V, Gonzalez-Angulo AM, Hortobagyi GN (2006) Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a,bN0M0): a review of the literature. J Clin Oncol 24:2113–2122. https://doi.org/10.1200/jco.2005.02.8035
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-Gene expression assay in breast Cancer. N Engl J Med 379:111–121. https://doi.org/10.1056/NEJMoa1804710
Lopes Cardozo JMN, Drukker CA, Rutgers EJT, Schmidt MK, Glas AM, Witteveen A, Cardoso F, Piccart M, Esserman LJ, Poncet C, van ‘t Veer LJ (2022) Outcome of patients with an Ultralow-Risk 70-Gene signature in the MINDACT Trial. J Clin Oncol 40:1335–1345. https://doi.org/10.1200/jco.21.02019
National Comprehensive Cancer Network. CCN Clinical Practice Guidelines in Oncology. Breast Cancer (2022) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 26, 2022
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194–1220. https://doi.org/10.1093/annonc/mdz173
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP, Gnant M (2021) Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32:1216–1235. https://doi.org/10.1016/j.annonc.2021.06.023
Shien T, Iwata H (2020) Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol 50:225–229. https://doi.org/10.1093/jjco/hyz213
Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, Margolese RG, Nesbitt L, Paik S, Pisansky TM, Wolmark N (2002) Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 20:4141–4149. https://doi.org/10.1200/jco.2002.11.101
Houvenaeghel G, Goncalves A, Classe JM, Garbay JR, Giard S, Charytensky H, Cohen M, Belichard C, Faure C, Uzan S, Hudry D, Azuar P, Villet R, Gimbergues P, Tunon de Lara C, Martino M, Lambaudie E, Coutant C, Dravet F, Chauvet MP, Chéreau Ewald E, Penault-Llorca F, Esterni B (2014) Characteristics and clinical outcome of T1 breast cancer: a multicenter retrospective cohort study. Ann Oncol 25:623–628. https://doi.org/10.1093/annonc/mdt532
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134:459–478. https://doi.org/10.1007/s10549-012-2114-5
Yussof I, Mohd Tahir NA, Hatah E, Mohamed Shah N (2022) Factors influencing five-year adherence to adjuvant endocrine therapy in breast cancer patients: a systematic review. Breast 62:22–35. https://doi.org/10.1016/j.breast.2022.01.012
Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer 98:1802–1810. https://doi.org/10.1002/cncr.11745
Ferreira AR, Di Meglio A, Pistilli B, Gbenou AS, El-Mouhebb M, Dauchy S, Charles C, Joly F, Everhard S, Lambertini M, Coutant C, Cottu P, Lerebours F, Petit T, Dalenc F, Rouanet P, Arnaud A, Martin A, Berille J, Ganz PA, Partridge AH, Delaloge S, Michiels S, Andre F, Vaz-Luis I (2019) Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol 30:1784–1795. https://doi.org/10.1093/annonc/mdz298
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 38:1346–1366. https://doi.org/10.1200/jco.19.02309
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A (2017) Management of small T1a/b breast cancer by tumor subtype. Breast Cancer Res Treat 163:111–118. https://doi.org/10.1007/s10549-017-4168-x
Fisher B, Dignam J, Tan-Chiu E, Anderson S, Fisher ER, Wittliff JL, Wolmark N (2001) Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst 93:112–120. https://doi.org/10.1093/jnci/93.2.112
(EBCTCG) EBCTCG (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–1467
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/s0140-6736(11)60993-8
(EBCTCG) EBCTCG (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/s0140-6736(15)61074-1
Adachi Y, Oze I, Sawaki M, Hattori M, Yoshimura A, Kotani H, Kataoka A, Sugino K, Horisawa N, Ozaki Y, Endo Y, Nozawa K, Takatsuka D, Iwata H (2021) Impact of adjuvant endocrine therapy on prognosis in small hormone receptor-positive, HER2-negative early breast cancer. Breast Cancer 28:1087–1095. https://doi.org/10.1007/s12282-021-01245-w
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF (2017) 20-Year risks of breast-Cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836–1846. https://doi.org/10.1056/NEJMoa1701830
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Alencar VHM, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou M-F, Inbar M, Khaled H, Kielanowska J, Kwan W-H, Mathew BS, Mittra I, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrútia G, Valentini M, Wang Y, Peto R (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816. https://doi.org/10.1016/S0140-6736(12)61963-1
Tryfonidis K, Zardavas D, Cardoso F (2014) Small breast cancers: when and how to treat. Cancer Treat Rev 40:1129–1136. https://doi.org/10.1016/j.ctrv.2014.09.004
Ganz PA, Petersen L, Bower JE, Crespi CM (2016) Impact of adjuvant endocrine therapy on quality of life and symptoms: Observational Data over 12 months from the mind-body study. J Clin Oncol 34:816–824. https://doi.org/10.1200/jco.2015.64.3866
Hu X, Walker MS, Stepanski E, Kaplan CM, Martin MY, Vidal GA, Schwartzberg LS, Graetz I (2022) Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with Early-Stage, hormone receptor-positive breast Cancer. JAMA Netw Open 5:e2225485. https://doi.org/10.1001/jamanetworkopen.2022.25485
Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, Fouladbakhsh JM, Gil B, Hershman DL, Mansfield S, Mussallem DM, Mustian KM, Price E, Rafte S, Cohen L (2018) Integrative therapies during and after breast Cancer Treatment: ASCO endorsement of the SIO Clinical Practice Guideline. J Clin Oncol 36:2647–2655. https://doi.org/10.1200/jco.2018.79.2721
Mao JJ, Ismaila N, Bao T, Barton D, Ben-Arye E, Garland EL, Greenlee H, Leblanc T, Lee RT, Lopez AM, Loprinzi C, Lyman GH, MacLeod J, Master VA, Ramchandran K, Wagner LI, Walker EM, Bruner DW, Witt CM, Bruera E (2022) Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline. J Clin Oncol 40:3998–4024. https://doi.org/10.1200/jco.22.01357
Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK, Kengla AT, Melnik MK, Jorgensen CW, Kreisle WH, Minasian LM, Fisch MJ, Henry NL, Crew KD (2018) Effect of acupuncture vs Sham acupuncture or Waitlist Control on Joint Pain related to aromatase inhibitors among women with early-stage breast Cancer: a Randomized Clinical Trial. JAMA 320:167–176. https://doi.org/10.1001/jama.2018.8907
Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, Sharer CW, Burton GV, Kuzma CS, Moseley A, Lew DL, Fisch MJ, Moinpour CM, Hershman DL, Wade JL 3rd (2018) Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in early-stage breast Cancer: SWOG S1202. J Clin Oncol 36:326–332. https://doi.org/10.1200/jco.2017.74.6651
Baglia ML, Lin IH, Cartmel B, Sanft T, Ligibel J, Hershman DL, Harrigan M, Ferrucci LM, Li FY, Irwin ML (2019) Endocrine-related quality of life in a randomized trial of exercise on aromatase inhibitor-induced arthralgias in breast cancer survivors. Cancer 125:2262–2271. https://doi.org/10.1002/cncr.32051
Hagen KB, Aas T, Kvaløy JT, Søiland H, Lind R (2019) Adherence to adjuvant endocrine therapy in postmenopausal breast cancer patients: a 5-year prospective study. Breast 44:52–58. https://doi.org/10.1016/j.breast.2019.01.003
Lambert LK, Balneaves LG, Howard AF, Gotay CC (2018) Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat 167:615–633. https://doi.org/10.1007/s10549-017-4561-5
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28:4120–4128. https://doi.org/10.1200/jco.2009.25.9655
Lemij AA, de Glas NA, Derks MGM, Bastiaannet E, Merkus JWS, Lans TE, van der Pol CC, van Dalen T, Vulink AJE, van Gerven L, Guicherit OR, Linthorst-Niers EMH, van den Bos F, Kroep JR, Liefers GJ, Portielje JEA (2022) Discontinuation of adjuvant endocrine therapy and impact on quality of life and functional status in older patients with breast cancer. Breast Cancer Res Treat 193:567–577. https://doi.org/10.1007/s10549-022-06583-7
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ (2019) Adjuvant endocrine therapy for women with hormone receptor-positive breast Cancer: ASCO Clinical Practice Guideline focused Update. J Clin Oncol 37:423–438. https://doi.org/10.1200/jco.18.01160
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrútia G, Valentini M, Wang Y, Peto R (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816. https://doi.org/10.1016/s0140-6736(12)61963-1
Goldvaser H, Barnes TA, Šeruga B, Cescon DW, Ocaña A, Ribnikar D, Amir E (2018) Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast Cancer: a systematic review and Meta-analysis. J Natl Cancer Inst 110. https://doi.org/10.1093/jnci/djx141
Zhao F, Ren D, Shen G, Ahmad R, Dong L, Du F, Zhao J (2020) Toxicity of extended adjuvant endocrine with aromatase inhibitors in patients with postmenopausal breast cancer: a systemtic review and Meta-analysis. Crit Rev Oncol Hematol 156:103114. https://doi.org/10.1016/j.critrevonc.2020.103114
Early Breast Cancer Trialists’, Collaborative G, Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707–1716. https://doi.org/10.1016/S0140-6736(11)61629-2
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375:209–219. https://doi.org/10.1056/NEJMoa1604700
Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, Putter H, van den Bosch J, Maartense E, van Leeuwen-Stok AE, Liefers GJ, Nortier JWR, Rutgers EJT, van de Velde CJH (2018) Optimal duration of extended adjuvant endocrine therapy for early breast Cancer; results of the IDEAL trial (BOOG 2006-05). J Natl Cancer Inst 110. https://doi.org/10.1093/jnci/djx134
Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer CE Jr, Rastogi P, Fehrenbacher L, Graham ML, Chia SK, Brufsky AM, Walshe JM, Soori GS, Dakhil SR, Seay TE, Wade JL 3rd, McCarron EC, Paik S, Swain SM, Wickerham DL, Wolmark N (2019) Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:88–99. https://doi.org/10.1016/s1470-2045(18)30621-1
Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, Floyd JD, Garber JE, Hofstatter EW, Khan SA, Katapodi MC, Pruthi S, Raab R, Runowicz CD, Somerfield MR (2019) Use of endocrine therapy for breast Cancer risk reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol 37:3152–3165. https://doi.org/10.1200/jco.19.01472
Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, Baaijens MH, Bogaerts J, Bartelink H (2012) Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 13:412–419. https://doi.org/10.1016/s1470-2045(12)70042-6
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232. https://doi.org/10.1056/NEJMoa020989
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241. https://doi.org/10.1056/NEJMoa022152
Lagendijk M, van Maaren MC, Saadatmand S, Strobbe LJA, Poortmans PMP, Koppert LB, Tilanus-Linthorst MMA, Siesling S (2018) Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer 142:165–175. https://doi.org/10.1002/ijc.31034
Acknowledgements
Participating institutions in this survey (from north to south) were: Hokkaido Cancer Center, Iwate Medical University Hospital, Tohoku University Hospital, Akita University Hospital, Fukushima Medical University Hospital, University of Tsukuba Hospital, Ibaraki Prefectural Central Hospital, Jichi Medical University Hospital, Gunma Prefectural Cancer Center, Saitama Cancer Center, Saitama Medical Center, National Cancer Center Hospital East, Chiba Cancer Center, National Cancer Center Hospital, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Center Hospital of the National Center for Global Health and Medicine, National Hospital Organization Tokyo Medical Center, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Toranomon Hospital, St. Luke’s International Hospital, Tokai University Hospital, Kanagawa Cancer Center, Kitasato University Hospital, Niigata Cancer Center Hospital, Shizuoka General Hospital, Hamamatsu University Hospital, Shizuoka Cancer Center Hospital, Aichi Cancer Center Hospital, Nagoya City University Hospital, National Hospital Organization Osaka National Hospital, Kansai Medical University Hospital, Yao Municipal Hospital, Okayama University Hospital, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Hiroshima University Hospital, National Hospital Organization Fukuyama Medical Center, Hiroshima City North Medical Center Asa Citizens Hospital, National Hospital Organization Shikoku Cancer Center, National Hospital Organization Kyushu Cancer Center, Kitakyushu Municipal Medical Center, National Hospital Organization Nagasaki Medical Center, and Social medical corporation Hakuaikai Sagara Hospital.
Funding
This study was partly supported by the National Cancer Center Research and Development Fund (23-A-19/26-A-4/29-A-3/2020-J-3) from the Ministry of Health, Labour and Welfare, and the Practical Research for Innovative Cancer Control (JP20-22ck0106593) from the Japan Agency for Medical Research and Development, AMED.
Open access funding provided by Okayama University.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Material preparation, data collection, and analysis were performed by SS, NK, HH, TS, and HI. The first draft of the manuscript was written by SS and all authors commented on the subsequent versions of the manuscript. All authors read and approved the final manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Nagoya City University (12-Feb-2021/No. 60-20-0182).
Consent to participate
Formal consent was not required for this type of study.
Consent for publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Material 1: Figure S1
. Study flow-chart

Supplementary Material 2: Figure S2
. Cumulative incidence of distant metastasis and effect of endocrine therapy according to risk factors. Low NG and negative Ly (A), Low NG and positive Ly (B), high NG and negative Ly (C), and high grade and positive Ly (D). NG, nuclear grade; Ly, lymphatic invasion

Supplementary Material 3: Figure S3
. Cumulative incidence of distant metastasis according to tumor size. T1a (A), T1b (B) tumors
Supplementary Material 4: Table S1
. Risk of Overall Survival in Patients With T1a/b Breast Cancer. Table S2. Distant Metastasis According to Risk Classification. Table S3. Risk of Ipsilateral Breast Tumor Recurrence in Patients With T1a/b Breast Cancer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sasada, S., Kondo, N., Hashimoto, H. et al. Prognostic impact of adjuvant endocrine therapy for estrogen receptor-positive and HER2-negative T1a/bN0M0 breast cancer. Breast Cancer Res Treat 202, 473–483 (2023). https://doi.org/10.1007/s10549-023-07097-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07097-6