Abstract
Purpose
Herein, we report the frequency and distribution of germline pathogenic variants (PVs) among females with breast cancer (BC) and at least one other non-BC who underwent multi-gene panel testing (MGPT). Among females with PVs diagnosed first with BC or ovarian cancer (OC), we sought to enumerate the frequency of subsequent PV-associated cancers.
Methods
Females with BC and cancer of ≥ 1 other site (multiple primary cancers, MPC) who underwent MGPT through Ambry Genetics from March 2012 to December 2016 were included if they had testing of at least 21 genes of interest (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, and TP53). Phenotypic data were abstracted from test requisition forms and clinical notes.
Results
Of 6,617 evaluable patients, most were White (70.8%) and median age at first cancer, second cancer, and MGPT was 49 (interquartile range [IQR]: 18), 59 (IQR: 16), and 63 (IQR: 16) years, respectively. PVs were found among 14.1% (932/6617) of the overall cohort and in 16.4% (440/2687) of females who were diagnosed first with BC. Among those, 55.2% (243/440) had an actionable PV associated with a subsequent cancer diagnosis including 150 OCs. Of the 2443 females with breast and ovarian cancer, few (n = 97, 9.5%) were diagnosed first with OC, limiting our analysis.
Conclusions
Females with MPC, including BC, have a high frequency of germline PVs (14.1%). These data delineate the opportunities for intercepting subsequent cancers associated with genetic risk among females diagnosed first with BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple primary cancers (MPCs) and, notably, subsequent cancer diagnoses among breast cancer survivors are multifactorial. While some are due to receipt of chemotherapy or radiation or exposure to a common carcinogen, others can be attributed to a genetic risk [1]. In prior work, we found that patients with MPCs who underwent germline genetic testing had a high rate of pathogenic variants (PVs) (> 10%) regardless of age at their second cancer diagnosis (< 50, 50–70 or > 70) [2]. Patients with breast cancer (BC) who develop a second BC are more likely to have PVs in germline BC genes (ATM, BRCA1, BRCA2, CHEK2, CDH1, NBN, NF1, PABL2, PTEN, and TP53) than those with one BC [3]. Additionally, patients with BC and any other primary cancer diagnosis are more likely to have a PV in a variety of cancer genes than those with a BC only (8.5% vs. 4.9%) [4]. Data remain limited on the frequency of PVs among BC survivors who develop a subsequent cancer diagnosis.
Germline genetic testing of female patients with BC is often limited to those with a strong cancer family history, early age at diagnosis, and specific tumor subtypes [5]. Family history of BRCA-associated cancers (breast, ovarian, pancreatic, and prostate) and risk models that prompt a referral for germline genetic testing (BOADICEA, BRCAPRO, Penn II, and Myriad) emphasize BRCA-related cancer risk [6]. Evidence supporting targeted therapies for early BC has led to calls for more liberal germline genetic testing of patients with BC at diagnosis to inform surgical and therapeutic options [7]. Moreover, as BC care and survivorship continue to improve, it is increasingly important to counsel BC patients on future cancer risks, including the risk of a second BC and the risk of a subsequent non-breast primary cancer.
Likewise, while genetic testing has been recommended for all patients with ovarian cancer (OC) in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) since 2010, there has been low compliance with this guideline [8]. Numerous quality improvement initiatives have been undertaken to address this testing gap [9, 10]. As survivorship in OC improves, owing in part to targeted and maintenance therapy [11, 12], subsequent cancers may emerge as an important issue for this patient population.
The primary aim of this study was to delineate the frequency and distribution of germline PVs/likely PVs among females with BC and a primary cancer of another non-breast site who underwent multi-gene panel testing (MGPT). We examined the frequency and distribution of PVs by combinations of cancers. We report PV frequencies by the order of cancer diagnosis (referred to as diagnostic order) for BC and OC. Next, we sought to elucidate the opportunities for reducing cancer morbidity and mortality through quantifying the frequency of subsequent cancers associated with patients’ PVs. We limited our analysis to subsequent cancers that are known to be associated with germline PVs whose outcomes are influenced by early detection or interception (e.g., BC and colorectal cancers), or which may be prevented by risk-reducing procedures (breast, colorectal, endometrial, and ovarian cancers). Among the subset of females with germline PVs who were diagnosed first with BC and separately with OC, we quantified the opportunities for cancer interception [13].
Methods
Subjects
A retrospective cross-sectional study of reported cancer history and genetic test results was conducted of tested individuals through a diagnostic testing laboratory (Ambry Genetics, Aliso Viejo, CA). Females were included if they had a personal diagnosis of BC and ≥ 1 non-BC diagnosis (excluding nonmelanoma skin cancers) and had MGPT between March 2012 and December 2016, inclusive of the following 21 genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, and TP53. Concurrent cancers at one site were counted once only (e.g., two colorectal cancers at the same age counted as one cancer). Patients with multiple PVs were enumerated and excluded from the analytic cohort and are the subject of a separate analysis.
Variant interpretation was performed based on the American College of Medical Genetics and Genomics and the Association of Medical Pathologists guidelines [14, 15]. PVs and likely PVs were both denoted as PVs in this study. The lower-risk variant, CHEK2 p.I157T, was distinguished from other PVs in CHEK2 and included in overall counts. Biallelic MUTYH was considered a PV and monoallelic MUTYH was not. Per NCCN Guidelines® for Breast/Ovarian Genetic/Familial High-Risk Assessment V1.2022 [16], we categorized BRCA1, BRCA2, CDH1, PALB2, PTEN, and TP53 as high-risk BC genes; ATM, BARD1, CHEK2 (excluding CHEK2 p.I157T), NBN 657del, and NF1 as moderate-risk BC genes; CHEK2 I157T, RAD51C, and RAD51D as “other BC” genes. The DNA Mismatch Repair (MMR) genes included the following: 3’ deletions in EPCAM, MLH1, MSH2, MSH6, and PMS2. We limited our analysis to these 21 genes because they are commonly tested for, often have distinct screening or risk-reducing strategies for cancer interception and most have been associated with more than one cancer type. This study was exempt from review by the Western Institutional Review Board.
Clinician-completed requisition forms and clinical documentation (pedigrees and chart notes) were abstracted for clinical characteristics including cancer history and demographics (sex, age at testing and at each tumor diagnosis).
Data analysis
Descriptive statistics are summarized as medians (interquartile range [IQR]) for continuous and proportions for categorical patient characteristics. T-tests were used to examine differences in ages at panel testing, first and second primary cancer diagnosis. For diagnostic order of BC, we performed chi-squared tests. We also performed chi-squared test for trend to examine whether there was a significant increasing/decreasing trend for the PV frequency by the diagnostic order for OC. All statistical tests were two-sided, and a P value of < 0.05 was considered statistically significant. A continuity correction was used for the 95% confidence interval (CI) when the numerator was < 10. All analyses were conducted with R v.3.3.3.
Results
Cohort characteristics
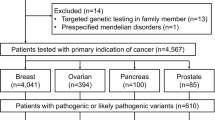
From March 2012 to December 2016, 9,820 patients with multiple primaries underwent germline multi-gene panel testing at Ambry Genetics that included all 21 genes of interest as previously described (Fig. 1) [7]. Patients with multiple PVs (n = 96) were (excluded from further analysis. Among the 9,714 remaining patients with MPCs (2–6 primary tumors), 6,617 (68.1%) had female BC. Among the analytic cohort (6617), most were White (70.8%) and had two primary tumors (84.5%). The median age at first cancer diagnosis was 49 (IQR 18), median age at second cancer diagnosis was 59 (IQR 16), and median age at testing was 63 years (IQR 16, Table 1).
There were 1,007 (15.2%) patients with bilateral BCs: 25.6% (n = 258) were diagnosed with synchronous bilateral BCs and the remaining 74.4% (n = 749) were asynchronous. Hormone receptor data were missing from 45.5% of BC cases. Among the 3,609 females with available hormone receptor subtype, 79.3% had estrogen-receptor-positive (ER +) and/or progesterone-receptor-positive (PR +) disease. Among the 3,525 females with hormone receptor subtype and human epidermal growth factor receptor 2 (HER2) subtype also available, 15.0% had triple negative breast cancer. Notably, 2443 (36.9%) also had diagnosis of OC, though only 1022 (18.3%) had only BC and OV among the 5594 patients with two cancer diagnoses only.
Overall, 14.1% (n = 932/6617) had one PV including CHEK2 I157T and biallelic MUTYH, 23.3% (n = 1541) had no PV but at least one variant of uncertain significance (VUS), and 61.6% (n = 4074) had negative testing for all 21 genes of interest. The most frequently detected germline PVs were in BRCA1 (2.3%, 95% CI 2.0–2.7%), BRCA2 (2.3%, 95% CI 2.0–2.7%), CHEK2 excluding I157T (2.0%, 95% CI 1.7–2.3%), ATM (1.5%, 95% CI 1.3–1.9%), and PALB2 (0.9%, 95% CI 0.7–1.1%) (Fig. 2; Online Resource, Supplemental Table 1). An additional 0.7% (n = 49) had CHEK2 I157T PV and an additional 1.7% (n = 110) had monoallelic MUTYH.
Cancer combinations
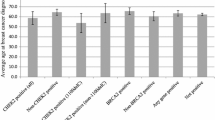
We examined the frequency of PVs by the combination of cancer types among females with only two cancers (Fig. 2). As anticipated, among females with BC and OC (n = 1022), PVs were most frequent in BRCA1 (8.6%, 95% CI 7.0–10.5%) and BRCA2 (5.8%, 95% CI 4.5–7.4%). For breast and colorectal cancers (n = 731), PVs were most frequent in CHEK2 excluding I157T (2.2%, 95% CI 1.4–3.5%), ATM (1.2%, 95% CI 0.6–2.4%), MLH1 (0.8%, 95% CI 0.3–1.9%), and PMS2 (0.7%, 95% CI 0.3–1.7%). Among 507 females with BC and thyroid cancer, CHEK2 PVs (n = 22, 4.3%, 95% CI 2.9–6.5%) were detected most frequently. For the combination of BC and pancreatic cancer (n = 175), PVs were most frequent in ATM (6.9%, 95% CI 4.0–11.6%) followed by BRCA2 (5.1%, 95% CI 2.5–9.8%). For the combination of BC and sarcoma (n = 150), PVs were most frequent in TP53 (3.3%, 95% CI 1.2–8.0%) followed distantly by BRIP1 and CHEK2. Among 80 females with BC and brain cancers, 11 had PVs of which PVs in BRCA2 (n = 4, 5.0%, 95% CI 1.6–13.0%) occurred most frequently. Among 40 females with BC and gastric cancer, 10 had PVs: BRCA2 (n = 3, 7.5%, 95% CI 2.0–21.5%) was the most frequent, followed by PALB2 (n = 2, 5.0%, 95% CI 0.9–18.2%) and CHEK2 (n = 2, 5.0%, 95% CI 0.9–18.2%). ATM, CDH1, and TP53 PVs accounted for the 3 remaining females with BC and gastric cancer with PVs (for each: n = 1, 2.5%).
PV frequency by order of breast cancer diagnosis
Of the 6617 females in this study, 5651 had evaluable data on the order of their first BC diagnosis (1st, 2nd, or ≥ 3rd cancer diagnosis). For 966 females, the diagnostic order of their (first, if multiple) BC was ambiguous, and therefore, they excluded from this analysis. The frequency of PVs was 16.4% (95% CI 15–17.8%) when BC was diagnosed 1st, 11.0% (95% CI 9.9–12.3%) when diagnosed 2nd, and 14.9% (95% CI 11.4–19.2%) when it was the > 3rd cancer diagnosis (p < 0.001, Fig. 3). The median age of BC was younger when BC diagnosis was the 1st cancer diagnosis (50, IQR 15) compared to when BC was the 2nd cancer diagnosis (58, IQR 17) or ≥ 3rd (64, IQR 17) cancer diagnosis. The frequency of PVs in high-risk BC genes (BRCA1, BRCA2, CDH1, PALB2, PTEN, and TP53) was greater for patients with BC as the 1st cancer diagnosis, 8.7% (95% CI 7.7–9.8%) compared to BC as a 2nd or ≥ 3rd cancer diagnosis, 4.1% (95% CI 3.4–4.9%) and 3.7% (95% CI 2.1–7.9%), respectively (p for trend < 0.001). The frequency of MMR PVs was highest for patients with BC as their ≥ 3rd cancer diagnosis, 5.3% (95% CI 3.3–8.3%) compared to 1st or 2nd cancer, 1.5% (95% CI 1.1–2.1%) and 1.7% (95% CI 1.3–2.3%), respectively (p for trend = 0.001). Females who were diagnosed with BC as their 2nd cancer had a younger median age at genetic testing (61, IQR 16) than those with BC as their 1st cancer diagnosis (65, IQR 14) (Online Resource, Supplemental Table 1).
PV frequency by order ovarian cancer diagnosis
The order of OC diagnosis (1st, 2nd or ≥ 3rd cancer) was available for 1,247 of the 2,443 females with OC in this cohort. The frequency of PVs was 14.4% (95% CI 8.8–22.8%) when OC was the 1st cancer diagnosis, 20.3% (95% CI 18.0–22.8%) when diagnosed second 2nd and 22.8% (95% CI 14.9–33.2%) when it was the ≥ 3rd cancer diagnosis (p for trend = 0.15 Online Resource, Supplemental Fig. 1). The median age of OC was younger when the OC diagnosis was the 1st cancer diagnosis (46, IQR 19), compared to when OC was the 2nd cancer diagnosis (63, IQR 17) or ≥ 3rd cancer diagnosis (67, IQR 14.5) (Online Resource, Supplemental Table 2).
Cancer interception
Among females with breast cancer as their first cancer diagnosis (n = 2687), 14.3% (n = 383) had a PV in a BC risk gene, 10.5% (n = 282) had a PV in an OC risk gene, and 1.5% (n = 41) with a PV in a MMR gene (Fig. 4). Among females with a PV in a BC risk gene (ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NBN- 657del, NF1, PALB2, PTEN, RAD51C, RAD51D, and TP53), 22.5% (86/383) had a subsequent BC diagnosis. Among females with a PV in an OC risk genes (BRCA1, BRCA2, BRIP1, PABL2, RAD51C, RAD51D and MMR genes), 53.2% (150/282) had a subsequent OC diagnosis. Among those with Lynch syndrome due to a PV in a MMR gene (MLH1, MSH2, MSH6, and PMS2), 61.0% (25/41) were subsequently diagnosed with endometrial cancer, 24.4% (10/41) with colorectal cancer, and 3 patients with both. In sum, 16.4% (440/2687) of patients with BC as their first cancer diagnosis were found to have a PV in a cancer susceptibility gene and this included two patients with biallelic MUTYH. Among these 438 females with an identified PV in a BC risk gene, OC risk gene, or MMR gene, 243 (55.5%) females had 271 subsequent diagnoses of breast, ovarian, endometrial, or colorectal cancer associated with their germline PV.
Subsequent genetic cancers among females first diagnosed with breast cancer. Legend: Pts, patients; PV(s), pathogenic or likely pathogenic variant(s). Top: Box 1 includes breast cancer genes (ATM, BARD1, BRCA1/2, CDH1, CHEK2, NBN 657del, NF1, PALB2, PTEN, RAD51C/D, and TP53). Box 2 includes ovarian cancer genes (BRCA1/2, BRIP1, PALB2, RAD51C/D and MMR genes. Box 3 includes mismatch repair genes (MLH1, MSH2, MSH6, and PMS2). Bottom: Venn diagrams display overlapped patients from above as follows: Boxes 1 and 2, n = 227; Boxes 2 and 3, n = 41; Boxes 4 and 5, n = 23; Boxes 5 and 6, n = 2; Boxes 6 and 7, n = 3. As we excluded patients with multiple mutations, there were no overlapped patients for Boxes 1 and 3
Among females diagnosed first with OC, 13.4% (13/97) had a PV in a BC risk gene or an MMR gene (Online Resource, Supplemental Fig. 2). Of the 10 patients who had a PV in a BC risk gene, 60.0% (n = 6) developed a subsequent BC.
Discussion
In this selected cohort of females with breast cancer and a non-breast cancer diagnosis, 14.1% (932/6617) had a single PV in one of 21 hereditary cancer genes. Owing to early detection and targeted therapies, BC survivorship has improved, and identifying BC patients with germline cancer susceptibility provides an opportunity to enhance their care through cancer prevention or interception.
Our findings expand on prior work where the frequency of non-BRCA1/BRCA2 PVs for women with MPCs (BC and another cancer diagnosis, including a second BC diagnosis) was 8.5% (47/551) among those followed in a high-risk BC program [2]. Herein, we report a similar rate of non-BRCA1/BRCA2 PVs (7.9% [526/6617], 95% CI 7.3–8.6%), despite differences in inclusion criteria. One difference between the cohorts was that the prior study had more females with bilateral or contralateral BC (44.1%, 243/551) compared to our study (15.2%, 1007/6617).
Most patients did not undergo germline MGPT until well after their second cancer diagnosis. The median age at first cancer diagnosis was 49 and was 10 years prior to the median age of second cancer diagnosis at 59. The median age of MGPT was 63 in this cohort, ascertained from 2012 to 2016. This lengthy interval to germline genetic testing is consistent with prior work [9, 17, 18]. One study evaluating genetic test rates over an overlapping time period (from 2012 to 2019) found that only 25.2% of eligible patients with BC and 34.3% of females with OC received germline genetic testing, and many experienced barriers to testing [8]. Prior to 2012, MGPT was not widely available, and utilization of MGPT has increased since then for patients with BC and OC [8].
The greatest prevalence of PVs by cancer combination was among females with BC in combination with gastric cancer (25.0%), OC (22.3%), and pancreatic cancer (20.0%). CHEK2 PVs were frequent in females with BC who also had either thyroid cancer or kidney cancer. This association has been previously described by our group and others [2, 19, 20]. The prevalence of PVs in ATM among females with BC and pancreatic cancer was greater than the prevalence of BRCA2 PVs. This finding, while unexpected, is consistent with recent data delineating the age-specific penetrance of pancreatic cancer among individuals with PVs in ATM.[21]
The prevalence of PVs varied by the order of diagnosis of BC (1st: 16.4%, 2nd: 11.0%, and ≥ 3rd: 14.9%) and these differences were statistically significant. However, in all groups, the PV prevalence was > 10%, indicating that females with BC and another cancer diagnosis have a high rate of harboring a PV in a cancer susceptibility gene. As anticipated, females diagnosed with BC as their first cancer had a younger median age and were more likely to harbor a PV in a high-risk BC gene (BRCA1, BRCA2, CDH1, PALB2, PTEN, and TP53). Interestingly, their median age of genetic testing was older (65) than patients with BC as their second cancer diagnosis (61). This is likely due to secular trends, such as temporal changes in access to and greater utilization of genetic testing for females with BC.
Among the 2,687 patients with a first diagnosis of BC, 16.4% harbored a PV in a BC risk gene (ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NBN- 657del, NF1, PALB2, PTEN, RAD51C, RAD51D, and TP53), 10.5% in an OC risk gene ((BRCA1, BRCA2, BRIP1, PABL2, RAD51C, RAD51D and MMR genes)), and 1.5% in an DNA MMR gene (MLH1, MSH2, MSH6, and PMS2). There were 271 subsequent cancers identified in 243 of the 438 females with PVs that may have been intercepted through precision surveillance and/or prevention. These are all cancers for which there is enhanced surveillance or risk reduction based on the PV as recommended by the NCCN Guidelines® [16]. For females with PVs in BRCA1, BRCA2, BRIP1, PABL2, RAD51C, RAD51D, and MMR genes, bilateral salpingo-oophorectomies are the standard of care. For females with PVs in MMR genes, colorectal and uterine cancers can be prevented through frequent colonoscopies and hysterectomy, respectively. Interception of these cancers in females with germline PVs is associated with reduced morbidity and reduced mortality.
Our understanding of subsequent BCs and contralateral BCs is evolving, and data show that females with a PV in BRCA1, BRCA2, PALB2, TP53, or the CHEK2 1100del PV have a high risk of a subsequent and/or contralateral BCs in particular, if their first BC was diagnosed at a young age [1, 3, 22,23,24]. Moreover, about one-fifth of subsequent BCs (22.5%, 86/383) were in females with a PV in a BC risk gene.
In 2019, the American Society of Breast Surgeons released a consensus statement that germline genetic testing should be available for all patients with BC, although they acknowledged this approach may not be feasible [25]. In June 2021, when the OlympiA study showed a role for adjuvant olaparib for patients with high-risk Stage 2–3 BC and a germline PV in BRCA1 or BRCA2,[7], the NCCN Guidelines® endorsed germline testing for any females with a new diagnosis of nonmetastatic BC who would be candidates for adjuvant PARP inhibitor therapy [26]. With the OlympiA results, there have been calls to expand testing to all patients with BC regardless of stage, tumor subtype, and clinical status [27, 28]. Experts have advocated for genetic testing to be listed on the WHO Essential Diagnostic List for patients with cancer regardless of race, ethnicity, ancestry, and geography [5]. Universal testing for all patients with cancer has been explored in research settings with many actionable results identified [29,30,31]. The President’s Cancer Panel recommends “an assessment of eligibility for germline genetic testing for all people diagnosed with cancer” [32] and calls grow for the implementation of germline genetic testing for all patients with solid organ cancers since the results impact clinical care [5, 29, 31].
Limitations of this study include the ascertainment biases inherent in a cohort selected for genetic testing for cancer predisposition. Our study population had to have survived their first cancer to be diagnosed with a subsequent cancer. Females with MPCs, including BC, may have been more likely to have germline genetic testing if diagnosed with their first cancer diagnosis at a younger age (median age of 48) or if they have a family history of cancer. The testing population is racially homogenesis (predominately White). In this cohort of females with BC, some may have had genetic testing with smaller panels at other laboratories (i.e., BRCA1/BRCA2 only) prior to having testing for these 21 genes. Variant classification and the clinical impact of specific variants may be refined over time (e.g., CHEK2 low-risk variants I157T, S428F, and T476M)[20]. Next, our analyses were limited to the 21 selected genes, and it is possible that if we evaluated more genes, such as BAP1, CDKN2A, FH, MEN1, MEN2, MITF, POT1, RET, SDHx, TERT, and VHL, additional cancers could be intercepted. For patients found to have genetic risk of melanoma, endocrine neoplasms, and/or renal cell carcinoma, there are efficacious guidelines for screening that should be utilized [33,34,35]. We have previously reported on the frequency of unexpected germline PVs among patients with BC [36, 37] and on intercepted genetic cancers [38]. Information on BC hormone receptor subtype was only available for some patients and therefore was not analyzed. Females with multiple PVs were removed from the analytic cohort and are the subject of a separate manuscript. While compelling, the subgroup analysis of opportunities for interception of subsequent cancers among females with PVs first diagnosed with BC or OC may be conservative. For example, we did not include females with multiple PVs, and females with ATM or BRCA2 PVs who later developed pancreatic cancer, despite progress in earlier detection of pancreatic cancer [39,40,41,42]. While we provide a conservative estimate of opportunities for cancer interception, this is reasonable, as some patients may not have access to intensive care for a germline PV. Despite these limitations, a strength of this work is that, short of counterfactual modeling[43] or randomized trials of genetic testing, the opportunities for subsequent cancer interception would be indeterminable.
The high prevalence (> 10%) of PVs among patients with breast cancer and a non-breast cancer diagnosis supports germline MGPT of this population, irrespective of diagnostic order of BC, age at their cancer diagnoses, breast tumor subtype, BC stage, and type of non-BC. With expanding indications for germline genetic testing in breast oncology, including identifying females with nonmetastatic Stage II-III disease who are candidates for adjuvant olaparib, more PVs will be identified. The identification of PVs in females with BC not only informs their cancer treatment, but also their risk for subsequent cancers, and opportunities for interception of those cancers for themselves and for their families. Since outcomes are worse for patients with a subsequent cancer diagnosis compared to patients with an initial cancer diagnosis for most cancer types [44], opportunities for reducing subsequent cancer morbidity and mortality in females with breast cancer and pathogenic variants are increasingly important.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
13 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10549-023-06928-w
References
Sung H, Freedman RA, Siegel RL, Hyun N, DeSantis CE, Ruddy KJ, Jemal A (2021) Risks of subsequent primary cancers among breast cancer survivors according to hormone receptor status. Cancer 127(18):3310–3324
Bychkovsky BL, Lo MT, Yussuf A, Horton C, Richardson M, LaDuca H, Garber JE, Rana HQ (2021) Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer 128(6):1275–1283. https://doi.org/10.1002/cncr.34056
Yao KK, Clifford J, Li S, LaDuca H, Hulick P, Gutierrez S, Black MH (2020) Prevalence of Germline Pathogenic and Likely pathogenic variants in patients with second breast cancers. JNCI Cancer Spectr 4(6):pkaa094
Maxwell KN, Wenz BM, Kulkarni A, Wubbenhorst B, D’Andrea K, Weathers B, Goodman N, Vijai J, Lilyquist J, Hart SN et al (2020) Mutation rates in cancer susceptibility genes in patients with breast cancer with multiple primary cancers. JCO Precis Oncol 4:916–925
Bychkovsky B, Rana HQ, Ademuyiwa F, Plichta J, Anderson K, Nogueira-Rodrigues A, Santa-Maria CA, Coffman LG, Marquez C, Das A et al (2022) Call for action: expanding global access to hereditary cancer genetic testing. Lancet Oncol 23(9):1124–1126
Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Doubeni CA, Epling JW Jr, Kubik M et al. From United States Preventative Services Task Force (USPSF), (2019) Risk Assessment, genetic counseling, and genetic testing for BRCA-related cancer: USPSTF recommendation statement. JAMA: J Am Med Assoc 322(7):652–665
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A, Balmana J et al (2021) Adjuvant Olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 384(25):2394–2405
Kurian AW, Ward KC, Abrahamse P, Bondarenko I, Hamilton AS, Deapen D, Morrow M, Berek JS, Hofer TP, Katz SJ (2021) Time trends in receipt of germline genetic testing and results for women diagnosed with breast cancer or ovarian cancer, 2012–2019. J Clin Oncol 39(15):1631–1640
Rana HQ, Kipnis L, Hehir K, Cronin A, Jaung T, Stokes SM, Fekrmandi F, Vatnick D, Matulonis UA, Garber JE et al (2021) Embedding a genetic counselor into oncology clinics improves testing rates and timeliness for women with ovarian cancer. Gynecol Oncol 160(2):457–463
Morrow A, Chan P, Tucker KM, Taylor N (2021) The design, implementation, and effectiveness of intervention strategies aimed at improving genetic referral practices: a systematic review of the literature. Genet Med 23(12):2239–2249
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS et al (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379(26):2495–2505
Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A et al (2021) Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22(12):1721–1731
Blackburn EH (2011) Cancer interception. Cancer Prev Res (Phila) 4(6):787–792
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17(5):405–424
Pesaran T, Karam R, Huether R, Li S, Farber-Katz S, Chamberlin A, Chong H, LaDuca H, Elliott A (2016) Beyond DNA: an integrated and functional approach for classifying germline variants in breast cancer genes. Int J Breast Cancer 2016:2469523
National Comprehensive Cancer Network (NCCN) (2023) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed 29 Oct 2022
Bednar EM, Oakley HD, Sun CC, Burke CC, Munsell MF, Westin SN, Lu KH (2017) A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol Oncol 146(2):399–404
Pederson HJ, Hussain N, Noss R, Yanda C, O’Rourke C, Eng C, Grobmyer SR (2018) Impact of an embedded genetic counselor on breast cancer treatment. Breast Cancer Res Treat 169(1):43–46
Naslund-Koch C, Nordestgaard BG, Bojesen SE (2016) Increased Risk for other cancers in addition to breast cancer for CHEK2*1100delC Heterozygotes estimated from the copenhagen general population study. J Clin Oncol 34(11):1208–1216
Bychkovsky BL, Agaoglu NB, Horton C, Zhou J, Yussuf A, Hemyari P, Richardson ME, Young C, LaDuca H, McGuinness DL et al (2022) Differences in Cancer phenotypes among frequent CHEK2 Variants and implications for clinical care-checking CHEK2. JAMA Oncol 8:1598
Hsu FC, Roberts NJ, Childs E, Porter N, Rabe KG, Borgida A, Ukaegbu C, Goggins MG, Hruban RH, Zogopoulos G et al (2021) Risk of Pancreatic cancer among individuals with pathogenic variants in the ATM gene. JAMA Oncol 7(11):1664–1668
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE, Milne RL, Andrieu N et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416
Weischer M, Nordestgaard BG, Pharoah P, Bolla MK, Nevanlinna H, Van’t Veer LJ, Garcia-Closas M, Hopper JL, Hall P, Andrulis IL et al (2012) CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J Clin Oncol 30(35):4308–4316
Yadav S, Boddicker N, Na J, Polley EC, Hu C, Hard SN, Gnanaolivu R, Larson N, Hottegaard S, Huang H, et al (2023) Contralateral breast cancer risk among carriers of germline pathogenic variants in ATM, BRCA1, BRCA2, CHEK2 and PALB2. J Clin Oncol. https://doi.org/10.1200/JCO.22.01239
Weiss A, Kuerer HM and Boughey JC. (2020) Genetic testing for all breast cancer patients: Is this becoming a reality? American College of Surgeons. Available at: https://bulletin.facs.org/2020/01/genetic-testing-for-all-breast-cancer-patients-is-this-becoming-a-reality/. Accessed 29 Oct 2022
National Comprehensive Cancer Network (NCCN) (2023) Breast cancer. Version 3.2023. Available at:https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 15 Mar 2023
Narod SA (2021) Adjuvant olaparib—should all patients with breast cancer have genetic testing? Nat Rev Clin Oncol 18(10):607–608
Mittal A, Pramanik R (2021) ASO author reflections: germline testing for all patients with breast cancer: has the time finally come? Ann Surg Oncol 29:1433
Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, Kunze KL, Golafshar M, Uson PLS Jr, Mountjoy L et al (2021) Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol 7(2):230–237
Bychkovsky BL and Rana HQ. Beyond BRCA1/2 2021 Two Large Studies Shed Light on Moderate Breast Cancer Genes. ASCO Daily News. July 28,. Available at: https://dailynews.ascopubs.org/do/beyond-brca1-2-two-large-studies-shed-light-moderate-breast-cancer-genes. Accessed 29 Oct 2022
Esplin ED, Nielsen SM, Bristow SL, Garber JE, Hampel H, Rana HQ, Samadder NJ, Shore ND, Nussbaum RL (2022) Universal germline genetic testing for hereditary cancer syndromes in patients with solid tumor cancer. JCO Precis Oncol 6:e2100516
President’s Cancer Panel. 2022 CLOSING GAPS IN CANCER SCREENING: Connecting People, Communities, and Systems to Improve Equity and Access. A Report from the President s Cancer Panel to the President of the United States. Bethesda (MD). https://prescancerpanel.cancer.gov/report/cancerscreening/pdf/PresCancerPanel_CancerScreening_Feb2022.pdf. Accessed 2 Feb 2023
Schultz KAP, Rednam SP, Kamihara J, Doros L, Achatz MI, Wasserman JD, Diller LR, Brugieres L, Druker H, Schneider KA et al (2017) PTEN, DICER1, FH, and their associated tumor susceptibility syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res 23(12):e76–e82
Amar L, Pacak K, Steichen O, Akker SA, Aylwin SJB, Baudin E, Buffet A, Burnichon N, Clifton-Bligh RJ, Dahia PLM et al (2021) International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat Rev Endocrinol 17(7):435–444
Al-Salameh A, Cadiot G, Calender A, Goudet P, Chanson P (2021) Clinical aspects of multiple endocrine neoplasia type 1. Nat Rev Endocrinol 17(4):207–224
Culver S, Kipnis L, Stokes S, Bychkovsky BL, Scheib R, Rana H, and Garber J. 2018 Casting a wide net: Finding actionable results in non-breast cancer (BC) genes on multi-gene panel testing (MGPT) in a BC cohort. San Antonio Breast Cancer Symposium (SABCS). Poster P4–3–02. .
Rohanizadegan M, Kipnis L, Stokes S, Bychkovsky BL, Scheib RG, Rana HQ, Garber JE. 2021 Casting a Wide Net: Finding actionable results in non-breast cancer genes on multi-gene panel testing in a breast cancer cohort. ASHG. Poster.
Ghazani AA, Breen KM, Dwan M, Barletta JA, Vatnick DR, Stokes SM, Block C, Doherty GM, Cohn AY, Marqusee E et al (2020) Unexpected pathogenic RET pV804M variant leads to the clinical diagnosis and management of medullary thyroid carcinoma. Am J Case Rep 21:e927415
Canto MI, Kerdsirichairat T, Yeo CJ, Hruban RH, Shin EJ, Almario JA, Blackford A, Ford M, Klein AP, Javed AA et al (2020) Surgical outcomes after Pancreatic resection of screening-detected lesions in individuals at high risk for developing pancreatic cancer. J Gastrointest Surg 24(5):1101–1110
Biller LH, Wolpin BM, Goggins M (2021) Inherited pancreatic cancer syndromes and high-risk screening. Surg Oncol Clin N Am 30(4):773–786
Overbeek KA, Goggins MG, Dbouk M, Levink IJM, Koopmann BDM, Chuidian M, Konings I, Paiella S, Earl J, Fockens P et al (2021) Timeline of development of pancreatic cancer and implications for successful early detection in high-risk individuals. Gastroenterology 162:772–785
Tanaka H, Tamura K, Abe T, Yoshida T, Macgregor-Das A, Dbouk M, Blackford AL, Borges M, Lennon AM, He J et al (2021) Serum carboxypeptidase activity and genotype-stratified CA19–9 to detect early-stage pancreatic cancer. Clin Gastroenterol Hepatol 20:2267–2275
Hofler M (2005) Causal inference based on counterfactuals. BMC Med Res Methodol 5:28
Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A (2020) Association of First primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA 324(24):2521–2535
Acknowledgements
The authors would like to acknowledge Kaitlyn T. Bifolck and Valerie H. Goldstein for their editorial support. The content is solely the responsibility of the authors.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BLB contributed to investigation, methodology, writing—original draft, writing—review and editing, and visualization. M-TL contributed to data curation, formal analysis, and methodology. AY contributed to data curation and formal analysis. CH contributed to resources, project administration, methodology, visualization, writing—review, and editing. PH contributed to project administration and methodology. HL contributed to data curation, resources, project administration, and methodology. JEG and RS contributed to conceptualization, writing—review, and editing. HQR contributed to conceptualization, investigation, methodology, writing—original draft, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
M-T.L., A.Y., C.H., P.H., and H.L. report employment at Ambry Genetics, a commercial lab. H.L. reports current employment at Color Health. J.E.G. reports institutional research funding from Myriad Genetics, Ambry Genetics and Invitae Genetics; consulting for Helix Genetics (compensation) and Earli (no compensation); leading two clinical trials for Astra-Zeneca; serving on the scientific advisory board of Konica Minolta (no compensation); conducting a sponsored lecture for Clinical Care Options, LLC; Editorial Services publications, President, Fellows of the AACR Academy, and member, Foundation Board of the American Association for Cancer Research; co-scientific director, Breast Cancer Research Foundation; board of directors, Facing Our Risk of Cancer Empowered; spousal consulting fees from Novartis Oncology, GTx Pharmaceuticals, Aleta BioTherapeutics and H3 Biomedicine, Inc.; a spousal advisory board membership at Oric Pharmaceuticals; and spousal scientific advisory board memberships at Kronos Bio, Susan G. Komen for the Cure, James P. Wilmot Foundation, Inc., Diane Helis Henry Medical Research Foundation, Adrienne Helis Melvin Medical Research Foundation, and Global Biological Standards Institute.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. This study was exempt from review by the Western Institutional Review Board.
Waiver of consent
This study was exempt from review by the Western Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous presentation: These data were presented at the SABCS 2021 Annual Meeting.
The original online version of this article was revised: In the original publication of the article, the reference citations in the text have been incorrectly processed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bychkovsky, B.L., Lo, MT., Yussuf, A. et al. Pathogenic variants among females with breast cancer and a non-breast cancer reveal opportunities for cancer interception. Breast Cancer Res Treat 200, 63–72 (2023). https://doi.org/10.1007/s10549-023-06870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06870-x