Abstract
Purpose
Screen-detected unilateral non-palpable breast cancer (NPBC) shows favorable prognosis, whereas bilateral breast cancer (BBC), especially synchronous BBC (SBBC) manifests worse survival than unilateral breast cancer (BC). It remains unclear whether screen-detected bilateral NPBC has compromised survival and requires intensified treatment or favorable prognosis and needs de-escalating therapy.
Methods
From 2003 to 2017, 1,075 consecutive NPBC patients were retrospectively reviewed. There were 988 patients with unilateral NPBC (UniNPBC), and 87 patients with ipsilateral NPBC + any contralateral BC [(N + AnyContra) PBC], including 32 patients with bilateral NPBC (BiNPBC) and 55 patients with ipsilateral NPBC + contralateral palpable cancer [(N + Contra) PBC]. Median follow-up time was 91 (48–227) months. Clinicopathological characteristics were compared between UniNPBC and BBC, whereas relapse-free survival (RFS) and overall survival (OS) among BBC subgroups. RFS and OS factors of BBC were identified.
Results
Compared to UniNPBC, patients with screen-detected bilateral BC had more invasive (85.1%, 74.8%), ER negative (26.4%, 17.1%), PR negative (36.8%, 23.5%), triple-negative (21.6%, 8.5%) BC as well as less breast conserving surgery (17.2%, 32.4%), radiotherapy (13.8%, 32.0%) and endocrine therapy (71.3%, 83.9%). 10 year RFS and OS rates of (N + AnyContra) PBC (72.8%, 81.5%), (N + Contra) PBC (60.6%, 73.9%), and synchronous (N + Contra) PBC (58.1%, 70.1%) were significantly compromised compared to UniNPBC (91.0%, 97.2%). RFS factors of BBC included pN3 (p = 0.048), lymphovascular invasion (p = 0.008) and existence of contralateral palpable interval BC (p = 0.008), while the OS relevant factor was pN3 (p = 0.018).
Conclusion
Screen-detected bilateral NPBC including SynBiNPBC and MetaBiNPBC showed good prognosis as UniNPBC so that the therapy of BiNPBC could be de-escalated and optimized according to UniNPBC. Contrarily, screen-detected ipsilateral NPBC with contralateral palpable BC [(N + Contra) PBC] manifested unfavorable survival worse than UniNPBC and synchronous (N + Contra) PBC had the worst survival among all subgroups, implying that these were actually bilateral interval BC and required intensified treatment.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the commonest malignancy worldwide and the leading cause of cancer death in Chinese women younger than 45 years [1,2,3]. It is well established that screen-detected BC has favorable biological behavior and prognosis compared to symptomatic interval breast cancer [4,5,6], which is also true in our previous study on screen-detected non-palpable breast cancer (NPBC) among Chinese asymptomatic women [7]. Meanwhile, we showed in our previous meta-analysis that bilateral breast cancer (BBC), especially synchronous BBC (SBBC) had worse survival compared to unilateral BC (UBC) [8]. However, most studies included in this meta-analysis did not investigate survival of screen-detected BBC, which has increased dramatically over the past 40 years [9]. It remains unclear whether screen-detected bilateral NPBC has compromised prognosis and requires intensified treatment or good survival as unilateral NPBC (UniNPBC) and needs de-escalating therapy. Additionally, it is also a question whether the survival of screen-detected bilateral NPBC would be diversified in view of synchronous or metachronous BBC.
With these questions, we carried out this retrospective study based on long-term follow-up outcomes of a consecutive hospital cohort, to elucidate the specific survival of screen-detected BBC, especially synchronous and metachronous bilateral NPBC and ipsilateral screen-detected NPBC with contralateral palpable interval BC, to provide general and specific data for prognostic evaluation and comparison of screen-detected BBC patient subgroups.
Patients and methods
Ethics statement
This retrospective study was approved by the Ethics Committee of the Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Sciences.
Patient cohort, criteria for SBBC vs MBBC diagnosis and follow-up
There were 1075 consecutive female NPBC patients diagnosed in Dept. Breast Surgery, PUMC Hospital from January 2003 to December 2017. There were 988 patients (91.9%) with unilateral screen-detected NPBC (UniNPBC), and 87 patients (8.1%) with BBC of ipsilateral NPBC + any contralateral BC [(N + AnyContra) PBC], including 32 patients with bilateral screen-detected NPBC (BiNPBC) and 55 patients with ipsilateral screen-detected NPBC and contralateral palpable interval cancer [(N + Contra) PBC]. The inclusion criteria were female BC patients 18–90 years old with at least NPBC on one side diagnosed with ultrasound or mammogram guided hook-wire excisional biopsy or core-needle biopsy. Exclusion criteria were male BC patients, patients in pregnancy, bilateral interval/palpable BC and patients < 18 or > 90 years old.
All immunohistochemistry (IHC) staining of ER and PR of BC in PUMC Hospital would be reported with the positivity of nuclear staining percentage. Before 2010, the ER- and PR-positive BC was defined as ER and PR positive staining ≥ 10% by IHC, while patients included from 2011 to 2017 were judged with criteria of ER and PR staining ≥ 1% in IHC as positive according to the guidelines from the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) [10]. When IHC staining of Her2 was (2 +), fluorescence in situ hybridization (FISH) was used to determine the Her2 status. Before the year of 2013, HER2 to centromere enumerator probe (CEP) 17 ratio on FISH ≥ 2.2 was taken as positive, 1.8–2.2 as equivocal and < 1.8 as negative. Since 2014, the FISH criteria was changed to ≥ 2.0 as positive and < 2.0 as negative [11].
Metachronous BBC (MBBC) was diagnosed if the interval between first and second BC diagnosis was > 6 months [8]. The intervals of the 29 MBBC patients were 12–164 (median 61) months. The 32 BiNPBC patients included 20 synchronous bilateral screen-detected NPBC (SynBiNPBC) and 12 metachronous bilateral NPBC (MetaBiNPBC). The 55 (N + Contra) PBC patients included 38 synchronous bilateral BC with ipsilateral NPBC and contralateral palpable BC [Syn (N + Contra) PBC] as well as 17 metachronous BBC [Meta (N + Contra) PBC]. Among the 17 Meta (N + Contra) PBC patients, 9 NPBC were diagnosed before the contralateral palpable BC (ContraPBC), whereas 8 NPBC came after the ContraPBC.
All patients were followed by telephone call, out-patient clinics records of follow-up examinations or by both measures. The follow-up time was 48–227 (median 91) months.
Comparsion of clinicopathological characteristics and survival
The clinicopathological characteristics were compared between UniNPBC and (N + AnyContra) PBC patients as well as between BiNPBC and (N + Contra) PBC tumors with exclusion of the ContraPBC. The comparisons of Her2 status and subtype were performed among invasive cancers only. The 10 year relapse-free survival (RFS) and overall survival (OS) were compared among UniNPBC, (N + AnyContra) PBC, BiNPBC and subgroups, (N + Contra) PBC and subgroups, all synchronous BBC and all metachronous BBC, as well as among SynBiNPBC, MetaBiNPBC, Syn (N + Contra) PBC, and Meta (N + Contra) PBC.
RFS of synchronous BBC was defined as the interval between diagnoses of index cancer and the first recurrence or metastasis. As for metachronous BBC, the development of contralateral BC would be taken as a relapse event. Thus, RFS of the first cancer was the time interval between its diagnosis and the first RFS event including second (contralateral) cancer, while RFS of the second cancer was the time interval of its diagnosis and the next relapse event. The RFS definition would be further discussed in the Discussion of this article.
Statistical analysis
The quantitative variables were compared with t-test, the categorical variables with chi-square tests, and survival outcomes by the Kaplan–Meier curve method. RFS and OS related prognostic factors of BBC [(N + AnyContra) PBC] were identified, respectively, by Kaplan–Meier univariate analyses and Cox multivariate analyses. The significance threshold was set at p < 0.05. R (v4.1.3) software was used for all of the statistical analyses.
Results
Comparison of clinicopathological characteristics between screen-detected unilateral and bilateral BC
Compared to UniNPBC patients, screen-detected bilateral BC patients [(N + AnyContra) PBC] had more invasive BC (85.1% vs. 74.8%, p = 0.033), ER negative BC (26.4% vs. 17.1%, p = 0.032), PR negative BC (36.8% vs. 23.5%, p = 0.007), triple-negative BC (TNBC, 18.4% vs. 6.4%, p = 0.008), less breast conserving surgery (BCS, 17.2% vs. 32.4%, p = 0.013), less radiotherapy (13.8% vs. 32.0%, p < 0.001), and less endocrine therapy (71.3% vs. 83.9%, p = 0.002) (Table 1). There were no significant differences in age at first BC diagnosis, TNM stage, histological grade, multi-focality, lymphovascular invasion (LVI), Ki67 index, chemotherapy, and Her2-targeted therapy (Table 1).
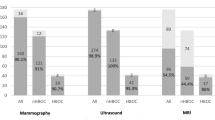
In view of the tumors, when the contralateral palpable BCs were excluded, the 64 screen-detected NPBC of the 32 BiNPBC patients were similar compared to the 55 screen-detected NPBC of 55 (N + AnyContra) PBC patients in terms of age at diagnosis, percentage of invasive BC, TNM stage, histological grade, multi-focality, ER, PR, Her2, Ki67 index, subtype, surgery, radiotherapy, Her2-targeted therapy, and endocrine therapy (Table 2). (N + AnyContra) PBC received more chemotherapy than BiNPBC (43.6% vs. 23.4%, p = 0.019) and showed an insignificant trend of more LVI (9.1% vs. 1.7%, synchronous 15.2% vs. 2.6%, p = 0.094) (Table 2, Fig. 1).
Survival comparison among screen-detected unilateral, bilateral, synchronous and metachronous subgroups of NPBC
In terms of 10-year RFS and OS rates, BiNPBC (94.7% and 92.9%), synchronous (SynBiNPBC, 100% and 100%), and metachronous BiNPBC (MetaBiNPBC, 90.9% and 90.0%) showed similar good prognosis as UniNPBC (91.0% and 97.2%, Table 3, Fig. 2). However, survival of (N + AnyContra) PBC (72.8% and 81.5%), (N + Contra) PBC (60.6% and 73.9%), synchronous (N + Contra) PBC (58.1 and 70.1%) and synchronous BBC [SynBiNPBC + Syn (N + Contra) PBC] (70.0% and 77.4%) were all significantly compromised when compared to those of UniNPBC (all the p < 0.001, Table 3, Figs. 2, 3). The 10-year RFS of BiNPBC was higher than 10-year OS rate due to RFS event happened later than 120 months. The 15-year RFS rate of BiNPBC was 84.2% (HR 65.3–100.0) and the 15-year OS rate 92.9% (HR 80.3–100.0). The 10-year RFS rate of metachronous (N + Contra) PBC (67.7%) and metachronous BBC [MetaBiNPBC + Meta (N + Contra) PBC, 77.1%] were worsened than that of UniNPBC (91.0%, both p < 0.001, Table 3), whereas there was no significant difference among 10-year OS of these three subgroups (87.5% and 89.7% vs. 97.2%, Table 3, Figs. 2, 3).
Comparison of RFS and OS between UniNPBC vs (N + AnyContra) PBC (A, B), UniNPBC vs BiNPBC (C, D), UniNPBC versus (N + Contra) PBC (E, F) and BiNPBC vs (N + Contra) PBC (G, H). BiNPBC showed similar good prognosis as UniNPBC (C, D), whereas survival of (N + AnyContra) PBC and (N + Contra) PBC were significantly worsened than UniNPBC (E–H)
Comparison of RFS and OS between UniNPBC vs synchronous (N + Contra) PBC (A, B), vs metachronous (N + Contra) PBC (C, D), vs all synchronous and metachronous BBC (E, F) and comparison among subgroups of BiNPBC and (N + Contra) PBC (G, H). Synchronous (N + Contra) showed the worst survival (C, D), while SynBiNPBC manifested the best prognosis (G, H)
As for subgroups of screen-detected BBC in terms of synchronous and metachronous BBC, SynBiNPBC showed the best 10-year RFS and OS rate of both 100%. Compared to SynBiNPBC, these prognostic counterparts were significantly worsened of MetaBiNPBC (90.9% and 90.0%), Syn (N + Contra) PBC (58.1% and 70.1%), and Meta (N + Contra) PBC (67.7% and 87.5%) (Table 4, Fig. 3). The 10-year RFS of MetaBiNPBC was higher than 10-year OS rate due to RFS event happened later than 120 months. The 15-year RFS rate of MetaBiNPBC was 77.9% (54.6–100.0) and the 15-year OS rate 90.0% (73.2–100) (Table 4).
Identification of RFS and OS prognostic factors of all screen-detected BBC
Among all screen-detected BBC, the RFS related prognostic factors included pN3 (p = 0.048), lymphovascular invasion (LVI, p = 0.008) and the existence of contralateral palpable interval BC (p = 0.008), while the OS relevant factor was pN3 (p = 0.018) (Table 5).
Discussion
The growing awareness, prolonged lifetime, advancements in diagnostic imaging, and improvements in detection rate from diversified screening had resulted in increased incidence of BBC and SBBC [2, 3, 12, 13]. Studies on survival of screen-detected BBC are difficult to conduct because there are several issues bringing complexity: (1) the heterogeneity of contralateral BC: for ipsilateral NPBC, the contralateral BC could be screen-detected NPBC, screen-detected symptomatic BC or interval BC; (2) the parameter of diagnostic interval: the contralateral BC could be synchronous or metachronous; (3) the parameter of diagnostic sequence: for ipsilateral NPBC with contralateral palpable or interval BC, would it be different whether the ipsilateral NPBC comes first or after the contralateral BC?
To our knowledge, our study was the first to investigate the long-term prognosis of screen-detected synchronous and metachronous BBC with the concern of contralateral palpable or non-palpable BC among Chinese women. The BC screening in China is quite diversified [3, 7, 12, 14, 15] so the screen-detected symptomatic BC and interval BC were usually difficult to differentiate and both documented as contralateral palpable BC in our study. The long-term follow-up of median 91 months was to ensure accurate evaluation of personalized prognosis and sufficient detection of metachronous BBC. To decipher the complexity of prognosis of screen-detected BBC, we choose interval of 6 months between detection of bilateral cancers as the criteria to differentiate SBBC versus MBBC to minimize the confounding effects on comparison of survival [8]. As for the third issue mentioned above, there were only 9 NPBC diagnosed before contralateral palpable BC, whereas 8 NPBC came after, which was too small case number for survival comparison. Studies suggested that risk of third primary cancers of non‐breast origin among women with BBC would also increase, indicating that BBC might be genetically susceptible to develop cancer [16]. Hence we chose RFS in addition to OS as prognostic endpoint so that the relapse events were breast cancer specific and diseases such as third primary cancers including thyroid, lung, pancreas, or cardiovascular diseases, etc. would be excluded as RFS events.
In our study, the development of contralateral BC in MBBC would also be taken as a relapse event. Otherwise, the survival outcome of the first tumor (ipsilateral BC) of MBBC would be over-estimated. For example, the contralateral BC showed different histology or subtype from ipsilateral BC, and then recurrence arose years later from the contralateral BC, if the development of contralateral BC was not taken as a relapse event, it was also unreasonable to take this recurrence from contralateral BC as a relapse event of the ipsilateral BC, then the RFS of the first tumor (ipsilateral BC) would be largely biased and over-estimated too much.
As for the clinicopathological features, study suggested that index cancers of bilateral screen-detected cancers and bilateral interval cancers show significant differences in tumor size, whereas nodal status, receptor status, and final surgical treatment are comparable [17]. Our study showed that the clinicopathological characteristics of screen-detected NPBC in those (N + Contra) PBC patients were similar to those of BiNPBC (Table 2). Taken together, it implied that screen-detected NPBC, either bilateral or ipsilateral, might be different clinical entities with contralateral palpable interval BC, even in the same patient and regardless of synchrony or metachrony.
There were controversies about whether adjuvant therapy for BBC should base on the higher risk tumor or the index tumor [18] and adjuvant chemotherapy might paradoxically both reduce the risk and worsen the prognosis of MBBC [13]. For screen-detected BBC, this paradox also included escalation or de-escalation of the treatment. Our study showed (N + Contra) PBC received more chemotherapy than BiNPBC due to existence of contralateral palpable BC (Table 2), however (N + Contra) PBC still showed worsened survival than BiNPBC, suggesting that the treatment of (N + Contra) PBC should be escalated. Similar to reports from other studies that majority of BBC patients (69.0–76.2%) would usually choose bilateral mastectomy even with young age [9, 19, 20], (N + AnyContra) PBC patients in our study also received more mastectomy compared to UniNPBC (79.3% vs. 67.6%, Table 1).
Study implied that women were more likely to have small breast cancer that was detected in screening than to have earlier detection of a tumor that was destined to become large [5]. In other words, early BC including NPBC could be detected small because they were good in biological behavior rather than they were good in prognosis because they were detected small. Our study results were highly coincided with this. Bilateral NPBC (BiNPBC) was actually screen-detected low-risk BC with similar prognosis as unilateral NPBC (UniNPBC) (Table 3, Fig. 2). Thus, the therapy of BiNPBC could be tailored according to UniNPBC and should not be intensified as those symptomatic SBBC. On the contrary, if the contralateral BC was palpable [(N + Contra) PBC], then it was in essence bilateral symptomatic interval BC with compromised survival (Table 3, Figs. 2, 3). The 10-year OS 70.1% of Syn(N + Contra)PBC (Table 4, Fig. 3) was close to the 10-year OS 71–77% of SBBC reported in our previous study [8]. Hence the treatment of Syn(N + Contra)PBC should be intensified as symptomatic SBBC and the therapy of Meta(N + Contra)PBC should be similar as unilateral symptomatic interval BC. Syn(N + Contra)PBC and SynBiNPBC belonged to distinct clinical entities with different prognoses and thus should be treated differently (Table 4, Figs. 2, 3). Taken together, these results implied that the survival ordered from poor to favorable might be like: Syn(N + Contra)PBC ≤ (N + Contra)PBC ≤ Meta (N + Contra) PBC ≌ symptomatic unilateral BC (UBC) < MetaBiNPBC ≤ SynBiNPBC ≌ BiNPBC ≌ UniNPBC.
The key strength of this study was that the clinicopathological features and survival outcomes were compared between both UniNPBC vs BBC and among subgroups of BBC with long follow-up time of 48–227 (median 91) months. There are several limitations in our study. Firstly, although majority of BBC could not fully be explained by BRCA carriership [18, 21], there was limited germline mutation data about BRCA1/2 and other BC related genes in the current study. Several studies suggest that BBC is one of the related clinical factors that increases the probability of BRCA mutations [9, 22] and remains one of the criteria for recommendation of genetic testing [23]. BRCA mutation rate was about 24% among Chinese women with BBC [22]. Interval breast cancers among BRCA mutation carriers have worse clinicopathologic features than screen-detected tumors, and require more aggressive medical and surgical therapy [6]. Association between BRCA mutation and survival of BBC and screen-detected BBC would be further studied in our future research. Secondly, due to the BC screening conditions in China [3, 7, 12, 14, 15], there was no clear-cut documentation of whether the contralateral palpable BC was screen-detected symptomatic or interval BC between regular screenings, which had overlap but were not identical clinical entities [24]. So they were both regarded as ContraPBC in this study. Thirdly, it was a retrospective single-center study based on hospital population with limited case number. Thus comparison between NPBC as first cancer and NPBC as second cancer among Meta(N + Contra)PBC could not be performed. Last but not the least, the patients enrolled in this study were collected over 15 years (2013.01–2017.12), although the follow-up time was long enough to identify the metachronous BBC with long interval, the improvement of neo/adjuvant therapy would inevitably bring bias to prognosis of BBC due to the heterogeneity in BC treatment over decades.
Conclusion
Compared to UniNPBC, patients with screen-detected bilateral BC had more invasive, ER negative, PR negative, triple-negative BC as well as less breast conserving surgery, radiotherapy, and endocrine therapy. Screen-detected bilateral NPBC including SynBiNPBC and MetaBiNPBC showed good prognosis as UniNPBC so that the therapy of BiNPBC could be de-escalated and optimized according to UniNPBC. Contrarily, screen-detected ipsilateral NPBC with contralateral palpable BC [(N + Contra) PBC] manifested unfavorable survival worse than UniNPBC (p < 0.001) and synchronous (N + Contra) PBC had the worst survival among all subgroups (p < 0.001), implying that these were actually bilateral interval BC and required intensified treatment.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132
Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE (2014) Breast cancer in China. Lancet Oncol 15(7):e279-289
Ahmed M, Douek M (2014) The management of screen-detected breast cancer. Anticancer Res 34(3):1141–1146
Welch HG, Prorok PC, O’Malley AJ, Kramer BS (2016) Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 375(15):1438–1447
Pilewskie M, Zabor EC, Gilbert E, Stempel M, Petruolo O, Mangino D, Robson M, Jochelson MS (2019) Differences between screen-detected and interval breast cancers among BRCA mutation carriers. Breast Cancer Res Treat 175(1):141–148
Pan B, Yao R, Zhu QL, Wang CJ, You SS, Zhang J, Xu QQ, Cai F, Shi J, Zhou YD et al (2016) Clinicopathological characteristics and long-term prognosis of screening detected non-palpable breast cancer by ultrasound in hospital-based Chinese population (2001–2014). Oncotarget 7(47):76840–76851
Pan B, Xu Y, Zhou YD, Yao R, Wu HW, Zhu QL, Wang CJ, Mao F, Lin Y, Shen SJ et al (2019) The prognostic comparison among unilateral, bilateral, synchronous bilateral, and metachronous bilateral breast cancer: a meta-analysis of studies from recent decade (2008–2018). Cancer Med 8(6):2908–2918
Sakai T, Ozkurt E, DeSantis S, Wong SM, Rosenbaum L, Zheng H, Golshan M (2019) National trends of synchronous bilateral breast cancer incidence in the United States. Breast Cancer Res Treat 178(1):161–167
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American society of clinical oncology/college Of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Song QK, Wang XL, Zhou XN, Yang HB, Li YC, Wu JP, Ren J, Lyerly HK (2015) Breast cancer challenges and screening in china: lessons from current registry data and population screening studies. Oncologist 20(7):773–779
Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, Dickman PW, Hall P (2007) Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol 25(27):4210–4216
Breast and Cervical Cancer Screening Project for rural women in China
Shen S, Zhou Y, Xu Y, Zhang B, Duan X, Huang R, Li B, Shi Y, Shao Z, Liao H et al (2015) A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br J Cancer 112(6):998–1004
Kwast AB, Liu L, Roukema JA, Voogd AC, Jobsen JJ, Coebergh JW, Soerjomataram I, Siesling S (2012) Increased risks of third primary cancers of non-breast origin among women with bilateral breast cancer. Br J Cancer 107(3):549–555
van Bommel R, Lameijer JRC, Voogd AC, Nederend J, Louwman MWJ, Setz-Pels W, Strobbe LJ, Tjan-Heijnen VCG, Duijm LE (2018) Tumour characteristics of bilateral screen-detected cancers and bilateral interval cancers in women participating at biennial screening mammography. Eur J Radiol 108:215–221
Holm M, Tjonneland A, Balslev E, Kroman N (2014) Prognosis of synchronous bilateral breast cancer: a review and meta-analysis of observational studies. Breast Cancer Res Treat 146(3):461–475
Pak LM, Gaither R, Rosenberg SM, Ruddy KJ, Tamimi RM, Peppercorn J, Schapira L, Borges VF, Come SE, Warner E et al (2021) Tumor phenotype and concordance in synchronous bilateral breast cancer in young women. Breast Cancer Res Treat 186(3):815–821
Narod SA (2014) Bilateral breast cancers. Nat Rev Clin Oncol 11(3):157–166
Imyanitov EN, Hanson KP (2003) Molecular pathogenesis of bilateral breast cancer. Cancer Lett 191(1):1–7
Wei H, Wang M, Ou J, Jiang W, Tian F, Sheng Y, Li H, Xu H, Zhang R, Guan A et al (2018) Multicenter cross-sectional screening of the BRCA gene for Chinese high hereditary risk breast cancer populations. Oncol Lett 15(6):9420–9428
Network NCC (2022) Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 2
Iwamoto T, Kumamaru H, Miyata H, Tomotaki A, Niikura N, Kawai M, Anan K, Hayashi N, Masuda S, Tsugawa K et al (2016) Distinct breast cancer characteristics between screen- and self-detected breast cancers recorded in the Japanese breast cancer registry. Breast Cancer Res Treat 156(3):485–494
Funding
This work was supported by the Natural Science Foundation of China (No. 81001183), the Science & Technology Research Project of Returned Visiting Scholar, Ministry of Human Resources and Social Security (2015), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1–014, the National High Level Hospital Clinical Research Funding 2022-PUMCH-A-165 and the National High Level Hospital Clinical Research Funding [Whole process management of bilateral breast cancer, PI: Yidong Zhou, co-PI: Bo Pan].
Author information
Authors and Affiliations
Contributions
BP, YX, and YZ contributed to research idea generation, study design, data collection and analysis, and manuscript writing. RY, XZ, and YX contributed to data collection and patients' follow-up. FM, YL, XHZ, SJ, and YLX contributed to methodology, patients’ treatment, and data collection. XR contributed to pathology relevant data collection and manuscript editing. MX and QLZ contributed to ultrasound relevant data collection, writing-review, and editing. LK contributed to mammography relevant data collection and writing-review. QS contributed to project administration, supervision, and writing-review.
Corresponding author
Ethics declarations
Competing interest
The authors have declared that no conflicts of interest.
Ethical approval
This retrospective study was performed in line with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, B., Xu, Y., Zhou, Y. et al. Long-term survival of screen-detected synchronous and metachronous bilateral non-palpable breast cancer among Chinese women: a hospital-based study (2003–2017). Breast Cancer Res Treat 196, 409–422 (2022). https://doi.org/10.1007/s10549-022-06747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06747-5