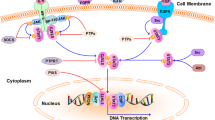
Abstract
Purpose
Triple-negative breast cancer (TNBC) represents the worst prognostic subtype of breast cancer and lacks targeted therapeutic drugs. Signal transducer and activator of transcription 3 (STAT3) is overexpressed and constitutively activated in TNBCs and associated with poor patient outcomes. However, no agents targeting STAT3 have been successfully developed and marketed. Selective Estrogen Receptor Modulators (SERMs) have been reported as potential inhibitors of the IL-6/STAT3 signaling pathway. Naphthalene compounds have good pharmacological activity and significant anti-cancer activity. In this study, we synthesized a new series of naphthalene derivatives with the general structure of SERM and evaluated their effects on TNBC and STAT3 signals.
Methods
A new series of compounds based on the scaffold of SERMs and an amino group were designed and screened based on the structure–activity relationship by MTT assay. The binding activity of SMY002 to STAT3 was predicted and validated by docking and SPR. The STAT3 signaling target and anti-cancer effects of SMY002 were evaluated with three TNBC cell lines and the mice transplanted tumor model.
Results
Among the compounds, SMY002 displayed the most potent activity, which could directly interact with STAT3 SH2-domain, and strongly inhibit the phosphorylation, dimerization, nuclear distribution, transcriptional activity, and target genes expression of STAT3. Furthermore, SMY002 markedly suppressed migration, invasion, survival, growth, and metastasis of TNBC cells in vitro and in vivo via down-regulating the expression of Cyclin D1 and MMP9.
Conclusions
SMY002 can significantly inhibit the growth and metastasis of TNBC cells by targeting the STAT3 signal.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- STAT3:
-
Signal transducer and activator of transcription 3
- TNBC:
-
Triple-negative breast cancer
- SERM:
-
Selective estrogen receptor modulator
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- GP130:
-
Glycoprotein 130 KD
- JAKs:
-
Janus kinases
- SH2:
-
Src homology domain 2
- MMPs:
-
Matrix-metalloproteases
- Tyr705:
-
Tyrosine 705
- IC50:
-
Half-maximal inhibitory concentration
- DMSO:
-
Dimethyl sulfoxide
- SPR:
-
Surface plasmon resonance
- MTT:
-
3-(4,5-Dimethylthiazol)-2,5-diphenyltetrazolium bromide
- DAPI:
-
4,6-Diamidino-2-phenylindole
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Kumar P, Aggarwal R (2016) An overview of triple-negative breast cancer[J]. Arch Gynecol Obstet 293(2):247–269. https://doi.org/10.1007/s00404-015-3859-y
Jhan JR, Andrechek ER (2017) Triple-negative breast cancer and the potential for targeted therapy[J]. Pharmacogenomics 18(17):1595–1609. https://doi.org/10.2217/pgs-2017-0117
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3[J]. Nat Rev Cancer 9(11):798–809. https://doi.org/10.1038/nrc2734
Darnell JJ, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins[J]. Science 264(5164):1415–1421. https://doi.org/10.1126/science.8197455
Katz M, Amit I, Yarden Y (2007) Regulation of MAPKs by growth factors and receptor tyrosine kinases[J]. Biochim Biophys Acta 1773(8):1161–1176. https://doi.org/10.1016/j.bbamcr.2007.01.002
Klemm JD, Schreiber SL, Crabtree GR (1998) Dimerization as a regulatory mechanism in signal transduction[J]. Annu Rev Immunol 16:569–592. https://doi.org/10.1146/annurev.immunol.16.1.569
Leslie K, Lang C, Devgan G et al (2006) Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3[J]. Cancer Res 66(5):2544–2552. https://doi.org/10.1158/0008-5472.CAN-05-2203
Grad JM, Zeng XR, Boise LH (2000) Regulation of Bcl-xL: a little bit of this and a little bit of STAT[J]. Curr Opinion Oncol 12(6):543–549. https://doi.org/10.1097/00001622-200011000-00006
Li W, Lee MR, Kim T et al (2018) Activated STAT3 may participate in tumor progression through increasing CD133/survivin expression in early stage of colon cancer[J]. Biochem Biophys Res Commun 497(1):354–361. https://doi.org/10.1016/j.bbrc.2018.02.084
Jia ZH, Jia Y, Guo FJ et al (2017) Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer[J]. PLoS ONE 12(8):e183622. https://doi.org/10.1371/journal.pone.0183622
Wang D, Zheng X, Fu B et al (2019) Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling[J]. EBioMedicine 46:119–132. https://doi.org/10.1016/j.ebiom.2019.07.058
Heichler C, Scheibe K, Schmied A et al (2020) STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis[J]. Gut 69(7):1269–1282. https://doi.org/10.1136/gutjnl-2019-319200
Qin JJ, Yan L, Zhang J et al (2019) STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review[J]. J Exp Clin Cancer Res 38(1):195. https://doi.org/10.1186/s13046-019-1206-z
Ren B, Kwah M, Liu C et al (2021) Resveratrol for cancer therapy: Challenges and future perspectives[J]. Cancer Lett 515:63–72. https://doi.org/10.1016/j.canlet.2021.05.001
Garg M, Shanmugam M, Bhardwaj V et al (2020) The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy[J]. Med Res Rev 41:1291–1336. https://doi.org/10.1002/med.21761
Li M, Yu H (2021) Identification of WP1066, an inhibitor of JAK2 and STAT3, as a KV 1.3 potassium channel blocker[J]. Br J Pharmacol 178(13):2617–2631. https://doi.org/10.1111/bph.15441
Li CH, Xu LL, Jian LL et al (2018) Stattic inhibits RANKL-mediated osteoclastogenesis by suppressing activation of STAT3 and NF-kappaB pathways[J]. Int Immunopharmacol 58:136–144. https://doi.org/10.1016/j.intimp.2018.03.021
Yang L, Lin S, Xu L et al (2019) Novel activators and small-molecule inhibitors of STAT3 in cancer[J]. Cytokine Growth Factor Rev 49:10–22. https://doi.org/10.1016/j.cytogfr.2019.10.005
Li H, Xiao H, Lin L et al (2014) Drug design targeting protein-protein interactions (PPIs) using multiple ligand simultaneous docking (MLSD) and drug repositioning: discovery of raloxifene and bazedoxifene as novel inhibitors of IL-6/GP130 interface[J]. J Med Chem 57(3):632–641. https://doi.org/10.1021/jm401144z
Tian J, Chen X, Fu S et al (2019) Bazedoxifene is a novel IL-6/GP130 inhibitor for treating triple-negative breast cancer[J]. Breast Cancer Res Treat 175(3):553–566. https://doi.org/10.1007/s10549-019-05183-2
Allen SL, Lundberg AS (2011) Amonafide: a potential role in treating acute myeloid leukemia[J]. Expert Opin Investig Drugs 20(7):995–1003. https://doi.org/10.1517/13543784.2011.585756
Casado A, Rosell R, Garcia-Gomez R et al (1996) Phase II study of mitonafide in non-small cell lung cancer (NSCLC)[J]. Invest New Drugs 14(4):415–417. https://doi.org/10.1007/BF00180820
Wang JR, Shen GN, Luo YH et al (2018) The compound 2-(naphthalene-2-thio)-5,8-dimethoxy-1,4-naphthoquinone induces apoptosis via reactive oxygen species-regulated mitogen-activated protein kinase, protein kinase B, and signal transducer and activator of transcription 3 signaling in human gastric cancer cells[J]. Drug Dev Res 79(6):295–306. https://doi.org/10.1002/ddr.21442
Xu X, Kasembeli MM, Jiang X et al (2009) Chemical probes that competitively and selectively inhibit Stat3 activation[J]. PLoS ONE 4(3):e4783. https://doi.org/10.1371/journal.pone.0004783
Shao H, Xu X, Mastrangelo MA et al (2004) Structural requirements for signal transducer and activator of transcription 3 binding to phosphotyrosine ligands containing the YXXQ motif[J]. J Biol Chem 279(18):18967–18973. https://doi.org/10.1074/jbc.M314037200
Grimme S, Antony J, Ehrlich S et al (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. J Chem Phys 132(15):154104. https://doi.org/10.1063/1.3382344
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading[J]. J Comput Chem 31(2):455–461
Wang W, Dong Z, Zhang J et al (2019) Acute and subacute toxicity assessment of oxyclozanide in wistar rats[J]. Front Vet Sci 6:294. https://doi.org/10.3389/fvets.2019.00294
Sau S, Mondal SK, Kashaw SK et al (2017) Combination of cationic dexamethasone derivative and STAT3 inhibitor (WP1066) for aggressive melanoma: a strategy for repurposing a phase I clinical trial drug[J]. Mol Cell Biochem 436(1–2):119–136. https://doi.org/10.1007/s11010-017-3084-z
Hong D, Kurzrock R, Kim Y et al (2015) AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer[J]. Sci Transl Med 7(314):185r–314r. https://doi.org/10.1126/scitranslmed.aac5272
Bharadwaj U, Eckols TK, Xu X et al (2016) Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma[J]. Oncotarget 7(18):26307–26330
Hayakawa F, Sugimoto K, Harada Y et al (2013) A novel STAT inhibitor, OPB-31121, has a significant anti-tumor effect on leukemia with STAT-addictive oncokinases[J]. Blood Cancer J 3:e166. https://doi.org/10.1038/bcj.2013.63
Ogura M, Uchida T, Terui Y et al (2015) Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies[J]. Cancer Sci 106(7):896–901. https://doi.org/10.1111/cas.12683
Doheny D, Sirkisoon S, Carpenter RL et al (2020) Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis[J]. Oncogene 39(42):6589–6605. https://doi.org/10.1038/s41388-020-01454-1
Gote V, Sharma AD, Pal D (2021) Hyaluronic acid-targeted stimuli-sensitive nanomicelles co-encapsulating paclitaxel and ritonavir to overcome multi-drug resistance in metastatic breast cancer and triple-negative breast cancer cells[J]. Int J Mol Sci 22(3):1257. https://doi.org/10.3390/ijms22031257
Zhu J, Li Z, Zhang G et al (2011) Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3 /AKT signalings[J]. PLoS ONE 6(8):e23720. https://doi.org/10.1371/journal.pone.0023720
Han D, Yu T, Dong N et al (2019) Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells[J]. J Exp Clin Cancer Res 38(1):289. https://doi.org/10.1186/s13046-019-1289-6
Acknowledgements
We acknowledge the National Natural Science Foundation of China for funding the project (Nos. 81803575, 31902287) and the Henan Science and Technology Department of China for financing the project (No. G20190126005). We also thank the Department of Science and Technology, Government of Kaifeng, China, for funding the project (No. 21SSF003)), the Key R&D and promotion projects of Kaifeng (No. 2203008).
Funding
This work was supported by grants from the High-End Foreign Experts Introduction Plan of Henan Science and Technology Department (No. G20190126005), Kaifeng Science and Technology Development Plan Project (No. 21SSF003), Youth Fund of National Natural Science Foundation of China (No. 81803575).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Zhengyan Yang contributed to conceptualization and writing-original draft. Hongyun Xu contributed to investigation and validation. Yupo Yang and Chaoqun Duan contributed to the synthesis and analysis of the compounds. Pai Zhang contributed to the data curation. Yang Wang contributed to resource collection. Kai Fu contributed to methodology and formal analysis. Yonghong Shen contributed to the writing-reviewing, and editing of the manuscript. Marvin Xuejun Xu contributed to funding acquisition and project administration. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Institutional Ethical Committee. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Xu, H., Yang, Y. et al. Synthesis and evaluation of naphthalene derivatives as potent STAT3 inhibitors and agents against triple-negative breast cancer growth and metastasis. Breast Cancer Res Treat 197, 255–267 (2023). https://doi.org/10.1007/s10549-022-06790-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06790-2