Abstract
Purpose
In breast conserving surgery, accurate lesion localization is essential for obtaining adequate surgical margins. Preoperative wire localization (WL) and radioactive seed localization (RSL) are widely accepted methods to guide surgical excision of nonpalpable breast lesions but are limited by logistical challenges, migration issues, and legislative complexities. Radiofrequency identification (RFID) technology may offer a viable alternative. The purpose of this study was to evaluate the feasibility, clinical acceptability, and safety of RFID surgical guidance for localization of nonpalpable breast cancer.
Methods
In a prospective multicentre cohort study, the first 100 RFID localization procedures were included. The primary outcome was the percentage of clear resection margins and re-excision rate. Secondary outcomes included procedure details, user experience, learningcurve, and adverse events.
Results
Between April 2019 and May 2021, 100 women underwent RFID guided breast conserving surgery. Clear resection margins were obtained in 89 out of 96 included patients (92.7%), re-excision was indicated in three patients (3.1%). Radiologists reported difficulties with the placement of the RFID tag, partially related to the relatively large needle-applicator (12-gauge). This led to the premature termination of the study in the hospital using RSL as regular care. The radiologist experience was improved after a manufacturer modification of the needle-applicator. Surgical localization involved a low learning curve. Adverse events (n = 33) included dislocation of the marker during insertion (8%) and hematomas (9%). The majority of adverse events (85%) occurred using the first-generation needle-applicator.
Conclusion
RFID technology is a potential alternative for non-radioactive and non-wire localization of nonpalpable breast lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The detection of nonpalpable breast cancer has increased by improved imaging and the introduction of population-based screening programmes [1]. Significantly more patients opt for breast conserving surgery (BCS) in combination with radiotherapy, as it is a proven alternative to mastectomy with respect to overall survival [2]. In BCS, accurate intraoperative lesion localization is essential for adequate surgical margins whilst sparing surrounding healthy tissue to achieve optimal cosmesis [3]. Preoperative wire localization and radioactive seed localization are widely accepted methods to guide surgical excision of nonpalpable breast lesions but have significant limitations [4].
Wire-guided localization (WL) has been the standard method for the surgical excision of nonpalpable tumours for several decades. WL is facilitated by an imaging guided insertion of a wire in the tumour by a radiologist. This technique requires careful logistical planning as the wire needs to be placed on the day of surgery, which can lead to significant workflow inefficiencies and delays. In addition, the entry site of the wire may differ from the ideal surgical approach. Other disadvantages of WL are patient anxiety caused by the wire protruding from the patient’s skin, the risk of migration, dislodgement or pneumothorax during placement [5]. Therefore, radioactive seed localization (RSL) has been introduced to improve the surgical treatment. During a short procedure, a radioactive seed (125I) is placed in the tumour in a short radiological procedure which subsequently is localised intraoperatively by a gamma probe. The RSL resection margins, re-excision, and reoperation rates are non-inferior to WL. However, the use of radioactive seeds is constrained by stringent nuclear regulations needing meticulous tracking of the radioactive seed legislation on top of a growing concern for availability of radioactive materials for medical use [6]. These disadvantages have limited the widespread adoption of the radioactive seed and have prompted research and development of non-radioactive seed.
Recently introduced radiofrequency identification (RFID) technology may offer a viable non-radioactive, non-wire alternative for localization of nonpalpable breast lesions during BCS. A number of single centre cohort studies [7,8,9,10,11] and a retrospective cohort analysis [12] have concluded that the RFID lesion localization can be used safely and effectively for non-palpable breast lesions. However, data to evaluate RFID localization process is currently lacking. Therefore, the current research evaluates the feasibility, clinical acceptability and safety of RFID surgical guidance for localization of nonpalpable breast cancer in a prospective multicentre cohort study.
Materials and methods
The design of this prospective multicentre cohort study, and technical specifications about the RFID technique were reported in detail earlier [13] (The Dutch Trial Register—NL8019) concluding an unattainable response assessment of neoadjuvant chemotherapy (NAC) for the patients with a RFID tag due to a significant MRI artefact. The study was approved by the Medical Research Ethical Committee of the University Medical Center Utrecht. Informed consent was obtained from all participants.
Study population
All adult breast cancer patients with nonpalpable, histologically proven, ductal carcinoma in situ or invasive breast cancer, scheduled for breast conserving surgery between April 2019 and May 2021 in Medisch Spectrum Twente (Enschede, the Netherlands) or Diakonessenhuis Utrecht (Utrecht, the Netherlands), were considered eligible for participation. A total of 100 patients were included in this feasibility study. Participants were recruited at the surgical outpatient clinic. The following exclusion criteria were applied: lesion located deeper than 7 cm assessed in supine position, pregnancy or lactating status, and multicentric breast cancer. Additional exclusion criterion was applied with respect to the standard of care for localization at each study site:
-
At Diakonessenhuis Utrecht (RSL) patients undergoing neoadjuvant chemotherapy treatment (NAC) were excluded, as a single procedure using RSL was preferred over a two-step localization procedure with a biopsy marker before NAC followed by preoperative RFID tag placement [13].
-
At Medisch Spectrum Twente (WL) patients undergoing NAC were not excluded. A biopsy marker was used during NAC, followed by preoperative RFID tag placement.
RFID tag placement procedure
The LOCalizer™ RFID tag (10.6 × 2 mm) (Fig. 1A) was inserted during an image guided procedure up to 30 days prior to surgery by an experienced breast radiologist or radiology resident under supervision (intended use > 30 days) [14]. The image guided procedure involved ultrasound (Fig. 1B) or stereotactic imaging. The RFID tag was inserted percutaneously through a small skin incision with a preloaded 12-gauge sterile needle-applicator after injection of local anaesthesia (lidocaine 2%). Each tag has a unique identification number and can be localised transcutaneous by a handheld reader making it easy to distinguish two different tags. The reader (illustrated in Fig. 1A) has a loop probe with a detection range of up to 7 cm, and an attachable single-use sterile pencil probe (8 mm tip diameter) with a detection range up to 3.5 cm for highly accurate localization. The audible and visual feedback provide the surgeon with real-time guidance during the excision procedure. During the course of this study, the manufacturer made a modification to the applicator needle allowing a comparison between the versions.
An overview of RFID localization procedure with A the RFID LOCalizer™ system (Faxitron, Hologic); B Ultrasound guided RFID tag placement; C Post placement mammography to confirm correct tag location (CC-view); D Transcutaneous detection with the loop probe; E Intraoperative detection with the surgical probe; F Specimen radiography (Trident, Hologic) showing the RFID tag, biopsy marker and specimen clips
The quality of RFID placement was assessed by post-placement mammography and classified as successful if inserted at 0–5 mm omnidirectional distance to the tumours or the pre-NAC placed biopsy marker. In case of unsuccessful placement an additional marker was used, i.e. WL or radioactive seed in cases when an additional marker was needed at a distance shorter than 2 cm (to prevent possible signal interference [14]), or an RFID tag. For cases with a complete radiological response after NAC, the RFID tag was inserted next to the biopsy marker placed in the tumour before the start of NAC. The complete decision three is illustrated in Fig. 2. The pain score after local anaesthesia was recorded by the Visual Analogue Scale (VAS) [15]. Directly after the procedure the radiologist completed a questionnaire assessing the procedure and system usability [13, 16]. The total duration of the procedure was recorded (from start of local anaesthesia to the deposition of the marker). Directly after the tag placement, a two-view mammography, i.e. craniocaudal (CC) and mediolateral oblique (MLO) was acquired to assess the position of the RFID tag (Fig. 1C). The shortest distance from RFID tag to lesion (or biopsy clip marker) was recorded.
RFID guided breast conserving surgery
All surgical procedures were performed by experienced breast cancer surgeons or surgery residents under direct supervision. The RFID tag was intraoperatively localised using the handheld portable reader device (Fig. 1A). Transcutaneous measurements were performed with the loop probe (Fig. 1D), measurements after incision were performed with a single-use sterile pencil probe (Fig. 1E). In addition to measurements with the portable reader device lumpectomy specimen radiography was performed to confirm successful retrieval of the RFID tag (Fig. 1F). Directly after the procedure the surgeon completed a questionnaire on the ease of the procedure and system usability [13] [16]. Duration of the procedure from primary incision until specimen retrieval was recorded. Postoperative complications were recorded up to 30 days postoperatively.
Pathology
Lumpectomy specimens were examined by experienced breast pathologists to record the following parameters: presence and location of the RFID tag, resection margins, specimen dimensions, and tumour dimensions. The respective volumes were calculated using the three radii (1/2 of the diameter assessed by pathology protocol), and the ellipsoid equation \(V=\frac{4}{3}\pi {r}_{1}{r}_{2}{r}_{3}.\) According to the Dutch breast cancer guidelines[17], surgical margins were classified as radical (no tumour in ink), focally irradical (tumour in 1 limited area ≤ 4 mm) and more than focal irradical [17]. Re-excision or mastectomy was recommended for patients with more than focal irradical margins, whilst in patients with focal irradical margins a radiation-boost will suffice.
Data analysis
All data was collected using Castor Electronic Data Capture. The radical (re)excision rate is expressed as a quotient between the total number of excisions and the total number of radical (re)excisions. Descriptive statistics were used to summarise the data by mean, median, interquartile range (IQR), and 95% confidence intervals (CI). Data was analysed using IBM SPSS statistics (IBM Corp., Armonk, NY, USA, version 28).
Results
Between April 2019 and May 2021, a total of 100 women (median age of 62, 54–69 IQR) underwent breast conserving surgery using RFID localization (Table 1): 91 procedures were performed at Medisch Spectrum Twente, and nine at the Diakonessen hospital Utrecht.
Placement procedure
Fourteen experienced breast radiologists performed 100 RFID procedures (7.1 procedures on average, range 1–25). A 92% success rate was achieved, summarised in Table 2. All radiologists were experienced with RSL, the radiologists from MST also with WL. In 8 procedures (all with ultrasound guidance), the placement failed due to dislocation during retraction of the needle-applicator. Dislocation of the tag occurred in BIRADS density categories A, B, and C. In two patients the distance between tag and lesion (15 mm and 18 mm) were accepted after consultation with the surgeon. Two patients received a second RFID tag, and four patients underwent WL. The median duration of the procedure was five minutes (IQR 3–10 min). The mean VAS pain score of the patient was 1.4 (range 0–6). Median time between tag placement and surgery was 7 days (IQR 4–12 days). 99 RFID tags were placed under ultrasound guidance. One patient received a stereotactic placement due to the dense breast tissue encountered during the initial biopsy procedure. Twelve patients underwent surgery after completing NAC.
Experience of the radiologists with respect to the RFID placement is illustrated in Fig. 3 Radiologists were positive about the RFID tag visibility on ultrasound and easy handling of the needle-applicator. However, they experienced difficulty penetrating through dense glandular tissue and solid tumour tissue which led to repeated punctures and manoeuvring of the needle-applicator through the tissue. In relation to this, they assessed the size of the RFID tag and accompanying 12-gauge needle-applicator as large, necessitating local anaesthesia and increasing the risk of bleeding/hematoma. For these reasons, radiologists in one study centre (Diakonessenhuis Utrecht) preferred the radioactive seed procedure over RFID localization leading to premature termination of the study at this site. In response, the needle-applicator was modified to allow for a smooth penetration of dense breast tissue and solid tumours. The modified needle-applicator, implemented after the first 50 procedures, led to an improved user satisfaction (Fig. 3) in the remaining site (MST). In addition, there was an improvement of the successful placement rate from 90% (45 out of 50) to 98% (49 out of 50). However, a substantial learning curve for the radiologists was observed (Fig. 3). Specifically, the radiologists with few or infrequent procedures had a preference towards RSL, whilst radiologists had no preference between RSL/WL and RFID seed after 10 RFID procedures and following frequent placements (monthly). In two patients, two RFID tags were placed to delineate an area of interest. In one patient with multifocal invasive carcinoma an area of 3.2 cm was delineated. In the other case two tags delineated an area of 4 cm DCIS grade 3. In one patient 1 RFID tag was placed between two areas of DCIS grade 3, which were marked with a biopsy clip marker. (Fig. 4).
Radiologist satisfaction questionnaire results after the first 50 procedures completed by 14 radiologists (left), and after 50 additional procedures completed by 6 radiologists after needle modification (right). For the first 50 procedures the questionnaire was completed directly after each procedure, whilst for the second 50 procedures the questionnaire was completed at the conclusion of the study. *Based on patients with a solid tumour
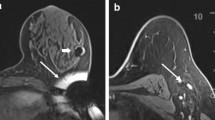
A Postplacement mammography (CC-view) of a patient with 2 RFID tags to delineate an area of multifocal breast cancer after NAC; B the corresponding specimen radiography (Trident, Hologic) after surgery, with the 2 RFID tag, 2 biopsy clip markers and marking sutures in situ; C postplacement mammography (CC-view) of a patient with 1 RFID tag in-between 2 clip markers which both designate an area of microcalcifications; D the corresponding specimen radiography (Trident, Hologic) after surgery, with the RFID tag, 2 biopsy clip markers and multiple specimen clips in situ
Surgical localization
As summarised in Table 2, eight surgeons performed RFID guided BCS in 96 patients (12 procedures on average, range 2–48). Out of the 100 patients included in the surgical evaluation, four individuals experienced dislocation of the RFID tag. In all cases, the dislocation of the RFID tag was detected immediately after its placement and confirmed by the post-placement mammography. Consequently, these patients were excluded from the surgical evaluation exclusively. As per site protocol, these patients received as an alternative method for lesion localization. Transcutaneous identification of the RFID tag with the loop probe was successful in all 96 participants that entered surgical localisation. Median duration of the surgical procedure was 17 min (IQR 12–20 min). All RFID tags were successfully retrieved. User experience with respect to surgery is illustrated in Fig. 5. Surgeons reported that after five to seven procedures, they felt sufficiently familiar with the RFID tag reflecting a signal from two poles (one at each end) instead of a small point source of radioactivity from a radioactive seed.
Histopathological results
Clear resection margins were obtained in 89 out of 96 patients (92.7%, 95% CI [86.2, 96.7]). Four patients (4.2%) had focally involved resection margins, i.e. 3 patients with DCIS and 1 patient with focal irradical invasive carcinoma (NST).
In three additional patients (3.1%), the resection margins were irradical and these patients required re-excision surgery. Despite a complete radiological response to NAC (per MRI), one patient with infiltrating lobular carcinoma (ILC) had an extensive irradical resection. One patient had irradical resection of invasive carcinoma, i.e. the tumour size was underestimated on preoperative imaging (mammography and ultrasound). The third re-excision was in a patient with extensive florid lobular carcinoma in situ (LCIS) which size was underestimated on preoperative imaging (Mammography and Ultrasound, MRI was not performed). In two additional patients, laceration was observed at multiple sites in the lumpectomy tissue reaching into the tumour, complicating evaluation of the surgical margins (supplementary information).
Adverse events
Two patients had a postoperative wound infection within 30 days after surgery amongst which one patient after an oncoplastic lumpectomy. Dislocation of the marker during insertion occurred in eight patients (7.7%). After tag insertion, nine patients experienced some bleeding or hematoma after the placement.
At histopathological assessment of the lumpectomy specimens, tissue damage was found in 14 patients (16%). These specimens showed evidence of mild inflammatory and foreign body reaction with fibrosis. In addition, areas of hematomas and large cavities/ruptured tissues were seen in the area where the RFID tag was positioned (supplementary information). The majority of these cases (85%) occurred before implementation of the modified needle-applicator.
Discussion
This study evaluated the RFID localization procedure in nonpalpable, histologically proven breast cancer including DCIS, and showed clear resection margins in 92.7% of all cases with re-excision indicated in total of 3.1% patients. Importantly, the first 50 procedures indicated difficulties regarding the penetration through dense breast tissue (BIRADS C & D) resulting in tissue damage (hematoma and lacerated tissue) in the area where the RFID tag was positioned. Specifically, multiple lump lacerations were observed in two patients which complicated evaluation of the surgical margins. As these patients did not undergo vacuum assisted biopsy, the tissue damage may be attributed to the RFID procedure. After introduction of the improved needle-applicator there was no tissue damage reported at histopathology. All of these is in line with previous RFID localization studies [9, 10]. Difficulties regarding the penetration led to technical modifications of the needle-applicator (by the manufacturer), i.e. a silicone coating was applied to the needle-applicator. After the modification of the needle-applicator these difficulties were resolved. Stereotactic guidance in one patient with dense breast (BIRADS category C) tissue on mammography (and difficult initial biopsy procedure) was proven successful using modified needle-applicator. Furthermore, supported by recent feasibility studies [10, 18] involving off-label use, it has been identified that the RFID has the potential for targeted excision of suspicious axillary lymph nodes.
The current-standard-of-care in the Netherlands allows for a maximum of 15% irradical excisions [17]. Country-wise statistics in 2020 showed 11.6% re-excision rate for carcinoma in situ, and 4% re-excision rate for invasive carcinoma [17]. This study showed an acceptable percentage of irradical excisions of 7.3%. With respect to 2020, this study showed impressive statistics, i.e. 3.7% re-excision rate for patients with carcinoma in situ, and 2.9% re-excision rate for invasive carcinoma. With respect to other localisation techniques, this study shows similar outcomes regarding resection rates. Specifically, a recent retrospective study by Liang et al. [19] reported negative margins in 91% for WL, 89% for radioactive seed localization, and 89% for magnetic seed. The average lump volume of 45cm3 in our study is slightly higher than the findings reported by McGugin et al. [12]. However, it is worth noting that our re-excision rate is significantly lower than in the mentioned article. Specifically, our study observed a re-excision rate of 3.7% for patients with carcinoma in situ and a re-excision rate of 2.9% for patients with invasive carcinoma, compared to a re-excision rate of 17% and 19% reported by McGugin et al. In comparison to our findings, recent research on magnetic seed localization demonstrates a greater lumpectomy volume of 68.5cm3 and higher re-excision rates of 14.4% in invasive carcinoma and 35.3% in DCIS [20].
Clinical challenges for the radiologists (the diameter of needle-applicator and MRI artefact) and surgeons (spatial insight) hampered initial clinical acceptability after the introduction of RFID localization. Therefore, a large non-inferiority trial comparing RFID localization to other localization techniques is necessary to motivate clinical acceptance. Furthermore, additional innovation of RFID is needed for longer-term RFID use in patients undergoing NAC. For the physicians accustomed to RSL (DU) precluded the use of the RIFD in patients receiving NAC, and the benefits of RFID localization did not outweigh the disadvantages of cumbersome localizer placement which resulted in premature termination of the study after nine procedures. However, for the physicians accustomed to WL (Medisch Spectrum Twente), the benefits were worth time and effort to implement RFID localization in clinical care which greatly simplified logistic process. Therefore, clinical acceptability may be related to the standard localization technique used prior to introduction of RFID localization.
In conclusion, RFID technology is a potential alternative for non-radioactive and non-wire localization of nonpalpable breast lesions.
Data availability
The datasets generated during the study will be available from the authors on reasonable request.
Abbreviations
- BCS:
-
Breast conserving surgery
- DCIS:
-
Ductal carcinoma in situ
- LCIS:
-
Lobular carcinoma in situ
- ILC:
-
Infiltrating lobular carcinoma
- NST:
-
No special type
- WL:
-
Wire localization
- RSL:
-
Radioactive seed localization
- RFID:
-
Radiofrequency identification
- NAC:
-
Neoadjuvant chemotherapy
- VAS:
-
Visual Analogue Scale
- CC:
-
Craniocaudal
- MLO:
-
Mediolateral oblique
- MRI:
-
Magnetic resonance imaging
- IQR:
-
Interquartile range
- CI:
-
Confidence interval
References
Welch HG, Prorok PC, O’Malley AJ, Kramer BS (2016) Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 375(15):1438–1447
van Maaren MC, Poortmans P, Siesling S (2016) Breast-conserving therapy versus mastectomy. Oncoscience 3(11–12):304–305
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347(16):1227–1232
Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA (2015) Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev (12):CD009206
Jensen SR, Luttenegger TJ (1979) Wire localization of nonpalpable breast lesions. Radiology 132(2):484–485
NEA (2019) 2019 Medical isotope demand and capacity projection for the 2019–2024 period, the supply of Medical Radioisotopes. OECD Publishing, Paris
Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M (2015) A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol 204(6):W720–W723
DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC (2019) Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat 175(1):165–170
Malter W, Holtschmidt J, Thangarajah F, Mallmann P, Krug B, Warm M, Eichler C (2019) First reported use of the Faxitron LOCalizer Radiofrequency Identification (RFID) System in Europe—a feasibility trial, surgical guide and review for non-palpable breast lesions. In Vivo 33(5):1559–1564
Lowes S, Bell A, Milligan R, Amonkar S, Leaver A (2020) Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Clin Radiol 75(12):942–949
Wazir U, Tayeh S, Perry N, Michell M, Malhotra A, Mokbel K (2020) Wireless breast localization using radio-frequency identification tags: the first reported european experience in breast cancer. In Vivo 34(1):233–238
McGugin C, Spivey T, Coopey S, Smith B, Kelly B, Gadd M, Hughes K, Dontchos B, Specht M (2019) Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat 177(3):735–739
den Dekker BM, Christenhusz A, van Dalen T, Jongen LM, van der Schaaf MC, Dassen AE, Pijnappel RM (2022) A multicenter prospective cohort study to evaluate feasibility of radio-frequency identification surgical guidance for nonpalpable breast lesions: design and rationale of the RFID Localizer 1 Trial. BMC Cancer 22(1):305
Hologic (2023) User manual localizer reader and RFID localization system. https://www.hologic.com/file/33961/download?token=InBEAoY8. Accessed 9 Jan 2023
Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, Harris JD (2018) Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev 2(3):e088
Brooke J (1995) SUS: a quick and dirty usability scale. Usability Eval Ind 189
Dica (2017) NABON Breast Cancer Audit Year Report 2017
Malter W, Eichler C, Hanstein B, Mallmann P, Holtschmidt J (2020) First reported use of radiofrequency identification (RFID) technique for targeted excision of suspicious axillary lymph nodes in early stage breast cancer—evaluation of feasibility and review of current recommendations. In Vivo 34(3):1207–1213
Liang DH, Black D, Yi M, Luo CK, Singh P, Sahin A, Scoggins ME, Moseley TW, Hunt KK (2022) Clinical outcomes using magnetic seeds as a non-wire, non-radioactive alternative for localization of non-palpable breast lesions. Ann Surg Oncol 29(6):3822–3828
Kelly BN, Webster AJ, Lamb L, Spivey T, Korotkin JE, Henriquez A, Gadd MA, Hughes KS, Lehman CR, Smith BL, Specht MC (2022) Magnetic seeds: an alternative to wire localization for nonpalpable breast lesions. Clin Breast Cancer 22(5):e700–e707
Funding
This work was supported by Hologic, grant number 1257. Hologic is not involved in the design of the study and collection, analysis, interpretation of data, writing the manuscript and in the decision to publish study results.
Author information
Authors and Affiliations
Contributions
AD, AC, and BD were involved in the study design. All authors have contributed to the manuscript and have approved the final manuscript for publication. First draft and data analysis was performed by AC, BD revised it critically.
Corresponding author
Ethics declarations
Conflict of interest
Author BD has received research support from Hologic. Author AD has received speaker and consultant honoraria from Hologic. Other authors declare that they have no competing interests.
Ethical approval
The study was approved by the Institutional Review Board of the University Medical Center Utrecht. The RFID Localizer 1 Trial is conducted in accordance with the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects (Fortaleza, Brazil, October 2013).
Consent to participate
Written informed consent is obtained from all participants.
Consent to publish
Written informed consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christenhusz, A., den Dekker, B.M., van Dalen, T. et al. Radiofrequency localization of nonpalpable breast cancer in a multicentre prospective cohort study: feasibility, clinical acceptability, and safety. Breast Cancer Res Treat 201, 67–75 (2023). https://doi.org/10.1007/s10549-023-07006-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07006-x