Abstract
Background
The efficacy of adjuvant endocrine therapy for hormone receptor-positive breast cancer has been previously established. However, significant adverse events related to endocrine therapy cannot be ignored. T1 breast cancer is expected to have a good prognosis. Therefore, adjuvant endocrine therapy for T1a breast cancer patients is controversial. Thus, in this study, we examined the effect of endocrine therapy on the prognosis of T1N0 hormone receptor-positive, HER2-negative breast cancer patients in each tumor size group, and re-considered the application of endocrine therapy.
Methods
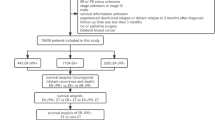
We retrospectively obtained clinical and pathological data from medical records of 7635 patients who underwent surgery for breast cancer at Aichi Cancer Hospital between January 2000 and December 2017. The primary end point of our analysis was disease-free survival (DFS). The secondary end points were distant disease-free survival (DDFS), overall survival (OS), and breast cancer-specific survival (BCSS). The log-rank test, cumulative survival generated curves with Kaplan–Meier methods and the hazard ratio (HR) calculated with a Cox regression model were used to assess the effects of endocrine therapy on prognosis.
Results
The 5-year DFS was worse in the non-endocrine therapy (non-ET) group (78%) than the endocrine therapy (ET) group (95%) in the T1c population (p < 0.001, HR 0.25). However, there was no statistically significant difference in DFS between the ET and the non-ET groups in T1a (ET 96% vs non-ET 93%, p = 0.9314, HR 0.94) and T1b (ET 96% vs non-ET 93%, p = 0.1481HR 0.53) breast cancer. The OS, DDFS, and BCSS of the patients also showed that endocrine therapy was associated with improvement of the prognosis in the T1c group, but not in the T1a and T1b groups.
Conclusions
Adjuvant endocrine therapy may be essential for T1c breast cancer patients. In contrast, this therapy should be discussed for T1a and T1b luminal breast cancer patients under some circumstances, such as suffering from adverse events.



Similar content being viewed by others
References
Kennedy T, Stewart AK, Bilimoria KY, Patel-Parekh L, Sener SF, Winchester DP. Treatment trends and factors associated with survival in T1aN0 and T1bN0 breast cancer patients. Ann Surg Oncol. 2007;14:2918–27.
Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ. 2015;351:h4901.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2018;124:2785–800.
Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378;771–84.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41.
Group BIGC, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–76.
van de Velde CJ, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel JM, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377:321–31.
Early Breast Cancer Trialists' Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2.
Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–80.
Whelan TJ, Pritchard KI. Managing patients on endocrine therapy: focus on quality-of-life issues. Clin Cancer Res. 2006;12:1056s-s1060.
National Comprehensive Cancer Network. Breast Cancer, https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 11 Nov.
Society JBC. The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer. Tokyo: Kanehara; 2018. p. 21–8.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81.
Hanna WM, Slodkowska E, Lu FI, Nafisi H, Nofech-Mozes S. Comparative analysis of human epidermal growth factor receptor 2 testing in breast cancer according to 2007 and 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations. J Clin Oncol. 2017;35:3039–45.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. Histopathology. 2002;41:151–2 (discussion 2–3).
Leslie H. Sobin MKG, Christian Wittekind. TNM classification of malignant tumours, 7th ed. 2011.
Hanrahan EO, Valero V, Gonzalez-Angulo AM, Hortobagyi GN. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a, bN0M0): a review of the literature. J Clin Oncol. 2006;24:2113–22.
Fisher B, Dignam J, Tan-Chiu E, Anderson S, Fisher ER, Wittliff JL, et al. Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst. 2001;93:112–20.
Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7.
Tao M, Ma D, Li Y, Zhou C, Li Y, Zhang Y, et al. Clinical significance of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011;129:247–54.
Nakagawa T, Martinez SR, Goto Y, Koyanagi K, Kitago M, Shingai T, et al. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin Cancer Res. 2007;13:4105–10.
Bansal C, Pujani M, Misra S, Srivastava AN, Singh US. Circulating tumor cells in breast cancer: correlation with clinicopathological parameters, hormone profile and MicroRNA polymorphisms. Turk Patoloji Derg. 2016;32:148–57.
Narod SA, Sopik V. Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res Treat. 2018;169:9–23.
Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat. 2018;170:647–56.
Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–9.
Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–73.
Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. 2016;387:866–73.
Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–56.
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol. 2004;22:4261–71.
Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, et al. Assessment of quality of life in MA17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23:6931–40.
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134:459–78.
Wagner LI, Zhao F, Goss PE, Chapman JW, Shepherd LE, Whelan TJ, et al. Patient-reported predictors of early treatment discontinuation: treatment-related symptoms and health-related quality of life among postmenopausal women with primary breast cancer randomized to anastrozole or exemestane on NCIC Clinical Trials Group (CCTG) MA.27 (E1Z03). Breast Cancer Res Treat. 2018;169:537–48.
Abraham J, Caldera H, Coleman R, Elias A, Goetz MP, Kittaneh M, et al. Endocrine therapy and related issues in hormone receptor-positive early breast cancer: a roundtable discussion by the breast cancer therapy expert group (BCTEG). Breast Cancer Res Treat. 2018;169:1–7.
Acknowledgements
The authors would like to thank Ms. Matsui and the doctors, nurses, and technical staff of Aichi Cancer Center Central Hospital for their daily support. We thank J. Iacona, Ph.D., from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
YA performed study planning and statistical analysis, and drafted the manuscript. YA, MS, MH, AY, HK, AK, KS, NH, YO, YE, KN, and TD participated in information and data collection. IO and HI helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Aichi Cancer Center Ethics Committee approved the study.
Informed consent
An opt-out approach was used with the disclosure of website.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

12282_2021_1245_MOESM1_ESM.jpg
The DDFS of ET group and non-ET group stratified according to tumor size. ET; endocrine therapy, DDFS; distant disease-free survival (JPG 121 kb)

12282_2021_1245_MOESM2_ESM.jpg
The BCSS of ET group and non-ET group stratified according to tumor size. ET; endocrine therapy, BCSS; breast cancer-specific survival (JPG 119 kb)
About this article
Cite this article
Adachi, Y., Oze, I., Sawaki, M. et al. Impact of adjuvant endocrine therapy on prognosis in small hormone receptor-positive, HER2-negative early breast cancer. Breast Cancer 28, 1087–1095 (2021). https://doi.org/10.1007/s12282-021-01245-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01245-w