Abstract
Purpose
The combination of targeting the CDK4/6 and estrogen receptor (ER) signaling pathways with palbociclib and fulvestrant is a proven therapeutic strategy for the treatment of ER-positive breast cancer. However, the poor physicochemical properties of fulvestrant require monthly intramuscular injections to patients, which limit the pharmacokinetic and pharmacodynamic activity of the compound. Therefore, an orally available compound that more rapidly reaches steady state may lead to a better clinical response in patients. Here, we report the identification of G1T48, a novel orally bioavailable, non-steroidal small molecule antagonist of ER.
Methods
The pharmacological effects and the antineoplastic mechanism of action of G1T48 on tumors was evaluated using human breast cancer cells (in vitro) and xenograft efficacy models (in vivo).
Results
G1T48 is a potent and efficacious inhibitor of estrogen-mediated transcription and proliferation in ER-positive breast cancer cells, similar to the pure antiestrogen fulvestrant. In addition, G1T48 can effectively suppress ER activity in multiple models of endocrine therapy resistance including those harboring ER mutations and growth factor activation. In vivo, G1T48 has robust antitumor activity in a model of estrogen-dependent breast cancer (MCF7) and significantly inhibited the growth of tamoxifen-resistant (TamR), long-term estrogen-deprived (LTED) and patient-derived xenograft tumors with an increased response being observed with the combination of G1T48 and the CDK4/6 inhibitor lerociclib.
Conclusions
These data show that G1T48 has the potential to be an efficacious oral antineoplastic agent in ER-positive breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estrogen receptor (ER/ESR1) is expressed in a majority of breast cancers, and drugs that inhibit ER signaling are the cornerstone of breast cancer pharmacotherapy for ER-positive/HER2-negative disease [1]. These targeted approaches include the Selective Estrogen Receptor Modulator (SERM) tamoxifen that acts as a competitive ER antagonist in the breast, and aromatase inhibitors (AIs) that inhibit aromatase, the enzyme responsible for estrogen production [2, 3]. However, the development of resistance limits the duration of meaningful therapeutic responses. Mechanisms of resistance to these endocrine therapies include cell cycle dysregulation and activation of alternative growth factor signaling pathways [1]. For example, activation of MAPK, PI3K, and GSK-3 can result in increased phosphorylation of ER or its attendant coregulatory proteins leading to ligand-independent ER activity and resistance [4,5,6,7]. Recently, genomic alterations in the ER gene itself, including amplification, translocation, and ligand binding domain mutations (most frequently ER-D538G and ER-Y537S) have emerged with AI therapy [1, 8,9,10].
After progression during tamoxifen and AI therapy, other endocrine treatments including the steroidal selective estrogen receptor downregulator (SERD) fulvestrant (Faslodex®) are generally used [11]. SERDs are a class of ER antagonists that in addition to competitively displacing estrogens, also trigger ER downregulation [12]. Although initially successful, the onset of resistance limits durable responses when used as a monotherapy. Therefore, in an effort to improve the therapeutic lifespan of endocrine treatments for metastatic breast cancer, combination regimens have been extensively studied. Clinical trials using a combination of AI or fulvestrant with pan-PI3K or mTOR inhibitors have been promising but inconclusive, and toxicity often remains an impediment to dose escalation [13,14,15,16]. Therefore, CDK4/6 inhibitors have emerged as a favored option when considering combination endocrine therapies [17,18,19,20]. However, the poor bioavailability of fulvestrant, coupled with its intramuscular route of administration and the long time to steady state blood levels, compromises its clinical use [21, 22]. Indeed, even at the higher clinical dose (500 mg) of fulvestrant, pharmacodynamic imaging suggests incomplete receptor saturation [23].
Collectively, these data highlight an unmet need for a safe, orally bioavailable SERD with appropriate pharmaceutical properties. Herein we describe the preclinical development of G1T48 (rintodestrant), an orally bioavailable, potent, and selective non-steroidal ER antagonist and downregulator [24]. G1T48 was found to robustly inhibit ER activity in multiple in vitro models of endocrine therapy resistance, including those harboring ER mutations or growth factor activation. Importantly, G1T48 demonstrated robust antitumor activity in an animal model of early stage estrogen-dependent breast cancer and suppressed the growth of tamoxifen- and estrogen deprivation-resistant xenograft tumors with increased efficacy observed for the combination of G1T48 and lerociclib, a newly developed CDK4/6 inhibitor [25, 26].
Methods
Reagents
Fulvestrant (CAS No: 129453–61-8, > 99% purity) was purchased from MedChem Express. Estradiol (E2) (E8875), lasofoxifene (SML1026), 4-hydroxytamoxifen (H7904), and tamoxifen (T5648) were purchased from Sigma. Raloxifene (2280) was purchased from Tocris. GDC-0810 (S7855), bazedoxifene (S2128), and AZD9496 (S8372) were purchased from Selleckchem. GW5638 (5638), GW7604 (7604), and RU 58,668 (RU) were provided by Donald McDonnell (Duke University). G1T48 was provided by G1 Therapeutics, Inc., as analytical grade compound.
RNA analysis
MCF7 cells were authenticated by short tandem repeat profiling, were tested for Mycoplasma and were not cultured for more than three months at a time [27]. MCF7 cells were plated in DMEM/F12 supplemented with 8% charcoal dextran treated FBS for 48 h. Cells were then treated for 24 h with ligand and RNA was isolated using the Aurum™ total RNA isolation kit (Bio-Rad, Hercules, CA). After cDNA synthesis (iScript kit, Bio-Rad) real-time PCR was performed using the Bio-Rad CFX384 real-time system. GAPDH mRNA expression was used to normalize all real-time data using the 2-ΔΔCT method [28]. For more detailed description of this method, please see Online Resource 1.
Proliferation
MCF7 cells were plated in DMEM/F12 supplemented with 8% charcoal dextran treated FBS in 96-well plates (5 K cells/well) for 48 h. Cells were treated with estradiol (0.1 nM) or insulin (20 μM) with or without test compound (dose response; 1.0–11 to 1.0–05 M) for 6 days. Plates were decanted and frozen at – 80°°C overnight prior to quantitation of DNA by fluorescence using Hoechst 33258.
Supplementary material
Detailed methods are available in Online Resource 1 for the following protocols: In-Cell Western, Radioactive Binding Assay, Chromatin Immunoprecipitation, Transcriptional Reporter Assays.
Murine studies
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Duke University or South Texas Accelerated Research Therapeutics (START, San Antonio, Texas) prior to initiating the experiment. For complete details, see Online Resource 1.
Results
G1T48 is similar to fulvestrant in its ability to downregulate the estrogen receptor and inhibit estrogen signaling in breast cancer cells
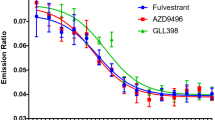
Novel ER antagonists with SERD activity have recently been described, but clinical development of these compounds has thus far been limited due to unanticipated side effects or for undisclosed reasons [29,30,31,32,33,34,35,36]. We sought to identify an orally bioavailable SERD using the chemical backbone of raloxifene as a starting point, since this SERM has demonstrated a favorable safety profile in the clinical setting of breast cancer prevention and osteoporosis treatment [37, 38]. G1T48 incorporates an acrylic acid side chain (Fig. 1a) [29, 31, 32, 34, 39, 40], and was the product of structure-guided investigations, driven by activity in breast cancer cell lines [24]. G1T48 was first assessed for its ability to downregulate ER when compared to several benchmark SERMs and SERDs including fulvestrant [12, 41]. Using In-Cell Western assays, G1T48 was found to downregulate ER with an efficacy modestly more potent than steroidal and other SERDs (e.g., fulvestrant, AZD9496; approximately 10% ER remaining after treatment) (Fig. 1b, online resource 2). Bazedoxifene (BZA), raloxifene (RAL), tamoxifen, 4-hydroxytamoxifen (4OHT), and lasofoxifene (laso) were also found to partially downregulate ER. These data demonstrate that in vitro G1T48 is a pure antiestrogen and selective estrogen receptor degrader (PA-SERD).
G1T48 is a potent selective estrogen receptor downregulator (SERD). a Chemical structures of G1T48 and benchmark SERMs and SERDs. b G1T48 downregulates the estrogen receptor in breast cancer cells. MCF7 cells were treated with ER ligands (10–12–10–6 M) for 18 h prior to fixation and detection of ER levels by In-Cell Western. *For tamoxifen and GW5638, dose response was 10–11–10–5 M. Error bars indicate the SD of triplicate samples
We next evaluated the ability of G1T48 to inhibit endogenous ER target gene transcription in MCF7 cells. As shown in Fig. 2a, G1T48 suppressed estrogen-mediated activation of the Trefoil Factor-1 (TFF1) mRNA similarly to fulvestrant and additional antiestrogens (4OHT, GDC-0810, AZD9496, RAL). The biochemical basis of G1T48-mediated ER antagonism was further evaluated using 3H-estradiol whole-cell competition assay. Results showed that G1T48 displaced radiolabeled agonist binding with potency greater than fulvestrant and similar to BZA (Fig. 2b). Radioligand binding assay (RBA) IC50 shows that G1T48 is a competitive ER antagonist (Online Resource 3).
G1T48 is a complete estrogen receptor antagonist. a G1T48 inhibits ER target gene expression in breast cancer cells. MCF7 cells were treated with ER antagonists (10–10–10–7 M) plus estradiol (E2; 10–9 M) for 18 h. TFF1 mRNA expression was analyzed by real-time PCR. GAPDH was used to normalize real-time PCR data. b G1T48 competes for estrogen binding to ER. MCF7 cells were treated with 10–10 M 3H-17β-E2 and competitor ligand (10–12–10–6 M) for 2 h. Cells were collected and radioactive counts were monitored on a Beckman LS 6000SC Scintillation counter. Error bars indicate the SD of duplicate samples. c G1T48 regulates ER target gene pharmacology similar to other SERDs. MCF7 breast cancer cells were treated with ER ligands (E2, fulvestrant, RU, RAL @ 100 nM; G1T48, 810, 9496, Laso, 4OHT, 7604, BZA @ 1.0 μM; 5638, Tam @ 10 μM) for 24 h. mRNA expression was analyzed by real-time PCR. GAPDH was used to normalize real-time PCR data. Heatmaps were generated from real-time PCR data after analysis with JMP pro software (SAS) using the Ward hierarchical clustering algorithm. d G1T48 blocks estrogen-dependent recruitment of ER to the TFF1 promoter. MCF7 cells were treated with ligand (E2: 5 × 10−10 M; ER antagonists: 10–6 M) as indicated for 45 min. Cells were fixed with formaldehyde and chromatin was immunoprecipitated with anti-ER antibody. Real-time PCR was used to assess the relative amount of ER bound to the TFF1 gene promoter. Error bars indicate the SD of triplicate samples
The inability of some ER antagonists, notably SERMs, to completely oppose the actions of estradiol is seen as a liability when being considered for the treatment of advanced therapy-resistant breast cancer. While data from Fig. 1b confirm G1T48 is a SERD, the potential remains for SERM activity, as G1T48 was developed based on compounds that exhibit both SERM and SERD activity. Therefore, we next considered the impact of G1T48 treatment on ER target genes that are differentially regulated by SERMs and SERDs [42]. As shown in Fig. 2c, compounds with SERM activity regulate these genes in a manner similar to the agonist estradiol, a reflection of their intrinsic agonist potential (red, green, and blue clusters). In contrast, G1T48 regulates these genes in a pattern that is consistent with compounds previously shown to downregulate ER (e.g., GW7604, fulvestrant, GW5638, RU 565899, GDC-0810, and AZD9496; orange, teal and purple clusters).
When bound by estrogen, ER is recruited to target gene promoters to activate or repress target gene transcription through recruitment of coregulator (coactivator or corepressor) proteins that modify chromatin structure [43]. We assessed the ability of G1T48 and benchmark SERMs or SERDs to inhibit estrogen-mediated recruitment of ER to the TFF1 promoter using chromatin immunoprecipitation (ChIP) assays. While estrogen and 4OHT treatment significantly increased ER binding to the TFF1 promoter (Fig. 2d), G1T48 inhibited the binding of ER to this promoter, with or without estrogen, with efficacy similar to fulvestrant, supporting the idea that G1T48 is an efficient ER antagonist in vitro.
We next evaluated the ER selectivity of G1T48 by assessing its ability to inhibit the transcriptional activity of related steroid hormone receptors androgen receptor (AR), progesterone receptor (PR), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR) using a cell-based reporter assay. When administered at doses up to 10 µM, G1T48 did not affect the transcriptional activities of AR, GR, MR, or PR (Online Resource 4), indicating that G1T48 is a highly selective antagonist of ER.
G1T48 inhibits the growth of ER-positive breast cancer cells
To examine the therapeutic potential of G1T48, we performed cell proliferation assays using multiple ER-positive breast cancer cell lines (Fig. 3). G1T48 significantly inhibited estrogen-mediated growth of MCF7 cells demonstrating approximately threefold higher potency when compared to fulvestrant (Fig. 3a, Online Resource 5). Additionally, G1T48 and benchmark antiestrogens also inhibited the estrogen-mediated growth of ER-positive BT474 and ZR-75-1 breast cancer cells, while no growth inhibition was observed in ER-negative MDA-MB-436 breast cancer cells (Fig. 3, Online Resource 5). Furthermore, G1T48 does not impact apoptosis in MCF7 breast cancer cells (Online Resource 6). Thus, G1T48 selectively inhibits the growth of ER-positive, but not ER-negative, breast cancer cells.
G1T48 inhibits ER-positive breast cancer cell growth. a ER-positive MCF7, b ER-positive BT474 , c ER-positive ZR-75–1 were treated for 7 days with 10–10 M E2 in addition to ER antagonists (10–11–10–5 M). d ER-negative MDA-MB-231 cells were treated for 7 days with ER antagonists (10–11–10–5 M). Cellular proliferation was assessed by measuring DNA content (Hoechst stain) and is presented as fold induction over vehicle control. Error bars indicate the SD of triplicate samples
G1T48 inhibits estrogen signaling in endocrine-resistant breast cancer models
In addition to the upregulation of growth factor signaling, a second key mechanism of resistance to aromatase inhibition is newly described mutations in the ligand binding domain of ER [8, 9], mutations that result in reduced potency for 4OHT and fulvestrant as compared to wild-type (wt) receptor [44,45,46,47,48,49,50]. To assess the activity of G1T48 on endocrine refractory ER mutants, we utilized a reporter gene assay in ER-negative SKBR3 breast cancer cells transfected with ER expression vectors (wtER or the two most common ER mutants, ER-Y537S or ER-D538G) (Fig. 4a). G1T48 was found to be a potent and effective inhibitor of both wtER and ER-D538G transcription. As has been previously reported [49], all antiestrogens tested, including G1T48, demonstrated reduced potency against ER mutant transcriptional activity when compared to wtER (Online Resource 7). To further understand the significance of these results, MCF7 cells expressing doxycycline inducible wtER, ER-D538G, or ER-Y537S were engineered and G1T48 was evaluated for its ability to inhibit the ER-dependent growth of these cells. As has been previously reported for 4OHT and fulvestrant (and also confirmed here), G1T48 exhibited an increased GI50 in cells expressing the ER-Y537S and ER-D538G mutations when compared to wtER (Fig. 4b, Online Resource 7) [44, 45, 47,48,49,50,51]. Collectively, these data highlights that G1T48, like other SERDs, may be useful at targeting some mutant receptors, a potential that can be further evaluated clinically.
G1T48 inhibits ER signaling in models of endocrine therapy resistance in vitro a SKBR3 breast cancer cells were transfected with an estrogen-responsive reporter gene together with ER (wtER, ER-Y537S, or ER- D538G) expression plasmids prior to 18 h of treatment with 17β-estradiol (1.0 nM) and ER antagonists (10–11–10–5 M). Firefly and renilla luciferase activity were then assessed using dual luciferase reagent. Error bars indicate the SD of triplicate samples. b G1T48 inhibits cell growth mediated by endocrine refractory ER mutants. MCF7 cells expressing ER variants ER-D538G and ER-Y537S were treated for 7 days with doxycycline plus increasing dose of antiestrogens (10–12–10–5 M). Cellular proliferation was assessed by measuring DNA content (Hoechst stain) and is presented as relative fluorescence units. Error bars indicate the SD of triplicate samples. c G1T48 inhibits growth factor-mediated breast cancer cell growth. MCF7 cells were treated for 7 days with insulin (20 nM) plus increasing dose of anti-estrogens (10–12–10–7 M). Cellular proliferation was assessed by measuring DNA content (Hoechst stain) and is presented as relative fluorescence units. Error bars indicate the SD of triplicate samples
Dysregulated growth factor signaling has emerged as a primary mechanism of resistance to tamoxifen and AI therapy [10]. Activation of these signaling pathways can alter the pharmacology of compounds like tamoxifen, converting them from antagonists to agonists through phosphorylation of ER or its attendant coregulator proteins [4,5,6,7]. G1T48 and comparator SERMs and SERDs were evaluated for their ability to inhibit insulin-mediated MCF7 cell proliferation, a model for endocrine therapy resistance. Compounds with SERD activity, including G1T48, effectively blocked growth factor-mediated cell growth, while compounds with SERM activity (e.g., 4OHT) were less effective (Fig. 4c). These data together support the potential for G1T48 to have efficacy in the treatment of AI or tamoxifen-resistant breast cancers having growth factor pathway activation.
Evaluation of the in vivo therapeutic efficacy of the SERD G1T48 and the CDK4/6 inhibitor lerociclib using estrogen-dependent and tamoxifen-resistant (TamR) breast cancer xenograft models
We next evaluated the therapeutic potential of G1T48 in ER-positive primary and endocrine refractory breast cancer models in vivo. G1T48 was first assessed, as a monotherapy or in combination with the CDK4/6 inhibitor lerociclib, for its impact on the growth of naïve MCF7 xenograft tumors (Fig. 5a). Ovariectomized estrogen-treated female nu/nu mice bearing MCF7 xenograft tumors were randomized to treatment with vehicle, lerociclib (50 mg/kg), and/or G1T48 (30 or 100 mg/kg). G1T48 treatment demonstrated dose-dependent repression of tumor growth. Combination of lerociclib and G1T48 was more effective than either monotherapy, demonstrating an added benefit to using these agents together. End of study tumor volumes are presented in Online Resource 8.
Combination strategy of G1T48 and the CDK4/6 inhibitor lerociclib inhibit estrogen-dependent and tamoxifen-resistant (TamR) breast cancer xenograft models in vivo a Ovariectomized estrogen-treated female nu/nu mice bearing MCF7 xenograft tumors were randomized to treatment with vehicle, lerociclib (50 mg/kg) or G1T48 (30 or 100 mg/kg), alone or together, p.o. daily for 28 days. 2-way ANOVA comparison of average tumor volumes throughout treatment, followed by Bonferroni multiple comparison test, indicated significant tumor growth inhibition by all treatments, as well as increased response to the combination of G1T48 (30 mg/kg) and lerociclib (50 mg/kg). Error bars represent SEM. b–d Ovariectomized tamoxifen-treated female nu/nu mice bearing TamR xenograft were randomized to treatment with vehicle, palbociclib (100 mg/kg), lerociclib (50 or 100 mg/kg) (b), fulvestrant (200 mg/kg), or G1T48 (30 or 100 mg/kg) (c), with lerociclib and G1T48 being tested alone and in combination (d), p.o. daily for 28 days. 2-way ANOVA comparison of average tumor volumes throughout treatment, followed by Bonferroni multiple comparison test, indicated significant tumor growth inhibition by all treatments, as well as increased response to the combination of G1T48 (30 mg/kg) and lerociclib. Error bars represent SEM
The TamR xenograft model exhibits tamoxifen-stimulated growth that can be inhibited by compounds with SERD activity with added benefit observed upon combination with CDK4/6 inhibitors [52]. Therefore, ovariectomized tamoxifen-treated mice bearing TamR xenografts were randomized to treatment with lerociclib (50 mg/kg or 100 mg/kg), G1T48 (30 mg/kg or 100 mg/kg), fulvestrant (200 mg/kg), or CDK4/6 inhibitor palbociclib (100 mg/kg) as monotherapies or a combination of lerociclib (50 mg/kg) and G1T48 (30 or 100 mg/kg). In this model system, lerociclib demonstrated efficacy equivalent to that of the mechanistic clinical comparator palbociclib (Fig. 5b). G1T48 was found to demonstrate dose-dependent inhibition of TamR tumor growth (Fig. 5c) albeit with less efficacy than fulvestrant. Interestingly, G1T48 treatment resulted in greater downregulation of intratumoral ER levels than fulvestrant despite less efficient inhibition of tumor growth (Online Resource 8). Finally, combination of G1T48 with lerociclib, using suboptimal doses of each inhibitor, resulted in tumor growth inhibition significantly greater than that observed for either compound as monotherapy (Fig. 5d). End of study tumor volumes are presented in Online Resource 10. Mouse body weight was largely unaffected by the treatment regimens in this study as presented in Online Resource 11.
Evaluation of the combined efficacy of lerociclib and G1T48 in a xenograft tumor model of resistance to estrogen deprivation in vivo
Although AIs have seen rapid adoption in the adjuvant setting, de novo and acquired resistance remains a persistent impediment to sustained clinical responses. We have developed an ER-positive model of aromatase resistance, termed long-term estrogen-deprived (LTED), to model this clinical situation [53]. In order to evaluate the combined efficacy of lerociclib and G1T48 in this model system, LTED xenograft tumors were orthotopically established in ovariectomized female nu/nu mice. When tumors measured 0.1–0.15 cm3 volume, G1T48 (5 mg/kg or 100 mg/kg) and lerociclib (50 mg/kg or 100 mg/kg) were administered alone and in combination, with fulvestrant (25 mg/kg) and palbociclib (100 mg/kg) included for comparison. As previously observed with the MCF7 parental and TamR models, G1T48 demonstrated dose-dependent inhibition of tumor growth (Fig. 6a). Additionally, the tumor growth inhibition after treatment with G1T48 and lerociclib was comparable to their clinical comparators (fulvestrant and palbociclib, respectively) (Fig. 6b). Combination of G1T48 with lerociclib suppressed tumor growth significantly compared to monotherapy (Fig. 6c) and resulted in tumor regression for a majority of tumors receiving the combined therapy regimen. End of study tumor volumes are presented in Online Resource 12.
Combination strategy of G1T48 and the CDK4/6 inhibitor lerociclib in vivo in an estrogen-deprived xenograft model. Ovariectomized vehicle-treated female nu/nu mice bearing LTED xenograft were randomized to treatment with vehicle, lerociclib (50 or 100 mg/kg) or palbociclib (100 mg/kg) (a) or G1T48 (5 or 100 mg/kg) or fulvestrant (25 mg/kg) (b), alone or together (c), p.o. daily for 28 days. 2-way ANOVA comparison of average tumor volumes throughout treatment, followed by Bonferroni multiple comparison test, indicated significant tumor growth inhibition by all treatments, as well as increased response to the combination of G1T48 (30 mg/kg) and lerociclib. Error bars represent SEM
Evaluation of the combined efficacy of lerociclib and G1T48 in a patient-derived xenograft model harboring the ER-Y537S Mutation
As described above, mutations in the LBD of ESR1 are an emerging mechanism of resistance to AIs. The efficacy of G1T48, as a mono- and combination therapy with lerociclib, was evaluated using a Patient-Derived Xenograft (PDX model) harboring the ER-Y537S mutation (Fig. 7a). Female athymic nu/nu mice were implanted with the ST2177 LUMB PDX tumor [33] and following treatment with G1T48, a dose-dependent decrease in tumor growth was observed. Intriguingly, G1T48 alone (30 and 100 mg/kg) or in combination with lerociclib was more efficacious than fulvestrant (Fig. 7a). Survival curve analysis demonstrated that the combination of 30 mg/kg G1T48 with lerociclib was more effective than monotherapy using either drug alone (Fig. 7b, c). Taken together these data highlight that G1T48 is either comparable or superior to fulvestrant in several models of endocrine therapy resistance, demonstrating its potential as a therapeutic agent.
Evaluation of the combined efficacy of lerociclib and G1T48 in a Patient-Derived Xenograft Model harboring the ER-Y537S Mutation. a Female nu/nu mice were engrafted with a START Patient-Derived Xenograft Model (START-PDX) model, designated ST2177, harboring an ER-Y537S mutation. Mice were randomized to vehicle, fulvestrant (5 mg/animal), G1T48 (30 or 100 mg/kg), lerociclib (50 mg/kg), or the combination of G1T48 and lerociclib and treated for 60 days. b, c Kaplan–Meier analysis is presented as time for tumors to reach endpoint (2.5 times original tumor volume). *Kaplan–Meier analysis followed by a Mantel–Cox test for significance demonstrated significantly greater tumor growth delay for these comparisons using an adjusted Bonferroni threshold of p < 0.012. Error bars represent SEM
Discussion
Targeting ER activity using therapies that directly oppose the mitogenic action of estrogen or that block estrogen synthesis is a proven strategy for the treatment and prevention of breast cancer. In locally advanced or metastatic disease, resistance to these therapies frequently emerges within two years, at which time treatment options are severely limited. Fulvestrant, a potent ER antagonist and downregulator, was initially approved for the treatment of endocrine therapy-resistant disease and more recently as first-line therapy for advanced ER-positive, HER2-negative breast cancer not previously treated with endocrine therapy. However, despite promising preclinical activity, the poorly controlled pharmacokinetics of fulvestrant remains a significant barrier to prolonged clinical efficacy. Clinical trials comparing high-dose (500 mg) to low-dose (250 mg) fulvestrant demonstrated superiority for the 500 mg dose in both first- and second-line settings, suggesting that increased target engagement can improve the outcome of ER degradation therapy [54, 55]. However, given its intramuscular route of administration, continued improvements in the clinical response to fulvestrant by further dose escalation appear unlikely. Therefore, development campaigns in this area have focused on the identification of orally bioavailable SERDs. The most active SERDs share common chemical features: either (a) a steroidal backbone (e.g., fulvestrant, RU58,668) or (b) an acrylic acid side chain (GW7604, GDC-0810, AZD9496) [29, 31, 32, 40, 56, 57]. Additional ER antagonists with novel chemical structures have also been reported to exhibit SERD properties [35, 36, 58], but none has yet gained FDA approval and some have been discontinued due to adverse effects or for undisclosed reasons [29,30,31,32,33,34,35,36, 40, 56]. We have identified a novel, orally bioavailable SERD, G1T48, that contains both a steroidal backbone and an acrylate side chain. G1T48 binds ER with low nanomolar affinity, inhibits estrogen-mediated target gene expression and breast cancer cell growth, and importantly blocks the tumor promoting effects of ER in both naïve and endocrine therapy-resistant animal models of breast cancer. Similar to AZD9496 and GDC-0810, G1T48 has good pharmacokinetic properties and maintains a more favorable side-effect profile compared to those reported for AZD9496 [56, 59, 60].
A hallmark feature of fulvestrant differentiating it from compounds like tamoxifen is that fulvestrant is a true antagonist with no agonist activity regardless of tissue context. By contrast, tamoxifen is a Selective Estrogen Receptor Modulator (SERM), demonstrating robust antagonist activity in the breast, but mimicking the agonist effect of estrogen in bone, the endometrium, and serum lipid profiles [61, 62]. This mechanistic difference between tamoxifen and fulvestrant can also be observed in breast cancer cells, where transcriptional profiling studies revealed that tamoxifen can regulate a subset of genes in a similar manner to estradiol. Our ER target gene regulation studies confirm the agonist activity of tamoxifen, with stimulation of SDK2, AGR2, and RAPGEL1 expression similar to the effect of estrogen treatment [42]. Compounds with SERD activity such as fulvestrant and AZD9496 did not increase these transcripts, consistent with a lack of agonist potential (i.e., pure antagonism). The transcriptional profile in breast cancer cells of G1T48 is most similar to fulvestrant and other SERDs. Interestingly, our studies revealed that there were modest differences in the transcriptional profiles even among the pure antagonist class of compounds, suggesting that they might engender different receptor conformations. The impact of these differences in ER target gene activation remain to be explored but could suggest that cross-resistance between different classes of SERDs can be avoided. Recent studies have indicated that in addition to receptor degradation, ER mobility is differentially impacted by sub-classes of SERMs and SERDs, and that compounds impeding mobility are more efficacious antagonists [35]. The impact of G1T48 on ER mobility is not currently known; however, our studies establish that G1T48 has very low intrinsic ER agonist activity.
Acquired resistance to endocrine therapy is complex and multifactorial; however, mutations in the ESR1 gene that result in ligand-independent receptor activity have emerged as a potential mechanism to account for approximately 30–40% of resistant disease following AI treatment [8, 44,45,46, 48,49,50,51]. It is significant, therefore, that G1T48 was found to suppress both the ligand-independent cell growth and transcriptional activity attributed to the two most prevalent endocrine refractory ER mutants, ER-Y537S and ER-D538G. Intriguingly, in contrast to the reconstituted transactivation assay in SKBR3, G1T48 was found to efficiently inhibit the growth of MCF7 cells engineered to overexpress the ER-Y537S variant. Cell context may contribute to this discrepancy; differential cofactor expression patterns in the two cell lines and/or the presence of endogenous wtER in MCF7 cells may influence G1T48 efficacy.
Long-term estrogen deprivation leading to a state of estrogen hypersensitivity is another means to model aromatase inhibitor therapy resistance. We have developed a new model of resistance to estrogen deprivation without ER mutation [53]. Using this model system, treatment with low-dose G1T48 (5 mg) resulted in incomplete tumor growth inhibition, while high-dose G1T48 (100 mg) as monotherapy resulted in tumor regression in the majority of animals, similar to fulvestrant, demonstrating the effectiveness of SERD therapy in this setting of resistant disease.
The combination of SERDs with CDK4/6 inhibitors has been evaluated clinically, most recently in the PALOMA-3 trial comparing the co-administration of the CDK4/6 inhibitor palbociclib (Ibrance®) with fulvestrant to fulvestrant alone. The results of this study demonstrated an overall survival benefit (median survival 34.9 months compared to 28 months) and a significant progression free survival rate (9.5 months vs 4.6 months) for the combination arm [17, 20]. These noteworthy improvements led to the 2016 FDA approval of palbociclib and fulvestrant combination therapy for ER-positive, HER-2- negative breast cancers progressing on other endocrine therapies. Further trials (MONALESSA-3 (NCT02422615) and MONARCH-2 (NCT02107703) have also demonstrated the utility of administering other CDK4/6 inhibitors with fulvestrant to improve patient outcomes [17,18,19,20]. The increased efficacy observed for the combination of G1T48 and lerociclib, as compared to monotherapy administration, in multiple in vivo breast cancer models sensitive or refractory to endocrine therapy treatment supports the potential utility of this regimen as an intervention in multiple stages of breast cancer treatment. Furthermore, lerociclib has been shown to promote less myelosuppression than palbociclib [25, 26]. Collectively, these data indicate that G1T48 has the potential to be an efficacious oral antineoplastic agent in ER+ breast cancer.
References
Ignatiadis M, Sotiriou C (2013) Luminal breast cancer: from biology to treatment. Nat Rev Clin Oncol 10(9):494–506
Maximov PY, Lee TM, Jordan VC (2013) The discovery and development of Selective Estrogen Receptor Modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8(2):135–155
Rydén L et al (2016) Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo - Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast 26:106–114
Font de Mora J, Brown M (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20(14):5041–5047
Campbell RA et al (2001) Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276(13):9817–9824
Yue W et al (2002) Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology 143(9):3221–3229
Medunjanin S et al (2005) Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor alpha and is involved in the regulation of receptor activity. J Biol Chem 280(38):33006–33014
Jeselsohn R et al (2015) ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 12(10):573–583
Ma CX et al (2015) Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 15(5):261–275
Tryfonidis K et al (2016) Endocrine treatment in breast cancer: cure, resistance and beyond. Cancer Treat Rev 50:68–81
Nathan MR, Schmid P (2017) A review of fulvestrant in breast cancer. Oncol Ther 5(1):17–29
McDonnell DP, Wardell SE, Norris JD (2015) Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J Med Chem 58(12):4883–4887
Krop IE et al (2016) Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 17(6):811–821
Baselga J et al (2017) Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18(7):904–916
Campone M et al (2018) Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: overall survival results from BELLE-2. Eur J Cancer 103:147–154
Di Leo A et al (2018) Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19(1):87–100
Cristofanilli M et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439
Sledge GW et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women With HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Slamon DJ et al (2018) Phase III Randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36(24):2465–2472
Turner NC et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936
Howell A, Sapunar F (2011) Fulvestrant revisited: efficacy and safety of the 500-mg dose. Clin Breast Cancer 11(4):204–210
Robertson JF et al (2004) Pharmacokinetic profile of intramuscular fulvestrant in advanced breast cancer. Clin Pharmacokinet 43(8):529–538
Heidari P et al (2015) Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res 21(6):1340–1347
Xiong R et al (2017) Novel selective estrogen receptor downregulators (SERDs) developed against treatment-resistant breast cancer. J Med Chem 60(4):1325–1342
Bisi JE et al (2017) Preclinical development of G1T38: A novel, potent and selective inhibitor of cyclin dependent kinases 4/6 for use as an oral antineoplastic in patients with CDK4/6 sensitive tumors. Oncotarget 8(26):42343–42358
Stice JP et al (2017) CDK4/6 Therapeutic intervention and viable alternative to taxanes in CRPC. Mol Cancer Res 15(6):660–669
Wright TM et al (2014) Delineation of a FOXA1/ERalpha/AGR2 regulatory loop that is dysregulated in endocrine therapy-resistant breast cancer. Mol Cancer Res 12(12):1829–1839
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) method. Methods 25:402–408
Lai A et al (2015) Identification of GDC-0810 (ARN-810), an orally bioavailable selective estrogen receptor degrader (SERD) that Demonstrates robust activity in tamoxifen-resistant breast cancer xenografts. J Med Chem 58(12):4888–4904
Wardell SE et al (2015) Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr Relat Cancer 22(5):713–724
Joseph JD et al (2016) The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. Elife 5:e15828
Weir HM et al (2016) AZD9496: An oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. Cancer Res 76(11):3307–3318
Bihani T et al (2017) Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER. Clin Cancer Res 23(16):4793–4804
Tria GS et al (2018) Discovery of LSZ102, a potent, orally bioavailable selective estrogen receptor degrader (SERD) for the treatment of estrogen receptor positive breast cancer. J Med Chem 61(7):2837–2864
Guan J et al (2019) Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell 178(4):949–963.e18
Kahraman M et al (2019) Maximizing ER-α degradation maximizes activity in a tamoxifen-resistant breast cancer model: identification of GDC-0927. ACS Med Chem Lett 10(1):50–55
Draper MW et al (1996) A controlled trial of raloxifene (LY139481) HCl: impact on bone turnover and serum lipid profile in healthy postmenopausal women. J Bone Miner Res 11(6):835–842
Vogel VG et al (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295(23):2727–2741
Wijayaratne AL et al (1999) Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140(12):5828–5840
De Savi C et al (2015) Optimization of a novel binding motif to (E)-3-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (AZD9496), a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. J Med Chem 58(20):8128–8140
Wardell SE, Nelson ER, McDonnell DP (2014) From empirical to mechanism-based discovery of clinically useful Selective Estrogen Receptor Modulators (SERMs). Steroids 90:30–38
Wardell SE, Kazmin D, McDonnell DP (2012) Research resource: transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol 26(7):1235–1248
Nagy L, Schwabe JW (2004) Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci 29(6):317–324
Bahreini A et al (2017) Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res 19(1):60
Harrod A et al (2017) Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene 36(16):2286–2296
Martin LA et al (2017) Discovery of naturally occurring ESR1 mutations in breast cancer cell lines modelling endocrine resistance. Nat Commun 8(1):1865
Merenbakh-Lamin K et al (2013) D538G mutation in estrogen receptor-α: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 73(23):6856–6864
Robinson DR et al (2013) Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45(12):1446–1451
Toy W et al (2013) ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 45(12):1439–1445
Toy W et al (2017) Activating ESR1 Mutations Differentially Impact the Efficacy of ER Antagonists. Cancer Discov 7(3):277–287
Jeselsohn R et al (2014) Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 20(7):1757–1767
Wardell SE et al (2015) Efficacy of SERD/SERM hybrid-CDK4/6 inhibitor combinations in models of endocrine therapy-resistant breast cancer. Clin Cancer Res 21(22):5121–5130
Wardell SE et al (2019) Pharmacokinetic and pharmacodynamic analysis of fulvestrant in preclinical models of breast cancer to assess the importance of its estrogen receptor-alpha degrader activity in antitumor efficacy. Breast Cancer Res Treat 179(1):67–77
Di Leo A et al (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28(30):4594–4600
Di Leo A et al. (2014) Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 106(1):djt337.
Hamilton EP et al (2018) A first-in-human study of the new oral selective estrogen receptor degrader AZD9496 for ER. Clin Cancer Res 24(15):3510–3518
Willson TM et al (1997) Dissection of the molecular mechanism of action of GW5638, a novel estrogen receptor ligand, provides insights into the role of estrogen receptor in bone. Endocrinology 138(9):3901–3911
Wardell SE et al (2013) Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res 19(9):2420–2431
Dees EC et al (2019) First-in-human dose-escalation study of g1t48, an oral selective estrogen receptor degrader (SERD), in postmenopausal women with ER+/Her2− locally advanced or metastatic breast cancer (ABC). European Society for Medical Oncology (ESMO), Barcelona, Spain
Cheung KWK et al (2019) GDC-0810 Pharmacokinetics and transporter-mediated drug interaction evaluation with an endogenous biomarker in the first-in-human, dose escalation study. Drug Metab Dispos 47(9):966–973
Love RR et al (1992) Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326(13):852–856
Love RR et al (1991) Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med 115(11):860–864
Acknowledgements
This work was supported by a sponsored research grant from G1 Therapeutics. The authors would like to thank the McDonnell laboratory for providing the space to perform the experiments and for topical and intellectual discussions about the research. The authors would also like to think Alexander Yllanes for his technical assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kaitlyn J. Andreano, Suzanne E. Wardell, Jennifer G. Baker, Taylor K. Desautels, Robert Baldi, Christina Chao, Kendall Heetderks, Yeeun Bae, Jessica A. Sorrentino and John D Norris. Design and bioassay-driven optimization of G1T48 was performed by Greg Thatcher, Rui Xiong, Debra Tonetti, Lauren Gutgesell, and Jiong Zhao. The first draft of the manuscript was written by Kaitlyn J. Andreano, Suzanne E. Wardell, Jennifer G. Baker and John D. Norris. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Jessica A. Sorrentino, Delita A. Thompson, John E. Bisi, and Jay C. Strum are employees of G1 Therapeutics, Inc. Delita A. Thompson, John E. Bisi, Jessica A. Sorrentino, and Jay C. Strum own stock in G1 Therapeutics, Inc. John D. Norris is a paid consultant for G1 Therapeutics, Inc. All other authors have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All procedures were approved by the Duke University Institutional Animal Care and Use Committee (IACUC) prior to initiating the experiment (protocol A011-16-01 and A272-18-12).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andreano, K.J., Wardell, S.E., Baker, J.G. et al. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat 180, 635–646 (2020). https://doi.org/10.1007/s10549-020-05575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05575-9