Abstract
Background
Evidence and consensus is lacking in international guidelines regarding axillary treatment recommendations for patients in whom a sentinel lymph node (SLN) cannot be visualized (non-vSLN) during the sentinel node procedure. In this study we aimed to determine the prevalence of non-vSLNs in a Dutch population of breast cancer patients and to examine predictors and survival rate for non-vSLN.
Methods
A nationwide, retrospective, population-based study was performed including 116,920 patients with invasive breast cancer who underwent a SLN procedure in the Netherlands between January 2005 and December 2013.
Results
Of the 76,472 clinically negative patients who underwent a SLN procedure, 1924 patients (2.5%) had a non-vSLN, of whom 1552 (80.7%) underwent an ALND. Multivariate analysis showed predictive factors for non-vSLN: older age (p < 0.001), diagnosis in the period 2005–2009 (p < 0.001), larger tumor size (p = 0.003), and extensive nodal involvement (p < 0.001). Multivariate survival analysis showed a significantly worse survival (HR 1.18, 95% CI 1.03–1.34, p = 0.015) for non-vSLNs patients. However, in the non-vSLN group, an ALND was not statistically significantly associated with a better survival (HR 0.96, 95% CI 0.53–1.75, p = 0.891).
Conclusion
Patients with non-vSLNs had less favorable disease characteristics and a worse survival compared to patients with a visualized SLN. Performing an ALND was not associated with a significantly better survival in patients with non-vSLNs. However, further research on the necessity of axillary treatment in this specific patient group is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, axillary lymph node dissection (ALND) has been the gold standard to determine the axillary lymph node status in patients with invasive breast cancer. However, an ALND can cause significant morbidity, such as lymphedema, dysesthesia, impairment of mobility, and pain [1,2,3,4]. Since the introduction of the sentinel lymph node (SLN) biopsy around 20 years ago, the indication to perform an ALND has constantly been under revision. It has already been established that, due to a low percentage of positive non-sentinel axillary lymph nodes, an ALND can be omitted in patients with a negative SLN and in SLN positive patients with micrometastases or isolated tumor cells [3, 5,6,7]. Moreover, the Z0011 trial, the AMAROS trial and the NSBAP-32 trial have shown that an ALND may be redundant in certain SLN positive patients with macrometastases and could be omitted completely or substituted by axillary radiotherapy [8,9,10].
However, none of the studies examining the necessity of the ALND included patients in whom the SLN procedure was unsuccessful, meaning that the SLN could not be visualized or retrieved (non-vSLN). The Dutch guideline recommends to perform an immediate ALND in case of a non-vSLN. However, review of the international guidelines reveals discrepancies in treatment recommendations in case of a non-vSLN. This illustrates the lack of consensus regarding the need to perform an ALND [11,12,13,14,15,16]. With the present study we aim to determine the prevalence of non-vSLNs in a Dutch population of breast cancer patients and to examine differences in clinicopathological characteristics, predictors, and overall survival between those in whom the SLN procedure was not successful (non-vSLN patients) versus patients in whom one or more SLN’s were successfully harvested (vSLN patients). We also examined whether performing an ALND is associated with a better survival in patients with a non-vSLN.
Patients and methods
Study population
In this nationwide, retrospective, population-based study we selected patients from the Netherlands Cancer Registry, which is a prospective database of all malignancies diagnosed in the Netherlands, based on notification by the Dutch nationwide pathology archive (PALGA) since 1989, containing information directly registered from the patients’ medical records in all hospitals in the Netherlands. The use of these data was approved by the NCR Committee of Privacy. We included patients with primary invasive breast cancer treated between January 2005 and December 2013, who had undergone a SLN procedure and did not have clinically palpable lymphadenopathy (cN0) or clinically apparent metastases (cM0). Patients receiving neo-adjuvant systemic treatment were excluded.
Sentinel node procedure
The Dutch guideline recommends performing the SLN procedure using a combination of preoperative lymphoscintigraphy with radioactive colloid and a preoperative injection of Patent Blue [11]. Lymphoscintigraphy was performed to visualize, locate and mark the sentinel nodes. At the start of the surgical procedure, usually about 0.5–1 ml of vital blue dye (Patent Blue V, 2.5% solution) was also injected. After incision, the blue lymphatics were visualized and a handheld gamma-detection probe was used to harvest the sentinel nodes [17]. The results of the sentinel node procedure were registered based on surgical and pathological reports. The procedure was considered unsuccessful if neither lymphoscintigraphy nor Patent Blue resulted in retrieval of a sentinel node.
Data analyses
The following information was available for all patients: age at time of diagnosis, year of diagnosis, side of the tumor, location of the tumor, clinical TNM-classification, type of surgery (mastectomy versus breast conserving operation), use of adjuvant systemic treatment (hormonal and/or chemotherapy), use of radiotherapy, date of follow-up/death (complete until January 2014), and vital status. The available data regarding the tumor included: pathological TNM-classification, tumor size, tumor morphology, tumor grade using the Nottingham-modification-scale, and hormone and HER2 receptor status. The location of the tumor was divided into: lateral (lateral lower and upper quadrant), medial (medial lower and upper quadrant), and central, including the nipple. The number of positive axillary lymph nodes was divided into negative, one or two positive lymph nodes (i.e., minimal nodal involvement), and three or more positive lymph node (i.e., extensive nodal involvement).
In the dataset, results of the SLN were reported in five histological categories: (1) negative, (2) isolated tumor cells (<0.2 mm), (3) micrometastases (0.2–2 mm), (4) macrometastases (>2 mm), and (5) non-visualized SLN. This variable was next recoded into: “negative” (categories 1 + 2), “positive” (categories 3 + 4), and “non-visualized” (category 5) [11]. For univariate and multivariate analyses the variable was recoded into “visualized” (categories 1 + 2 + 3 + 4 = vSLN group) versus “non-visualized” (category 5 = non-vSLN group).
In univariate analyses, the Chi square test was used to compare differences in patient and tumor characteristics between non-vSLN versus vSLN patients and in non-vSLN patients who received an ALND versus those who did not. Variables with a p value of <0.1 in the univariate analyses were included in multivariate logistic regression analyses in a stepwise backward fashion to identify predictive factors for a non-vSLN. Survival analyses were conducted using the Kaplan–Meier method. The log-rank test was used to compare survival curves. A Cox regression analysis was performed to calculate the Hazard Ratio (HR), adjusting for potential confounders, identified by the univariate log-rank tests. A p value of <0.05 was considered statistically significant in the univariate and multivariate analyses.
Results
Prevalence and predictors of non-visualized sentinel nodes
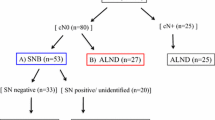
Figure 1 shows a flow chart of the patient selection. During the years 2005 until 2013, a total of 116,920 patients were diagnosed with invasive breast cancer in the Netherlands. After applying the in- and exclusion criteria, a total of 76,472 (65.4%) patients who had undergone a SLN procedure remained available for the study. Their median age at diagnosis was 60 years, ranging from 19 to 98 years. In 6912 (9%) patients the SLN procedure was performed, but details on the outcome were missing. Of the remaining 69,560 patients, 16,344 (23.5%) had a positive SLN biopsy of whom 15,014 (91.9%) received adjuvant therapy. An ALND was performed in 11,957 (73.2%) patients of whom 13,354 (81.7%) patients had minimal nodal involvement and 2984 (18.3%) patients had extensive nodal involvement. A negative SLN was found in 51,292 (73.7%) patients. The SLN group consisted of a total of 67,636 (97.2%) patients, and was compared with the 1924 (2.5%) patients in the non-vSLN group (See Fig. 1). Of the 1924 patients with non-vSLNs, 1035 (53.8%) received adjuvant systemic therapy and 1552 (80.7%) underwent an ALND, of whom 1213 (63.0%) did not have lymph node metastases, 207 (10.8%) had minimal nodal involvement, and 221 (11.5%) had extensive nodal disease. Table 1 shows the distribution of patient and tumor characteristics between non-vSLNs patients versus those with vSLNs and the results of univariate analyses.
In univariate analyses the following factors were associated with a statistically significantly higher (p < 0.10) prevalence of non-vSLNs: older age, a diagnosis in the period 2005–2009, mastectomy, a larger tumor size, extensive nodal involvement, and the absence of systemic therapy and radiotherapy. These factors were included in the multivariate logistic regression analysis, which showed that being diagnosed between in the period 2005–2009, being older, having larger tumors, and more often having extensive nodal involvement were predictors for a non-vSLN (Table 2).
Survival of patients with visualized versus non-visualized sentinel nodes
The median follow-up time of all patients was 3.3 years, with a maximum of 9 years. A total of 4802 patients had died, of whom 244 (5.1%) had non-vSLNs (p < 0.001). Survival analyses showed a 5-year survival rate of 91.3% (95% CI 91.1–91.5) for the vSLN group versus 86.1% (95% CI 84.2–88.2) for the non-vSLN group (p < 0.001) (Fig. 2). In the multivariate Cox regression analysis, adjusting for age at diagnosis, year of diagnosis, type of surgery, tumor size, number of positive lymph nodes, adjuvant systemic therapy, and radiotherapy, a worse survival was observed for patients with non-vSLNs compared to those with vSLN patients, with a hazard ratio (HR) of 1.18 (95% CI 1.03–1.34, p = 0.015).
We also performed a sensitivity analyses, in which we excluded patients of 70 years or older. Multivariate Cox regression analyses, adjusting for age, year of diagnosis, type of surgery, tumor size, progesterone status, number of positive lymph nodes, and adjuvant systemic therapy, showed that even after excluding these older patients, patients with a non-vSLN still had a worse overall survival compared to those with a vSLN (HR 1.42 95% CI 1.15–1.75 ).
ALND in patients with non-vSLN and association with overall survival
Of the 1924 patients with a non-vSLN 1552 (80.7%) underwent an ALND. Table 3 shows the distribution of patient and tumor characteristics in non-vSLN patients with versus without an ALND and the results of univariate and multivariate logistic regression analyses comparing both groups (with and without ALND). The results of the multivariate analyses showed that patients who underwent an ALND were more often diagnosed in the years 2005–2009 and had larger and more often multifocal tumors.
The 5-year survival rate of patients with an ALND was 85.6% (95% CI 79.2–92.0), compared to 86.0% (95% CI 84.0–88.0) for those without an ALND (p = 0.692) (Fig. 3). A multivariate Cox regression analysis, adjusting for age, year of diagnosis, tumor location, tumor size, ER and PR-status, and adjuvant systemic therapy, did not show a statistically significant difference in survival between patients with and without ALND (HR 0.96, 95% CI 0.53–1.75, p = 0.891).
Discussion
The present study shows that the SLN could not be visualized in 2.5% of all clinically node negative Dutch breast cancer patients who underwent a SLN procedure. Patients with a non-vSLN were older, were more often diagnosed in the earlier period of 2005–2009, had larger tumors, and were more likely to have extensive nodal involvement, compared to patients who had undergone a successful SLN procedure. Multivariate survival analysis, correcting for the most relevant confounding factors, showed a significantly poorer 5-year survival rate for patients with a non-vSLN versus those with a successful SLN procedure. Moreover, the majority of non-vSLN patients underwent an ALND. These patients more often had larger and multifocal tumors and were more likely to be diagnosed in the early period of 2005–2009, compared to those who did not undergo an ALND. Thus, the present data indicate that this specific group of patients in whom a SLN cannot be successfully visualized and harvested, represents a different breast cancer population.
It has been reported that several factors could influence the success rate of the SLN procedure. First, next to older age, a high body weight appears to result in an increased likelihood for a non-vSLN. It has been hypothesized that lymph nodes in older or more obese patients consist of more fat which decreases the nodes’ capacity for colloid uptake [18,19,20]. Although there was no information on body weight in the present study, this study did show an increased likelihood of non-vSLN with increasing age. Secondly, studies have confirmed our findings that a larger tumor size increases the risk of a non-vSLN. Some have reported that a central location of the breast tumor may increase the chance of a non-vSLN, though this could not be confirmed in the present study [19, 21]. Thirdly, a high number of positive lymph nodes and macrometastases is reported to also decrease the success rate of the SLN procedure, which is confirmed in this study [22,23,24]. This may be caused by blockage of the lymphatic pathways by the enlarged lymph nodes, which causes the lymphatic system to create alternative pathways. Finally, other factors that have been associated with the rate of SLN visualization refer to various procedural factors. SLN identification and visualization is lower during repeat SLNB in patients who previously underwent a SLNB or ALND [25]. The SNARB study (Sentinel Node and Recurrent Breast Cancer) by Vugts et al, which is a multicenter study on the feasibility and clinical usefulness of the repeat SLNB, showed that the limited SLN visualization may be caused by previous radiotherapeutic treatment. In addition, it was advised to inject a larger amount of radioactive dye and a 1-day protocol for lymphoscintigraphy was proposed instead of the current 2-day protocol to increase the visualization rate [26]. Studies also show that the success rate of the SLN procedure increases when both the lymphoscintigraphy and Patent Blue methods are used and when a larger amount of radioactive dye is injected [23, 27,28,29]. Finally, the experience of the surgeon in performing the SLN procedure is important in finding and identifying the lymphatic pathways and the SLN [30]. However, these latter factors were not addressed in the present study.
A clinically important question is whether patients with a non-vSLN should undergo an ALND. International guidelines differ in their treatment recommendations in case of a non-vSLN, if treatment options are mentioned at all. The European ESMO guidelines and the British NICE-guidelines both do not mention the possibility of a non-vSLN or its implications at all [12, 13]. The Dutch NABON-guideline, the American ASCO-guideline, and the Australian guideline all recommend to perform an ALND in case of a non-vSLN [11, 14, 15]. The NCCN guideline agrees with this statement; however, a footnote is added which states that in case of treatment with adjuvant radiation therapy, an extended radiation field to the axilla may also be sufficient [16]. Clinical data to substantiate these statements are very scarce.
Thus, the question remains whether the ALND is required in case of a non-vSLN. Obviously, axillary treatment can be omitted in case of a negative SLN. More recently, research also showed that the ALND could also be omitted in selected SLN positive patients [8,9,10]. The Z0011 trial formulated criteria to select SLN positive patients in whom the axillary treatment could be omitted without affecting (disease-free) survival [8]. However, the applicability of these criteria to non-vSLN patients is uncertain. In a previous study applying the Z0011 based criteria on a large cohort of Dutch breast cancer patients, more than half of the subgroup of non-vSLN patients who had undergone an ALND appeared to have no axillary lymph node metastases[31]. On the other hand, although in that study 37.2% of the patients with non-vSLNs had extensive nodal involvement, no statistically significant improved survival after an ALND could be shown. The present study, however, indicates that in non-vSLN patients extensive nodal involvement may be present more often.
The strength of the present study is the combination between analyses of a large national database of breast cancer patients regarding differences in characteristics and survival between vSLN patients and non-vSLN patients, with additional analyses on the impact of an ALND on the overall survival in the non-vSLN group. However, this study also has some limitations. Due to the use of a national database, some data were missing. Furthermore, the SLN procedure was registered as having been performed when either a lymphoscintigraphy and/or the Patent Blue technique was used. However, it was unknown whether both techniques or only one was used in the individual cases. Finally, there was of course a risk of selection bias when comparing the survival in the non-vSLN group between patients who did or did not receive an ALND. Also, due to the relatively small number of patients in these subgroups, confidence intervals of the hazard ratios were wide and a clinically relevant difference in survival thus cannot be ruled out.
Conclusion
In 2.5% of the patients who underwent a SLN procedure, the sentinel node could not be visualized and harvested. These non-vSLN patients had a worse survival compared to patients with a successful SLN procedure. Subsequently, 80.7% of these non-vSLN patients underwent an ALND. However, an ALND was not statistically significantly associated with a better survival. Therefore, we conclude that performing an ALND in patients with a non-vSLN is disputable, and that more confirmative research is needed to reach consensus regarding recommendations for axillary treatment in these patients.
References
Coufal O, Pavlik T, Fabian P et al (2009) Predicting non-sentinel lymph node status after positive sentinel biopsy in breast cancer: what model performs the best in a Czech population? Pathol Oncol Res 15:733–740
Wada N, Imoto S (2008) Clinical evidence of breast cancer micrometastasis in the era of sentinel node biopsy. Int J Clin Oncol 13:24–32
Gur AS, Unal B, Johnson R et al (2009) Predictive probability of four different breast cancer nomograms for nonsentinel axillary lymph node metastasis in positive sentinel node biopsy. J Am Coll Surg 208:229–235
Coutant C, Morel O, Antoine M et al (2007) Is axillary lymph node dissection always necessary in breast cancer patients with a positive sentinel node? J Chir (Paris) 144:492–501
Cripe MH, Beran LC, Liang WC et al (2006) The likelihood of additional nodal disease following a positive sentinel lymph node biopsy in breast cancer patients: validation of a nomogram. Am J Surg 192:484–487
Galimberti V, Cole BF, Zurrida S et al (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14:297–305
Galimberti V, Manika A, Maisonneuve P et al (2014) Long-term follow-up of 5262 breast cancer patients with negative sentinel node and no axillary dissection confirms low rate of axillary disease. Eur J Surg Oncol 40:1203–1208
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575
Krag DN, Anderson SJ, Julian TB et al (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8:881–888
Rutgers EJT, Donker M, Straver ME et al (2013) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: final analysis of the EORTC AMAROS trial. J Clin Oncol 31
NABON national guideline breast cancer 2.0; Comprehensive Cancer Centre Netherlands (2012). www.oncoline.nl/mammacarcinoom. Accessed 14 June 2016
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E et al (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–v30
Murray N, Winstanley J, Bennett A et al (2009) Diagnosis and treatment of advanced breast cancer: summary of NICE guidance. BMJ 338:b509
Lyman GH, Giuliano AE, Somerfield MR et al (2005) American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 23:7703–7720
National Breast and Ovarian Cancer Centre (NBOCC) Australia. Recommendations for use of Sentinel node biopsy in early (operable) breast cancer (June 2008); Cancer Australia 2011. www.canceraustralia.gov.au. Accessed 15 June 2016
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines); Breast Cancer (2016). www.nccn.com. Accessed 14 June 2016
Roumen RM, Valkenburg JG, Geuskens LM (1997) Lymphoscintigraphy and feasibility of sentinel node biopsy in 83 patients with primary breast cancer. Eur J Surg Oncol 23:495–502
Chagpar A, Middleton LP, Sahin AA et al (2005) Clinical outcome of patients with lymph node-negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer 103:1581–1586
Soran A, Falk J, Bonaventura M et al (2007) Does failure to visualize a sentinel node on preoperative lymphoscintigraphy predict a greater likelihood of axillary lymph node positivity? J Am Coll Surg 205:66–71
Dordea M, Colvin H, Cox P et al (2013) Clinical and histopathological factors affecting failed sentinel node localization in axillary staging for breast cancer. Surgeon 11:63–66
Goyal A, Newcombe RG, Chhabra A et al (2006) Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer–results of the ALMANAC validation phase. Breast Cancer Res Treat 99:203–208
Brenot-Rossi I, Houvenaeghel G, Jacquemier J et al (2003) Nonvisualization of axillary sentinel node during lymphoscintigraphy: is there a pathologic significance in breast cancer? J Nucl Med 44:1232–1237
Rousseau C, Classe JM, Campion L et al (2005) The impact of nonvisualization of sentinel nodes on lymphoscintigraphy in breast cancer. Ann Surg Oncol 12:533–538
Tanis PJ, Nieweg OE, Valdes Olmos RA et al (2002) Impact of non-axillary sentinel node biopsy on staging and treatment of breast cancer patients. Br J Cancer 87:705–710
Maaskant-Braat AJ, Roumen RM, Voogd AC et al (2013) Sentinel Node and Recurrent Breast Cancer (SNARB): results of a nationwide registration study. Ann Surg Oncol 20:620–626
Vugts G, Maaskant-Braat AJ, Voogd AC et al (2015) Improving the success rate of repeat sentinel node biopsy in recurrent breast cancer. Ann Surg Oncol 22(Suppl 3):S529–S535
Goyal A, Newcombe RG, Chhabra A et al (2006) Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer–results of the ALMANAC validation phase. Breast Cancer Res Treat 99:203–208
McMasters KM, Wong SL, Martin RC et al (2001) Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg 233:676–687
Heuts EM, van der Ent FW, van der Pol HA et al (2009) Additional tracer injection to improve the technical success rate of lymphoscintigraphy for sentinel node biopsy in breast cancer. Ann Surg Oncol 16:1156–1163
Straalman K, Kristoffersen US, Galatius H et al (2008) Factors influencing sentinel lymph node identification failure in breast cancer surgery. Breast 17:167–171
Verheuvel NC, Voogd AC, Tjan-Heijnen VC, et al (2016) Potential impact of application of Z0011 derived criteria to omit axillary lymph node dissection in node positive breast cancer patients. Eur J Surg Oncol 42:1162–1168
Acknowledgements
The authors thank the registration teams of the Comprehensive Cancer Centre Netherlands and Comprehensive Cancer Centre South for the collection of data for the Netherlands Cancer Registry and the scientific staff of the Comprehensive Cancer Center Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Verheuvel, N.C., Voogd, A.C., Tjan-Heijnen, V.C.G. et al. Non-visualized sentinel nodes in breast cancer patients; prevalence, risk factors, and prognosis. Breast Cancer Res Treat 167, 147–156 (2018). https://doi.org/10.1007/s10549-017-4483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4483-2