Abstract
Purpose
Over 50% of breast cancer patients prescribed a 5-year course of daily oral adjuvant endocrine therapy (ET) are nonadherent. We investigated the role of costs and cancer medication delivery mode and other medication delivery factors on adherence.
Methods
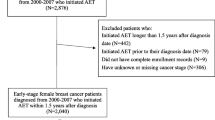
We conducted a retrospective cohort study of commercially insured and Medicare advantage patients with newly diagnosed breast cancer in 2007–2015 who initiated ET. We examined the association between 12-month ET adherence (proportion of days covered by fills ≥ 0.80) and ET copayments, 90-day prescription refill use, mail order pharmacy use, number of pharmacies, and synchronization of medications. We used regression models to estimate nonadherence risk ratios adjusted for demographics (age, income, race, urbanicity), comorbidities, total medications, primary cancer treatments, and generic AI availability. Sensitivity analyses were conducted using alternative specifications for independent variables.
Results
Mail order users had higher adherence in both commercial and Medicare-insured cohorts. Commercially insured patients who used mail order were more likely to be adherent if they had low copayments (< $5) and 90-day prescription refills. For commercially insured patients who used local pharmacies, use of one pharmacy and better synchronized refills were also associated with adherence. Among Medicare patients who used mail order pharmacies, only low copayments were associated with adherence, while among Medicare patients using local pharmacies both low copayments and 90-day prescriptions were associated with ET adherence.
Conclusion
Out-of-pocket costs, medication delivery mode, and other pharmacy-related medication delivery factors are associated with adherence to breast cancer ET. Future work should investigate whether interventions aimed at streamlining medication delivery could improve adherence for breast cancer patients.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562. https://doi.org/10.1200/jco.2007.11.5451
Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27:3445–3451. https://doi.org/10.1200/JCO.2008.19.2419
Chlebowski RTGM (2006) Adherence to endocrine therapy for breast cancer. Oncology 71:1–9
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134:459–478. https://doi.org/10.1007/s10549-012-2114-5
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537. https://doi.org/10.1007/s10549-010-1132-4
Lester RT, Mills EJ, Kariri A, Ritvo P, Chung M, Jack W, Habyarimana J, Karanja S, Barasa S, Nguti R et al (2009) The HAART cell phone adherence trial (WelTel Kenya1): a randomized controlled trial protocol. Trials 10:87. https://doi.org/10.1186/1745-6215-10-87
Gray TAFC, Harper R, Spencer AF, Campbell M, Henson DB et al (2012) Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomized controlled trial. Eye 26:407–417
Haynes RBSD, Gibson ES, Taylor DW, Hackett BC, Roberts RS et al (1976) Improvement of medication compliance in uncontrolled hypertension. Lancet 1:1264–1268
Ellis DA, Naar-King S, Chen X, Moltz K, Cunningham PB, Idalski-Carcone A (2012) Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: findings from a randomized controlled trial. Ann Behav Med 44:207–215
Simoni JMHD, Frick PA, Pearson CR, Andrasik MP, Dunbar PJ et al (2009) Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr 52:465–473
Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S et al (2014) Interventions for enhancing medication adherence. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000011.pub4
Choudhry NK, Krumme AA, Ercole PM, Girdish C, Tong AY, Khan NF, Brennan TA, Matlin OS, Shrank WH, Franklin JM (2017) Effect of reminder devices on medication adherence: the remind randomized clinical trial. JAMA Intern Med 177:624–631. https://doi.org/10.1001/jamainternmed.2016.9627
Volpp KG, Troxel AB, Mehta SJ, Norton L, Zhu J, Lim R, Wang W, Marcus N, Terwiesch C, Caldarella K et al (2017) Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: the heartstrong randomized clinical trial. JAMA Intern Med 177:1093–1101. https://doi.org/10.1001/jamainternmed.2017.2449
Hershman DL, Unger JM, Hillyer GC, Moseley A, Arnold KB, Dakhil SR, Esparaz BT, Kuan MC, Graham ML 2nd, Lackowski DM et al (2020) Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol 38:2122–2129. https://doi.org/10.1200/JCO.19.02699
https://www.nytimes.com/2021/11/02/us/politics/prescription-drug-prices-medicare.html (accessed Nov 15, 2021).
Choudhry NK, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, Pakes J, Brennan TA, Shrank WH (2011) The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med 171:814–822
Iyengar RN (2013) Rochelle Henderson R, Jay Visaria J, Frazee SG. Dispensing channel and medication adherence: evidence across 3 therapy classes. Am J Managed Care 19.
Iyengar RN, Balagere DS, Henderson RR, LeFrancois AL, Rabbitt RM, Frazee SG (2014) Association between dispensing channel and medication adherence among medicare beneficiaries taking medications to treat diabetes, high blood pressure, or high blood cholesterol. J Manag Care Spec Pharm 20:851–861. https://doi.org/10.18553/jmcp.2014.20.8.851
Adams AS, Uratsu C, Dyer W, Magid D, O’Connor P, Beck A, Butler M, Ho PM, Schmittdiel JA (2013) Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users. JAMA Intern Med 173:54–61. https://doi.org/10.1001/2013.jamainternmed.955
Marcum ZA, Driessen J, Thorpe CT, Gellad WF, Donohue JM (2014) Effect of multiple pharmacy use on medication adherence and drug-drug interactions in older adults with Medicare Part D. J Am Geriatr Soc 62:244–252. https://doi.org/10.1111/jgs.12645
Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH (2014) Optum Labs: building a novel node in the learning health care system. Health Aff 33:1187–1194
Abraham NS, Noseworthy PA, Inselman J, Herrin J, Yao X, Sangaralingham LR, Cornish G, Ngufor C, Shah ND (2020) Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Clin Gastroenterol Hepatol 18(337–346):e319
Ruddy KJ, Sangaralingham LR, Van Houten H, Nowsheen S, Sandhu N, Moslehi J, Neuman H, Jemal A, Haddad TC, Blaes AH (2020) Utilization of cardiac surveillance tests in survivors of breast cancer and lymphoma after anthracycline-based chemotherapy. Circ Cardiovasc Qual Outcomes. 13:e005984
Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL (2004) An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res 39:1733–1749. https://doi.org/10.1111/j.1475-6773.2004.00315.x
Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF (2013) Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 51:S11–S21. https://doi.org/10.1097/MLR.0b013e31829b1d2a
Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, Grann VR, Hershman DL (2011) Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol 29:2534–2542. https://doi.org/10.1200/jco.2010.33.3179
Biggers A, Shi Y, Charlson J, Smith EC, Smallwood AJ, Nattinger AB, Laud PW, Neuner JM (2016) Medicare D subsidies and racial disparities in persistence and adherence with hormonal therapy. J Clin Oncol 34:4398–4404. https://doi.org/10.1200/JCO.2016.67.3350
Elixhauser A, Steiner C, Harris R, Coffey R (1998) Comorbidity measures for use with administrative data. Med Care 36:8–17
Neuner JM, Fergestrom NM, Laud PW, Nattinger AB, Beyer KMM, Flynn KE, Pezzin LE (2019) The association of pharmacy fill synchronization with breast cancer endocrine therapy adherence. Cancer 125:3960–3965. https://doi.org/10.1002/cncr.32433
Fernandez EV, McDaniel JA, Carroll NV (2016) Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm 22:1247–1259
Iyengar RN, Balagere DS, Henderson RR, LeFrancois AL, Rabbitt RM, Frazee SG (2014) Association between dispensing channel and medication adherence among Medicare beneficiaries taking medications to treat diabetes, high blood pressure, or high blood cholesterol. J Manag Care Pharm 20:851–861. https://doi.org/10.18553/jmcp.2014.20.8.851
Hershman DL, Tsui J, Meyer J, Glied S, Hillyer GC, Wright JD, Neugut AI (2014) The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer I(106):1–9
Neuner JM, Kamaraju S, Charlson JA, Wozniak EM, Smith EC, Biggers A, Smallwood AJ, Laud PW, Pezzin LE (2015) The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv130
Girdish C, Shrank W, Freytag S, Chen D, Gebhard D, Bunton A, Choudhry N, Polinski J (2017) The impact of a retail prescription synchronization program on medication adherence. J Am Pharmacists Assoc 57(579–584):e571. https://doi.org/10.1016/j.japh.2017.05.016
Choudhry NK, Isaac T, Lauffenburger JC, Gopalakrishnan C, Lee M, Vachon A, Iliadis TL, Hollands W, Elman S, Kraft JM et al (2018) Effect of a remotely delivered tailored multicomponent approach to enhance medication taking for patients with hyperlipidemia, hypertension, and diabetes: the STIC2IT cluster randomized clinical trial. JAMA Intern Med 178:1182–1189. https://doi.org/10.1001/jamainternmed.2018.3189
Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, Stearns V, Clauw DJ, Williams DA, Henry NL (2014) Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 120:2403–2411. https://doi.org/10.1002/cncr.28756
Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH (2015) Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist 20:598–604. https://doi.org/10.1634/theoncologist.2015-0007
Stanton AL, Petrie KJ, Partridge AH (2014) Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat 145:525–534. https://doi.org/10.1007/s10549-014-2961-3
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30:936–942. https://doi.org/10.1200/jco.2011.38.0261
Cahir C, Guinan E, Dombrowski SU, Sharp L, Bennett K (2015) Identifying the determinants of adjuvant hormonal therapy medication taking behaviour in women with stages I-III breast cancer: a systematic review and meta-analysis. Patient Educ Couns. https://doi.org/10.1016/j.pec.2015.05.013
Spencer JC, Reeve BB, Troester MA, Wheeler SB (2020) Factors associated with endocrine therapy non-adherence in breast cancer survivors. Psychooncology 29:647–654
Funding
NIH/Minority Health and Health Disparities MD01-0728.
Author information
Authors and Affiliations
Contributions
Conceptualization; JMN, PWL, LEP, KJR, PWL, ANW. Data curation; JMN, NJ, KJR Formal analysis; JMN, NF, LEP, PWL Funding acquisition; JMN. Roles/Writing—original draft; JMN Writing—review & editing; all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Consent to participate
This study used only deidentified data and was judged to be exempt by the Medical College of Wisconsin IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neuner, J.M., Fergestrom, N., Pezzin, L.E. et al. Medication delivery factors and adjuvant endocrine therapy adherence in breast cancer. Breast Cancer Res Treat 197, 223–233 (2023). https://doi.org/10.1007/s10549-022-06704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06704-2