Abstract
Background
Knowledge of a germline pathogenic/likely pathogenic variant (PV) may inform breast cancer management. BRCA1/2 PV often impact surgical decisions, but data for multi-gene panel testing are lacking. Expedited genetic testing reduces turn-around times based on request for treatment-related decision making. This report aims to describe the clinical utility of expedited multi-gene panel testing for patients with newly diagnosed breast cancer.
Methods
Clinical and demographic information were reviewed for patients with newly diagnosed female breast cancer undergoing expedited panel testing between 2013 and 2017. The National Comprehensive Cancer Network guidelines (NCCN, version 1.2018) were evaluated in terms of published management recommendations for the genes in which PVs were identified.
Results
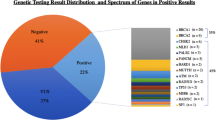
The overall PV yield was 9.5% (678/7127) for women undergoing expedited panel testing, with 700 PVs identified among 678 women. PVs were identified in genes other than BRCA1/2 in 55.9% (391/700) of cases. The NCCN guidelines recommend management for the genes in which 96.6% (676/700) of PVs are identified. The NCCN guidelines also recommend risk-reducing mastectomy for 46.0% (322/700) of PVs identified. An additional 45.6% (319/700) of PVs were identified in genes for which NCCN recommends mastectomy based on family history. In addition, 49.9% (349/700) of PVs were in genes with NCCN guidelines recommending prophylactic surgery for tissues other than breast.
Conclusion
A majority of the patients with newly diagnosed breast cancer were candidates for surgical intervention according to the NCCN guidelines, and half of these patients would have been missed if only BRCA1/2 testing had been ordered. Expedited multi-gene hereditary cancer panel testing should be considered as a first-line approach to provide comprehensive information for breast cancer management.

Similar content being viewed by others
References
Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:3660–7. https://doi.org/10.1200/jco.2015.63.0996.
Lynce F, Isaacs C. How far do we go with genetic evaluation? Gene, panel, and tumor testing. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Meet. 2016;35:e72–78. https://doi.org/10.14694/edbk_160391.
Southey MC, Winship I, Nguyen-Dumont T. PALB2: research reaching to clinical outcomes for women with breast cancer. Hered Cancer Clin Pract. 2016;14:9. https://doi.org/10.1186/s13053-016-0049-2.
Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35:2232–9. https://doi.org/10.1200/jco.2016.71.6480.
Chiba A, Hoskin TL, Hallberg EJ, et al. Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol. 2016;23:3232–8. https://doi.org/10.1245/s10434-016-5328-7.
Evans DGR, Lalloo F, Hopwood P, et al. Surgical decisions made by 158 women with hereditary breast cancer aged < 50 years. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2005;31:1112–8. https://doi.org/10.1016/j.ejso.2005.05.007.
Lokich E, Stuckey A, Raker C, Wilbur JS, Laprise J, Gass J. Preoperative genetic testing affects surgical decision making in breast cancer patients. Gynecol Oncol. 2014;134:326–30. https://doi.org/10.1016/j.ygyno.2014.05.028.
Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:1823–9. https://doi.org/10.1200/jco.2004.04.086.
Weitzel JN, McCaffrey SM, Nedelcu R, MacDonald DJ, Blazer KR, Cullinane CA. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg Chic Ill 1960. 2003;138:1323–8; discussion 1329. https://doi.org/10.1001/archsurg.138.12.1323.
Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res Phila Pa. 2010;3:1026–34. https://doi.org/10.1158/1940-6207.capr-09-0130.
Murphy AE, Hussain L, Ho C, et al. Preoperative panel testing for hereditary cancer syndromes does not significantly impact time to surgery for newly diagnosed breast cancer patients compared with BRCA1/2 testing. Ann Surg Oncol. 2017;24:3055–9. https://doi.org/10.1245/s10434-017-5957-5.
Kapoor NS, Curcio LD, Blakemore CA, et al. Multigene panel testing detects equal rates of pathogenic BRCA1/2 mutations and has a higher diagnostic yield compared to limited BRCA1/2 analysis alone in patients at risk for hereditary breast cancer. Ann Surg Oncol. 2015;22:3282–8. https://doi.org/10.1245/s10434-015-4754-2.
O’Leary E, Iacoboni D, Holle J, et al. Expanded gene panel use for women with breast cancer: identification and intervention beyond breast cancer risk. Ann Surg Oncol. 2017;24:3060–6. https://doi.org/10.1245/s10434-017-5963-7.
Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226:560–5. https://doi.org/10.1016/j.jamcollsurg.2017.12.037.
Ricker C, Culver JO, Lowstuter K, et al. Increased yield of actionable mutations using multi-gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genet. 2016;209:130–7. https://doi.org/10.1016/j.cancergen.2015.12.013.
Yadav S, Reeves A, Campian S, Sufka A, Zakalik D. Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis. Hered Cancer Clin Pract. 2017;15:11. https://doi.org/10.1186/s13053-017-0071-z.
Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–94. https://doi.org/10.6004/jnccn.2010.0043.
The American Society of Breast Surgeons Board of Directors. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. March 2017. Retrieved 12 March 2018 at https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf.
Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205–10. https://doi.org/10.1073/pnas.1415979111.
King M-C, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091–2. https://doi.org/10.1001/jama.2014.12483.
Norum J, Grindedal EM, Heramb C, et al. BRCA mutation carrier detection. a model-based cost-effectiveness analysis comparing the traditional family history approach and the testing of all patients with breast cancer. ESMO Open. 2018;3:e000328. https://doi.org/10.1136/esmoopen-2018-000328.
Roberts ME, Jackson SA, Susswein LR, et al. MSH6 and PMS2 germline pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med Off J Am Coll Med Genet. January 2018. https://doi.org/10.1038/gim.2017.254.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015;17:405–24. https://doi.org/10.1038/gim.2015.30.
Sequence Variant Nomenclature. Retrieved 18 April 2018 at http://varnomen.hgvs.org/.
Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–9. https://doi.org/10.1038/ng879.
Neuhausen S, Dunning A, Steele L, et al. Role of CHEK2*1100delC in unselected series of non-BRCA1/2 male breast cancers. Int J Cancer. 2004;108:477–8. https://doi.org/10.1002/ijc.11385.
Schmidt MK, Hogervorst F, van Hien R, et al. Age- and tumor subtype-specific breast cancer risk estimates for CHEK2*1100delC carriers. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:2750–60. https://doi.org/10.1200/jco.2016.66.5844.
NCCN Guidelines. Genetic/Familial High-Risk Assessment: Colorectal. Version 3.2017. Retrieved 18 October 2017 at https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
Cybulski C, Górski B, Huzarski T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75:1131–5. https://doi.org/10.1086/426403.
Teodorczyk U, Cybulski C, Wokołorczyk D, et al. The risk of gastric cancer in carriers of CHEK2 mutations. Fam Cancer. 2013;12:473–8. https://doi.org/10.1007/s10689-012-9599-2.
Leedom TP, LaDuca H, McFarland R, Li S, Dolinsky JS, Chao EC. Breast cancer risk is similar for CHEK2 founder and non-founder mutation carriers. Cancer Genet. 2016;209:403–7. https://doi.org/10.1016/j.cancergen.2016.08.005.
Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53. https://doi.org/10.1056/nejmoa1603144.
Thompson ER, Rowley SM, Li N, et al. Panel testing for familial breast cancer: calibrating the tension between research and clinical care. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:1455–9. https://doi.org/10.1200/jco.2015.63.7454.
Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190–6. https://doi.org/10.1001/jamaoncol.2017.0424.
Slavin TP, Maxwell KN, Lilyquist J, et al. The contribution of pathogenic variants in breast cancer susceptibility genes to familial breast cancer risk. NPJ Breast Cancer. 2017;3:22. https://doi.org/10.1038/s41523-017-0024-8.
Antoniou AC, Casadei S, Heikkinen T, et al. Breast cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. https://doi.org/10.1056/nejmoa1400382.
NCCN Guidelines. Gastric Cancer. Version 5.2017. Retrieved 18 October 2017 at https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74. https://doi.org/10.1136/jmedgenet-2015-103094.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theobald, K.A., Susswein, L.R., Marshall, M.L. et al. Utility of Expedited Hereditary Cancer Testing in the Surgical Management of Patients with a New Breast Cancer Diagnosis. Ann Surg Oncol 25, 3556–3562 (2018). https://doi.org/10.1245/s10434-018-6581-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6581-8