Abstract
Purpose
The status of human epidermal growth factor receptor 2 (HER2) is important for treatment decision-making of breast cancer and was commonly determined by core needle biopsy (CNB). The concordance of CNB with surgical excision biopsy (SEB) has been verified, but remain unclear according to the newly developed classification of HER2 status. Our study aimed to re-evaluate the diagnostic value of CNB for determining HER2 status in breast cancer, especially in the HER2-low population.
Methods
Eligible breast cancer patients in West China Hospital between January 1, 2007 and December 31, 2021 were enrolled consecutively and data were extracted from the Hospital Information System. The agreement of HER2 status between CNB and SEB was calculated by concordance rate and κ statistics, as well as the sensitivity, specificity, positive, and negative predictive values (PPV & NPV). Logistic models were used to explore potential factors associated with the discordance between both tests.
Results
Of 1829 eligible patients, 1097 (60.0%) and 1358 (74.2%) were consistent between CNB and SEB by pathological and clinical classifications, respectively, with κ value being 0.46 (0.43–0.49) and 0.57 (0.53–0.60). The sensitivity (50.9%–52.7%) and PPV (50.5%-55.2%) of CNB were especially low among IHC 1+ and 2+/ISH - subgroups by pathological classifications; however, it showed the highest sensitivity (77.5%) and the lowest specificity (73.9%) in HER2-low population by clinical classifications. Advanced N stages might be a stable indicator for the discordance between both tests.
Conclusion
The diagnostic value of CNB was limited for determining HER2 status in breast cancer, especially in HER2-low population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2), which determines histopathological molecular subtypes along with estrogen receptor (ER) and progesterone receptor (PR), is an important indicator for the treatment and prognosis of breast cancer. The HER2 status is mainly assessed using immunohistochemistry (IHC) [1] and was divided into HER2 negative (IHC score 0 and 1+), HER2 equivocal (IHC score 2+), and HER2 positive (IHC score 3+) [2]. In situ hybridization (ISH) is used further for HER2-equivocal cases; tumors that are ISH positive and negative are classified as HER2 positive and negative, respectively [3].
However, in recent years, this traditional classification of HER2 status has been challenged and from a new perspective, the concept of HER2-low (IHC 1+ or 2+/ISH -) tumors was proposed considering this new subtype may possess distinct features [4]. Existing population-based study evidences showed that, comparing to HER2-negative patients, patients with HER2-low tumors would have better overall and disease-free survival outcomes [5], as well as lower pathological complete response [6]. Besides, one comparative analysis of signal pathway mutations derived from second-generation sequencing (NGS) of gene panels among breast cancer patients and demonstrated that there might be more gene mutations in the PI3K-Akt signaling pathway of HER2-low tumors than HER2-positive and HER2-negative tumors [7].
Based on HER2 status, the treatment plan including whether and how to receive anti-HER2 therapy would be developed. For a long time, anti-HER2 therapy, such as dual blockade with trastuzumab+pertuzumab and TDM-1, is only prescribed for HER2-positive breast cancer patients [8], but latest research showed that trastuzumab deruxtecan (T-DXd) shall also benefit patients with HER2-low breast cancer [9, 10], which has been validated by one multicenter, randomized controlled trial (RCT) enrolling 557 patients with HER2-low breast cancer (DESTINY-Breast 04), presenting patients treated by T-DXd had significantly longer progression-free and overall survival time than those by only chemotherapy treatment [11]. This suggested that how to distinguish HER2-low breast cancer patients from HER2-positive or -negative patients is essential for decision-making regarding whether to receive anti-HER2 treatment and selecting appropriate types of anti-HER2 agents, e.g., T-DXd prescribed for HER2-low patients.
Currently, the HER2 status is mainly determined by detecting specimens from core needle biopsy (CNB) and surgical excision biopsy (SEB) [12]. Although SEB is always regarded as gold standards, CNB is more convenient, cost-effective, and is the only alternative detection method nowadays for patients with advanced cancer or those before neoadjuvant treatment, making it become a conventional procedure in many countries [13]. Till now, there has been several studies investigating the concordance between CNB and SEB, but the concordance rate of HER2 status is ambiguous, varying from 56 to 98.3% [14,15,16,17] and those potential factors influencing the agreement of both tests remain unclear.
In view of the development regarding new classification of HER2 status, the comparative effectiveness of HER2-low breast cancer patients from treatment by T-DXd, and the outstanding value of CNB among patients with advanced cancer or those before neoadjuvant therapy, it is urgent for us to further verify the concordance of CNB and SEB, especially to evaluate the diagnostic value of CNB among patients with HER2-low breast cancer. To address all these questions, we conducted this retrospective study based on medical records of female breast cancer from one general referral hospital in southwest China, based on which, we also investigated the epidemiological characteristics of HER2-low tumors comparing with those with positive and negative HER2 expression and explored the possible influential factors that might be associated with the discordance between CNB and SEB.
Methods
Design and patients
This retrospective study was conducted at Breast Center in West China Hospital in Sichuan province, China. All eligible patients diagnosed with breast cancer between January 1, 2007 and December 31, 2021 were enrolled consecutively and medical records of included patients were identified from the Hospital Information System (HIS) database. The data we collected were mainly necessary demographical, clinical, and pathological characteristics, including age, tumor location, histological type, grade, stage (T & N), surgery type, ER, and PR status. The study was approved by the institutional review board in West China Hospital (No. 2022 [671]) and exempt of patient informed consent.
Eligible patients should meet all the following criteria: female; diagnosed with invasive breast cancer; and underwent both CNB and SEB, with both specimens processed by IHC staining to determine HER2 status. Patients were excluded if they met any of the following criteria: received neoadjuvant systemic therapy (NAST); received ipsilateral breast/chest radiotherapy before operation; or IHC 2+ tumors with a gene amplification reported by fluorescence in situ hybridization (FISH) only in CNB or SEB. The reason for excluding patients receiving NAST is, compared to the previous CNB, the tumor tends to present size and pathological changes on SEB resulting from neoadjuvant treatment [18, 19].
Specimen processing
CNBs were obtained with ultrasound guided 14- or 16-gauge core needle. Conventionally, 4-6 samples of the same patient would be acquired (from center to peripheral sections of the lesion) and then fixed in 4% neutral formaldehyde for 8-12 h. SEB were obtained following lumpectomy or mastectomy, first cut tumor samples (removed during the surgery) into 5-mm sections, and last fixation (4% neutral formaldehyde) for 8-24 h. Later, tissue samples were embedded into paraffin and cut into 4-μm-thick sections (Leica RM 2245) prior to analysis. Routinely, Hematoxylin–eosin (HE) staining was performed on each section. IHC analysis were performed with specific antibody (Ventana 4B5) against HER2. The Multimer-Technology based ultraView universal DAB detection kit (Ventana) was used for IHC staining of slides prepared from formalin fixed paraffin embedded tissue on BenchMark Ultra (Ventana), an IHC automatic dyeing system. For each slide, the automated Benchmark system would put it through a series of user-defined de-paraffinization and antigen retrieval steps before commencing with the antibody staining. The primary antibody was either applied automatically in a pre-diluted dispenser or otherwise manually titrated onto the slide. Then the pre-diluted dispensers of the ultraView Universal DAB Detection Kit provided all reagents required for staining, and the staining was proceeding in the BenchMark Ultra system automatically.
IHC test scoring
Slides from the original specimens were reviewed. The evaluation of HER2 status by IHC test scoring were independently performed by two professors with at least 10-year abundant academic and clinical experience in breast pathology. The consistency rate of evaluation results was about 95%, and any inconsistency will be resolved through consensus on a multi-head microscope. The IHC scoring of HER2 status ranges from 0 to 3+ based on membranous staining [3]. 0 refers to no staining or weak membrane staining in < 10% of tumor cells, while incomplete or weak/barely visible membrane staining in ≥ 10% of tumor cells is 1+. 2+ means weak to moderate complete membrane staining in > 10% of tumor cells. Strong intact membrane staining observed in > 10% of tumor cells is regarded as 3+. For sections scoring 2+ in CNBs and SEBs, FISH would be applied for further pathological confirmation. When HER2 gene amplification was reported by FISH, the HER2 status was regarded as positive [3]. IHC 1+ and IHC 2+/FISH - cases were categorized into HER2-low tumors [4]. Since heterogeneity might exist in the results of FISH [20], we cannot ensure the precise status of HER2 when gene amplification was reported by FISH only in CNB or SEB, thus we excluded patients with HER2 gene amplification in only one of the specimens (CNB/SEB).
Statistical analysis
Our dataset had no missing data across variables of our interest. We described demographical, clinical, and pathological characteristics by the HER2 status (negative, low, and positive) in SEB. The numbers and percentages were used for categorical variables, while mean and standard deviation (SD) for continuous variables. The Chi-square test or Fisher’s exact test was used to compare the differences of characteristics between patients with different HER-2 statuses.
The agreement of HER2 status by both pathological and clinical classification between CNB and SEB was measured by concordance rate and kappa (κ) statistics and determined κ values with > 0.80, 0.61-0.80, 0.41-0.6, 0.21-0.4, and < 0.2 as excellent, substantial, moderate, fair, and poor, respectively. The Sankey diagrams was used to visualize the concordance of HER2 status between both tests. The sensitivity, specificity, positive (PPV), and negative predictive values (NPV) of CNB in different pathological and clinical categories of HER2 status by SEB were calculated to comprehensively investigate the value of CNB.
To explore potential influencing factors that might result in discordance between CNB and SEB, we conducted both univariable and multivariable logistic regression analysis, by setting the consistency of CNB and SEB on pathological or clinical classification as reference of dependent variable and included different independent variables in multivariable model according to three strategies: full model, stepwise backward based on Akaike information criterion (AIC), and univariable analysis with P < 0.05, with the area under the ROC curve used for model evaluation. The odds ratio (OR) and 95% confidence interval (CI) of each included factor were calculated.
We conducted all the statistical analyses by SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and R software (R version 4.0.4). The test of two-side P-value less than 0.05 was defined as statistically significant.
Results
Patient characteristics
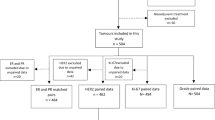
Initially, we identified 5915 breast cancer patients in target hospital and included a total of 1829 eligible patients for analysis (Fig. 1). There were 1016 (55.5%) patients diagnosed as HER2 low in SEB (IHC 1+ and 2+/ISH -), with 462 (25.3%) and 351 (19.2%) patients as HER2 negative and positive, respectively. The demographical, clinical, and pathological characteristics of patients are shown in Table 1. The average age of included patients were 51.1 years (SD: 11.5); 23 (1.3%) located in bilateral breast; 1727 (94.4%) were invasive ductal carcinoma; and histological grade 3, T3-T4 stages, and N2-N3 stages accounted for 51.3%, 9.1%, and 21.2%, respectively. 1658 (90.7%) patients underwent mastectomy surgery. 1324 (72.4%) and 1230 (67.2%) patients were diagnosed as ER positive and PR positive in SEB. Compared to those with HER2-negative or positive tumors, patients with HER2-low tumors were less inclined to be histological Grade 3 and T3-T4 stage; but more likely to be ER and PR positive; all differences were shown statistically significant with P < 0.05. Besides, it seemed that HER2 expression level might increase with age but remained to be verified. (Table 1).
Concordance of HER2 status between CNB and SEB
Figures 2 and 3 illustrate the concordance of HER2 status between CNB and SEB on pathological and clinical classifications, respectively. The concordance rate of HER2 status between CNB and SEB on pathological classification were 1097/1829 (60.0%) overall with κ being 0.46 (0.43-0.49). When we categorized IHC 1+ and IHC 2+/ISH - cases into a HER2-low group, the concordance rate (1358/1829; 74.2%) among three groups (negative/low/positive) increased compared to the initial value of four classes, with a κ value of 0.57 (0.53-0.60) indicating moderate consistency. As shown in Table 2 and Table 3, 15.3% (155/1016) and 7.3% (74/1016) HER2-low tumors would be misclassified as HER2-negative and HER2-positive (3+) tumors by CNB, respectively; meanwhile, 28.4% (131/462) HER2-negative and 23.1% (81/351) HER2-positive tumors would be misclassified as HER2-low tumors by CNB.
Stratified by HER2 status in SEB (reference standard), the sensitivity and PPV of CNB were generally low, especially among IHC 1+ and 2+/ISH - groups, presenting the sensitivity 50.9%-52.7% and PPV 50.5%-55.2%; whereas, the specificity and NPV reached about 80% and more across all HER2 status (Table 4). When incorporating IHC 1+ and 2+/ISH - cases into HER2-low group; however, it showed the highest sensitivity (77.5%) and the lowest specificity (73.9%). This indicated that initial low sensitivities mainly resulted from the poor ability of CNB to distinguish between IHC 1+ and 2+/ISH - tumors, and the CNB was limited in accurately excluding HER2-low tumors.
Factors associated with HER2 discordance between CNB and SEB
A total of 732 (40.0%) patients occurred discordance between CNB and SEB basing on pathological classification, while 471 (25.8%) basing on clinical classification and their distribution across variables of interest are shown in Table 5 and Table 6. The univariable and multivariable analyses showed that advanced N stages were inclined to be a stable indicative factor for the discordance of HER2 status by both pathological and clinical classification between these two tests (N1, N2-3 vs. N0 OR [95%CI] by univariable analysis: pathological discordance 1.34[1.08-1.67], 1.45[1.14-1.85]) and clinical discordance 1.27[0.99-1.63], 1.57[1.21-2.04]).
Since the histological grade and ER status were found significantly associated with PR status (chi-square test: χ2 = 892.8, 152.4, both P < 0.05), these two variables were not included in the following multivariable analysis to avoid collinearity. The multivariable model 1 including all potential influencing factors, model 2 developed by stepwise backward method, and model 3 adjusting only variables based on univariable analysis, all showed no obvious differences from those indicated by univariable analysis, with the area under the ROC curve of these three models being 0.58, 0.56, and 0.56 by pathological classification and 0.59, 0.58, and 0.57 by clinical classification, which suggested that there might be some important unobservable variables contributing to the discordance between CNB and SEB. In addition, it suggested that the PR-positive status and bilateral tumor location were associated with more discordance of HER2 status by pathological and clinical classification, respectively; while the invasive ductal cancer, compared with other histological type, was associated with less discordance of HER2 status by clinical classification.
Discussion
Focusing on the newly proposed concept of HER2-low (IHC 1+ or 2+/ISH -) tumors, and verified effectiveness of trastuzumab deruxtecan (T-DXd) treating HER2-low breast cancer patients by a large RCT, our study addressed the diagnostic value of CNB in determining HER2 status, especially in HER2-low population, based on which, we found moderate concordances between CNB and SEB using either pathological or clinical classification (Concordance rate: 60% and 74%; Kappa value: 0.46 and 0.57); the specificity and NPV of CNB calculated according to three categories seemed not enough for accurately excluding HER2-low tumors, and the CNB might be poor to distinguish IHC 1+ and 2+/ISH - tumors. In addition, our study demonstrated the unique epidemiological characteristics of HER2-low patients comparing with those with HER2-negative or positive tumors and suggested the discordance between CNB and SEB might be more inclined to occur in patients with advanced N stages and some other specific tumor characteristics, but more potential influencing factors remained to be explored.
CNB, as a more convenient and less painful biopsy method, has been widely used in clinical diagnosis and molecular subtyping of breast cancer. Since it obtains tissue instead of merely cells, pathologists could perform IHC staining and other tests on these specimens and determine HER2 status as well as other biomarkers of lesions. Notably, CNB could play an important role in therapy decision-making for patients with advanced tumors or those who would receive neoadjuvant therapy, because surgical excision specimens could not be acquired in all these specific populations, the determinations of biomarkers can only rely on the results of CNB.
The accurate identification of HER2 status means much for the treatment and prognosis of breast cancer. Previously, anti-HER2 therapy can only be used for HER2-positive tumors, but current guidelines recommended such therapy can also be used for those with low HER2 status. Additionally, drugs targeting tumor cells with different HER2 statuses are not the same. HER2-positive tumors can be treated with monoclonal antibodies, tyrosine kinase inhibitors (TKI), antibody - drug conjugates (ADC), and others, while for HER2-low tumors, only T-DXd has proven to be effective [11]. Therefore, if CNB makes an incorrect judgment, it will result in loss of possible treatment, misuse of drugs, and unexpected side effects, thus leading to missing the best time for treatment and waste of medical resources.
The accuracy and reliability of CNBs have been reported in several studies. Meattini et al. reviewed several literatures and summarized the concordance of biomarkers (ER, PR, HER2, and Ki67) between CNB and SEB as 61.5-99.1% [21]. If we focus only on the HER2 state, the consistency varies greatly. In a comparative study of 209 breast cancer patients, the authors calculated HER2 concordances between CNB and SEB of only 56% (κ = 0.392) [17]. However, two other studies supported the reliability and accuracy of CNB in evaluating HER2 status, with a concordance rate of 96.3% (n = 298, κ = 0.894) and 84.8% (n = 1372, κ = 0.684), respectively [22, 23]. In our study, we analyzed the data and evaluated the concordance according to both the pathological and clinical classifications. The pathological classification is based on specialist consensus and guidelines, while the clinical classification is what most scholars used in their studies and what guides the anti-HER2 therapy nowadays. Our findings indicated a limited diagnostic value of CNB reaching an overall concordance rate of 60% (κ = 0.46) by pathological classification and 74.2% (κ = 0.57) by clinical classification, still relatively lower than previous similar studies basing on traditional classification. In our opinion, there exist no unified standards or definition for an appropriate diagnostic value and varied depending on trade-off about the consequences of misdiagnosis, cost of undergoing diagnosis, preferences, and values of clinicians and patients. Specifically for our study, the higher the consistency of CNB with SEB, the better the diagnostic value of CNB when setting SEB as the gold standard.
Specifically, HER2-low tumors might be misclassified as HER2-negative (15.3%) and HER2-positive (7.3%) tumors; HER2-negative (28.4%) and HER2-positive (23.1%) tumors might also be misclassified as HER2-low tumors by CNB. Besides, we investigated the validity of CNB at each IHC score level by SEB (reference standard). Interestingly, among IHC 1+ and 2+/ISH - subgroups, the sensitivity (50.9%-52.7%) and PPV (50.5%-55.2%) were especially low; however, in incorporated HER2-low group (IHC 1+ and 2+/ISH -), the sensitivity elevated while the specificity decreased. This indicated that the low sensitivity of CNB was caused mainly by the poor discrimination between IHC 1+ and 2+/ISH - tumors, although it might not affect treatment decision-making now because anti-HER2 therapy was recommended for both populations. It is noteworthy that the specificity and NPV of CNB in HER2-low tumors were low, which means the CNB is limited accurately excluding HER2-low tumors, thus quite a few HER2-negative tumors might be classified as HER2-low tumors. Incorrect determination may affect therapy decisions for patients, which can lead to improper treatment, severe adverse outcomes, and waste of medical resources. Therefore, caution should be exercised in the utilization of CNB in HER2-low tumors, especially with the increasing demand of effective treatment based on accurate diagnosis, and the current dilemma remains the need for a reliable and sensitive quantitative assay to verify the status of HER2 [24].
Several studies have provided traditional explanations about the discordance between HER2 statuses of CNB and SEB, which included the heterogeneity of tumors, delayed fixation in SEB, and peripheral sampling in CNB [25, 26]. In HER2-low tumors, the role of these factors may be more pronounced, thus affecting the pathological interpretation. Previous studies have shown that HER2-low tumors (especially those scored as IHC 2+) have stronger heterogeneity [27, 28], and HER2-low can be found in both hormone receptor-positive and triple-negative breast cancers [29]. Moreover, HER2-low tumors are more susceptible to poor specimen sampling and processing. One study reported that up to 85% of the patients who scored IHC 0 in local laboratories would be evaluated as IHC 1+ or 2+ in central laboratories, emphasizing the importance of high-quality and standard HER2 evaluations [30]. Variations in staining of HER2-low cases led by different antibodies and kits should also be considered. A global multicenter study used different assays to re-interpret IHC results in traditionally defined HER2-negative patients and reported a HER2-low concordance of 85.1% in Ventana 4B5 assay, while 78.2% in non-Ventana 4B5 assay [31]. In another study, the HER2 status was assessed by Ventana 4B5 and HercepTest antibodies, showing the detection rate of HER2-low tumors was 27.4% and 9.2%, respectively [32]. It hinted that Ventana 4B5 antibody may be more applicable for HER2-low detection, as well as helping developing better detection methods to identify patients with HER2-low tumors who may benefit from targeted therapy. Additionally, pathologists have considerable uncertainty in the interpretation of HER2-low cases, especially between HER2-low and HER2-negative tumors. A study reported that 15% pathologists were in dispute over whether HER2 was evaluated as IHC 1+ or 0 [33], while another study demonstrated a concordance of merely 26% between IHC 0 and IHC 1+ tumors among 18 pathologists [34].
Our study also aimed to explore the pathological and clinical characteristics that may influence the concordance of HER2 status between CNB and SEB. Although the univariable and multivariable analyses by both classifications showed results with small differences, we found that advanced N stages (i.e., lymph node involvement) might be a stable indicator for the discordance between CNB and SEB, while other factors still need further verification, and some potential important factors have not been explored. It is reported that in patients with positive lymph nodes, specific genes exhibited characteristic expression patterns were related to tumor heterogeneity [35], suggesting tumors with lymph node metastases tend to be more heterogeneous in their primary focus than those without axillary involvement. Thus, tumors with a higher N stage tend to feature stronger invasiveness and heterogeneity in their primary cancers, causing IHC discordance when tested at different times.
To solve problems regarding the limited diagnostic value of CNB and insufficient detection methods for determining HER2 status, especially in HER2-low tumors, we proposed several suggestions as follows. One is to improve the quality of CNB by standardized specimen sampling and processing, as well as precise slide interpretation by pathologists; if possible, it is better to confirm the results of HER2 status by SEB. For some special conditions, such as determining HER2 status among patients with advanced cancer or before neoadjuvant therapy, it is necessary to develop some new detection method, or improve the accuracy of CNB, or even IHC itself, by technological innovation or combination with other detection methods. Since low expression of HER2 based on mRNA and protein is common in all breast cancers [36], if we can determine the HER2 expression threshold of anticancer effects of T-DXd, more precise therapy decisions will be made. Whether the current HER2 detection methods are sensitive enough to accurately define the threshold or lower limit of HER2 expression in the beneficial population is an issue worth paying attention to in future.
This might be the first population-based study addressing the diagnostic value of CNB among HER2-low breast cancer patients, which is very important for clinical decision-making contributing to the effectiveness of treatment and prognosis of breast cancer patients. Besides, we used not only the concordance rate, kappa value as usual, but also calculated the sensitivity, specificity, positive, and negative predictive values across different HER2 statuses to comprehensively evaluate the agreement of CNB and SEB on both pathological and clinical classifications.
Our study has some limitations. First, this was a retrospective study in a single hospital, making it subject to selection bias and limited generalizable of such conclusion. Second, there may be variations in specific sampling operations and specimen processing in clinical work resulting from human, machine, or environment that could not be completely avoided. Third, the characteristics of breast cancer patients were not sufficient, limited by data availability in our HIS system, some important variables associated with the discordance of CNB and SEB might not be observed and determined. Therefore, our future work will focus on dealing with these critical issues by collecting more abundant information of extended, heterogeneous population through linking to other data sources or purpose-oriented survey, as well as conducting large, prospective, multicenter studies if possible. Besides, variations in specific sampling operations and specimen processing may exist but will not make a big influence with laboratory work conducted by the department of pathology in West China Hospital, which has been certificated by CAP (College of American Pathologists) and implements strict quality control supporting academic research.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HER2:
-
Human epidermal growth factor receptor 2
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- T-DXd:
-
Trastuzumab deruxtecan
- CNB:
-
Core needle biopsy
- SEB:
-
Surgical excision biopsy
- NAST:
-
Neoadjuvant systemic therapy
- ISH:
-
Fluorescence in situ hybridization
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Farshid G, Bilous M, Morey A, Fox S, Lakhani S, Loi S et al (2019) ASCO/CAP 2018 breast cancer HER2 testing guidelines: summary of pertinent recommendations for practice in Australia. Pathology 51(4):345–348. https://doi.org/10.1016/j.pathol.2019.02.004
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142(11):1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/jco.2018.77.8738
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38(17):1951–1962. https://doi.org/10.1200/jco.19.02488
Li Y, Abudureheiyimu N, Mo H, Guan X, Lin S, Wang Z et al (2021) In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center. China Front Oncol 11:774577. https://doi.org/10.3389/fonc.2021.774577
Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22(8):1151–1161. https://doi.org/10.1016/s1470-2045(21)00301-6
Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L et al (2022) Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med 20(1):142. https://doi.org/10.1186/s12916-022-02346-9
Cesca MG, Vian L, Cristóvão-Ferreira S, Pondé N, de Azambuja E (2020) HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev 88:102033. https://doi.org/10.1016/j.ctrv.2020.102033
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib Study. J Clin Oncol 38(17):1887–1896. https://doi.org/10.1200/jco.19.02318
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20(8):1124–1135. https://doi.org/10.1016/s1470-2045(19)30328-6
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa2203690
Schreuder, Tjan-Heijnen, Vivianne CG, Westenend, Pieter J, Verloop, et al Clinical Auditing as an Instrument for Quality Improvement in Breast Cancer Care in the Netherlands: The National NABON Breast Cancer Audit.
Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K (2010) Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 152(4):238–246. https://doi.org/10.7326/0003-4819-152-1-201001050-00190
Cahill RA, Walsh D, Landers RJ, Watson RG (2006) Preoperative profiling of symptomatic breast cancer by diagnostic core biopsy. Ann Surg Oncol 13(1):45–51. https://doi.org/10.1245/aso.2006.03.047
Chen J, Wang Z, Lv Q, Du Z, Tan Q, Zhang D et al (2017) Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: a report of 1003 cases. Pathol Oncol Res 23(4):769–775. https://doi.org/10.1007/s12253-017-0187-5
Lorgis V, Algros MP, Villanueva C, Chaigneau L, Thierry-Vuillemin A, Nguyen T et al (2011) Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast 20(3):284–287. https://doi.org/10.1016/j.breast.2010.12.007
Ough M, Velasco J, Hieken TJ (2011) A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am J Surg 201(5):692–694. https://doi.org/10.1016/j.amjsurg.2010.02.015
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/s0140-6736(13)62422-8
Berghuis AMS, van Deurzen CHM, Koffijberg H, Terstappen L, Sleijfer S (2019) Real-world data on discordance between estrogen, progesterone, and HER2 receptor expression on diagnostic tumor biopsy versus tumor resection material. Breast Cancer Res Treat 175(2):451–458. https://doi.org/10.1007/s10549-019-05141-y
Polónia A, Caramelo A (2021) HER2 in situ hybridization test in breast cancer: quantifying margins of error and genetic heterogeneity. Mod Pathol 34(8):1478–1486. https://doi.org/10.1038/s41379-021-00813-x
Meattini I, Bicchierai G, Saieva C, De Benedetto D, Desideri I, Becherini C et al (2017) Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature. Eur J Surg Oncol 43(4):642–648. https://doi.org/10.1016/j.ejso.2016.10.025
Chen X, Sun L, Mao Y, Zhu S, Wu J, Huang O et al (2013) Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer 13:390. https://doi.org/10.1186/1471-2407-13-390
You K, Park S, Ryu JM, Kim I, Lee SK, Yu J et al (2017) Comparison of core needle biopsy and surgical specimens in determining intrinsic biological subtypes of breast cancer with immunohistochemistry. J Breast Cancer 20(3):297–303. https://doi.org/10.4048/jbc.2017.20.3.297
Hurvitz SA (2022) DESTINY-changing results for advanced breast cancer. N Engl J Med 387(1):75–76. https://doi.org/10.1056/NEJMe2206661
Douglas-Jones AG, Collett N, Morgan JM, Jasani B (2001) Comparison of core oestrogen receptor (ER) assay with excised tumour: intratumoral distribution of ER in breast carcinoma. J Clin Pathol 54(12):951–955. https://doi.org/10.1136/jcp.54.12.951
Uy GB, Laudico AV, Carnate JM Jr, Lim FG, Fernandez AM, Rivera RR et al (2010) Breast cancer hormone receptor assay results of core needle biopsy and modified radical mastectomy specimens from the same patients. Clin Breast Cancer 10(2):154–159. https://doi.org/10.3816/CBC.2010.n.021
Marchiò C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A (2021) Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 72:123–135. https://doi.org/10.1016/j.semcancer.2020.02.016
Buckley NE, Forde C, McArt DG, Boyle DP, Mullan PB, James JA et al (2016) Quantification of HER2 heterogeneity in breast cancer-implications for identification of sub-dominant clones for personalised treatment. Sci Rep 6:23383. https://doi.org/10.1038/srep23383
Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J et al (2022) Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.2286
Lambein K, Van Bockstal M, Vandemaele L, Geenen S, Rottiers I, Nuyts A et al (2013) Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: clinical and pathobiological relevance. Am J Clin Pathol 140(4):561–566. https://doi.org/10.1309/ajcp4a7ktayhzsoe
Viale G, Niikura N, Tokunaga E, Aleynikova O, Hayashi N, Sohn J et al (2022) Retrospective study to estimate the prevalence of HER2-low breast cancer (BC) and describe its clinicopathological characteristics. J Clin Oncol 40(16):1087. https://doi.org/10.1200/JCO.2022.40.16_suppl.1087
Scott M, Vandenberghe ME, Scorer P, Boothman A-M, Barker C (2021) Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J Clin Oncol 39(15):1021. https://doi.org/10.1200/JCO.2021.39.15_suppl.1021
Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B et al (2021) Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7(1):1. https://doi.org/10.1038/s41523-020-00208-2
Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K et al (2022) Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol 8(4):1–4. https://doi.org/10.1001/jamaoncol.2021.7239
Angarita FA, Oshi M, Yamada A, Yan L, Matsuyama R, Edge SB et al (2022) Low RUFY3 expression level is associated with lymph node metastasis in older women with invasive breast cancer. Breast Cancer Res Treat 192(1):19–32. https://doi.org/10.1007/s10549-021-06482-3
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712. https://doi.org/10.1126/science.2470152
Acknowledgements
Not applicable.
Funding
This study is supported by the funding from the National Natural Science Foundation of China (32071284), West China Hospital, Sichuan University (2022HXFH021) and the National Clinical Center for Geriatrics, West China Hospital, Sichuan University (Z20192011).
Author information
Authors and Affiliations
Contributions
RC analyzed and interpreted the patient data. RC, YQ, and YH were major contributors in writing the manuscript. WL, RY, XZ, YW, QL, ZW, XS, BW, and JC had made substantial contributions to the design of the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The study was approved by the institutional review board in West China Hospital (No. 2022 [671]) and exempt of patient informed consent.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, R., Qi, Y., Huang, Y. et al. Diagnostic value of core needle biopsy for determining HER2 status in breast cancer, especially in the HER2-low population. Breast Cancer Res Treat 197, 189–200 (2023). https://doi.org/10.1007/s10549-022-06781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06781-3