Abstract
Purpose
Sentinel node biopsy (SNB) after neoadjuvant therapy (NAT) for breast cancer remains controversial. We conducted a retrospective study of patients who underwent SNB after NAT to evaluate the effectiveness of this procedure.
Methods
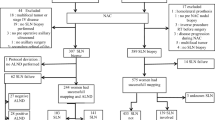
A consecutive 105 women with locally advanced breast cancer (cT1–4, cN0–3, M0) were treated with NAT between 2006 and 2015. The subjects were 80 of these patients who became or remained clinically node-negative after NAT, 53 of whom had axillary management determined by SNB (group A) and the other 27 underwent axillary lymph node dissection (ALND) without SNB (group B). SNB was performed using a modified dye method.
Results
The sentinel node (SN) identification rate was 94.3% and the mean number of removed SNs was 2.4. ALND was avoided in 33 patients, who were confirmed as SN-negative. There was no difference in recurrence-free and overall survival rates between groups A and B (p = 0.71 and p = 0.46, respectively) during the median follow-up time of 63 months. Of the 33 patients who did not undergo ALND, 10 suffered recurrence (33%). One patient (3%) had recurrence in an axillary lymph node and four had recurrence in a supraclavicular lymph node.
Conclusion
Axillary SNB after NAT did not affect the axillary failure rate or the prognosis. SNB may be a reliable procedure, even after NAT.


Similar content being viewed by others
References
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis. JAMA. 2011;305:569–75.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74.
Denkert C, Houber J, Loibl S, Prinzler J, Kronenwett R, Darb-Esfahani S, et al. Her2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013;15:R11.
Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–8.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lanpatinib with trastuzumab for Her2-positive early breast cancer (NeoALTTO): a randomized, open label, multicentre, phase 3 trial. Lancet. 2012;379:633–40.
Rubio IT. Sentinel lymph node biopsy after neoadjuvant treatment in breast cancer: work in progress. Eur J Surg Oncol. 2016;42:326–36.
van Nijnatten TJA, Schipper RJ, Lobbes MBI, Nelemans PJ, Beets-Tan RGH, Smidt ML. The diagnostic performance of sentinel lymph node biopsy in pathologically confirmed node positive breast cancer patients after neoadjuvant systemic therapy: a systemic review and meta-analysis. Eur J Surg Oncol. 2015;41:1278–87.
Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a meta-analysis. Cancer. 2006;106:4–16.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomized phase 3 trial. Lancet Oncol. 2010;11:927–33.
Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:695–700.
International Union Against Cancer UICC. In: Sobin LH, Witterkind CH, editos. TNM classification of malignant tumors, 6th ed. New York: Wiley-Liss; 2002.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2011;22:1736–47.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Ogawa Y, Ikeda K, Ogisawa K, Tokunaga S, Fukushima H, Inoue T, et al. Outcome of sentinel lymph node biopsy in breast cancer using dye alone: a single center review with a median follow-up of 5 years. Surg Today. 2014;44:1633–7.
Boughey JC, Ballmann KV, Hunt KK, et al. Axillary ultrasound after neoadjuvant chemotherapy in patients presenting with node-positive breast cancer (T0-T4, N1-2, M0) and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 trial (Alliance). J Clin Oncol. 2015;33:3386–93.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. Version3 (2015) http://www.nccn.org. Accessed 7 Mar 2017.
Takei H, Yoshida T, Kurosumi M, Inoue K, Matsumoto H, Hayashi Y, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy predicts pathological axillary lymph node status in breast cancer patients with clinically positive axillary lymph nodes at presentation. Int J Oncol. 2013;18:547–53.
Kida K, Ishikawa T, Yamada A, Shimizu D, Tanabe M, Sasaki T, et al. A prospective feasibility study of sentinel node biopsy by modified indigocarmine blue dye methods after neoadjuvant chemotherapy for breast cancer. Eur J Surg Oncol. 2015;41:566–70.
Forrest AP, Everington D, McDonald CC, Steele RJ, Chetty U, Stewart HJ. The Edinburgh randomized trial of axillary sampling or clearance after mastectomy. Br J Surg. 1995;82:1504–8.
Brady EW. Sentinel lymph node mapping following neoadjuvant chemotherapy for breast cancer. Breast J. 2002;8:97–100.
Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011;117:4606–16.
Galimberti V, FontanaSK Ribreiro, Maisonneuve P, Steccanela F, Vento AR, Intra M, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol. 2016;42:361–8.
Kim JY, Kim MK, Lee JE, Jung Y, Bae SY, Lee SK, et al. Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initial cytology-proven axillary node metastasis. J Breast Cancer. 2015;18:22–8.
Parks S, Lee JE, Paik H-J, Ryu JM, Bae SY, Lee SK, et al. Feasibility and prognostic effect of sentinel lymph node biopsy after neoadjuvant chemotherapy in cytology-proven, node positive breast cancer. Clin Breast Cancer. 2016;16:299–304.
Huang EH, Strom EA, Valero V, Fornage B, Perkins GH, Oh JL, et al. Locoregional treatment outcomes for breast cancer patients with ipsilateral supraclavicular metastasis at diagnosis. Int J Radiat Oncol Biol Phys. 2007;67:490–6.
Yamauchi C, Sekiguchi K, Nishioka A, Arahira S, Yoshimura M, Ogo E, et al. The Japanese Breast Cancer Society Clinical Practice Guideline for radiation treatment of breast cancer, 2015 edition. Breast Cancer. 2016;23:378–90.
http://meetinglibrary.asco.org/content/132077-144. Accessed 7 Mar 2017.
https://clinicaltrials.gov/ct2/show/NCT01901094. Accessed 7 Mar 2017.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Acknowledgements
We received no grants for this study. We thank Dr. Bunzo Nakata of Kashihara Municipal Hospital for his advice on our revision. Approved Number for this study by the ethical committee of Osaka City General Hospital: 1612092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Ogawa, Y., Ikeda, K., Watanabe, C. et al. Sentinel node biopsy for axillary management after neoadjuvant therapy for breast cancer: a single-center retrospective analysis with long follow-up. Surg Today 48, 87–94 (2018). https://doi.org/10.1007/s00595-017-1558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1558-y