Abstract
This study aims to stratify prognosis of triple-negative breast cancer (TNBC) patients using pre-treatment 18F-FDG-PET/CT, alone and with correlation to immunohistochemistry biomarkers. 200 consecutive TNBC breast cancer patients treated between 2008 and 2012 were retrieved. Among the full cohort, 79 patients had pre-treatment 18F-FDG-PET/CT scans. Immunostaining status of basal biomarkers (EGFR, CK5/6) and other clinicopathological variables were obtained. Three PET image features were evaluated: maximum uptake values (SUVmax), mean uptake (SUVmean), and metabolic volume (SUVvol) defined by SUV > 2.5. All variables were analyzed versus disease-free survival (DFS) using univariate and multivariate Cox analysis, Kaplan-Meier curves, and log-rank tests. The optimal cutoff points of variables were estimated using time-dependent survival receiver operating characteristic (ROC) analysis. All PET features significantly correlated with proliferation marker Ki-67 (all p < 0.010). SUVmax stratified the prognosis of TNBC patients with optimal cutoff derived by ROC analysis (≤3.5 vs. >3.5, AUC = 0.654, p = 0.006). SUVmax and EGFR were significant prognostic factors in univariate and multivariate Cox analyses. To integrate prognosis of biological and imaging markers, patients were first stratified by EGFR into low (≤15 %) and high (>15 %) risk groups. Further, SUVmax was used as a variable to stratify the two EGFR groups. In the high EGFR group, patients with high FDG uptake (SUVmax > 3.5) had worse survival outcome (median DFS = 7.6 months) than those patients with low FDG uptake (SUVmax ≤ 3.5, median DFS = 11.6 months). In the low EGFR group, high SUVmax also indicated worse survival outcome (17.2 months) than low SUVmax (22.8 months). The risk stratification with integrative EGFR and PET was statistically significant with log-rank p ≪ 0.001. Pre-treatment 18F-FDG-PET/CT imaging has significant prognostic value for predicting survival outcome of TNBC patients. Integrated with basal-biomarker EGFR, PET imaging can further stratify patient risks in the pre-treatment stage and help select appropriate treatment strategies for individual patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancers (TNBCs) are a heterogeneous group of tumors generally characterized by poor patient survival and lack of targeted therapeutics [1–3]. The majority of TNBCs are aggressive basal-like subtypes presenting with larger tumors of higher grade, and increased numbers of involved nodes. A minority of TNBCs, e.g., luminal androgen receptor, are a less aggressive subtype associated with better survival outcome [4–7]. Stratification of TNBC prognosis would be highly desired since some patients with better prognosis might be benefit from appropriate targeted treatment [4–6]. Gene profiling selection would be a direct approach to this need [7], however, classifying a single cancer into a gene expression subtype is impracticable in clinical practice [8–10]. An efficient approach would use immunohistochemistry (IHC) surrogates and other clinical available information to stratify TNBC patients upfront of treatment.
18-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG-PET/CT) has been used at the time of diagnosis of breast cancer to evaluate tumor staging, especially in the setting of metastasis prior to surgery or other treatment [12–14]. Recent studies show that FDG-PET has prognostic value to classify different breast cancer phenotypes [15]. Basu et al. [16] reported that tumor uptake value of 18F-PET for TNBC is significantly different compared to other phenotypes. Groheux et al. [17–19] have also showed that uptake values significantly change in TNBC patients who have been treated with neoadjuvant therapy. However, no PET imaging study to date has reported on the stratification of prognosis among TNBC patients. Given the heterogeneity of imaging signatures across the different breast cancer subtypes [15], it is feasible to use PET imaging to stratify TNBC patients through association with expression status of prognostic biomarkers.
This study aims to stratify prognostic TNBC patients using pre-treatment PET/CT alone and with correlation to immunohistochemistry biomarkers. We retrospectively evaluate the relationships among tumor metabolic activities and clinicopathological factors, and identify the prognostic value of PET image in predicting patient survival outcome. An integrative approach of imaging and tissue biomarkers is proposed to identify global tumor metabolic/biological status and to stratify individual patient risk. Since both PET and IHC biomarkers can be clinically available before starting treatment, the proposed method also provides a solution to screen potential patients for clinical trial-targeted therapy [4].
Materials and methods
Patients
After approval by the institutional review board, information was retrospectively collected on 200 consecutive TNBC patients who had undergone a resection of primary tumors at our medical center from 2008 to 2012. No consent was needed in the study. All patients were histologically confirmed as invasive carcinoma, and all patients received excisional biopsy, radical mastectomy, or modified radical mastectomy with lymph node dissection as primary treatment. 167 of these patients were treated with chemotherapy and/or radiotherapy. All tissue samples were examined by immunohistochemistry, and all samples with negative status of ER, PR, and HER2/neu were included.
Among the full cohort, 79 patients had pre-treatment PET/CT scans. To reduce the bias introduced by the limited numbers of patients with PET/CT images, the prognostic values of clinicopathological variables were examined in both full cohort of patients (n = 200) and subgroup patients with PET/CT images (n = 79). The resultant significant variables were used to correlate with the image features of PET images.
PET/CT imaging and image analysis
Patients fasted for 6 h prior to PET/CT imaging, and blood glucose level had to be less than 7 mmol/l. The dose of intravenous 18F-FDG was given based on body mass, using a reference of 370 MBq for 65 kg and not exceeding 555 MBq. After administration of 18F-FDG, patients rested in a quiet room for 60 min. CT and PET images were then acquired in a Gemini PET/CT scanner (germanium oxyorthosilicate-based PET + 16-slice CT; Philips Healthcare, Bothell, Washington). The patients were scanned from the level of the mid-thigh to the base of the skull with their arms raised. CT imaging was performed with a 16-slice multi-detector scanner using the following parameters: 0.5 s per rotation, 100 mA tube current, 120 kVp tube voltage, 5 mm slice thickness, and 4.25 mm slice interval. PET images were acquired using 4-mm slice thickness and 5 min per bed position for the area of interest and 3 min per bed position for all other areas. Acquired images were iteratively reconstructed with CT-based attenuation correction.
PET/CT images were analyzed with VelocityAI 3.0 (Varian Medical Systems, Palo Alto, CA). 18-F FDG uptake was measured by the standard uptake value (SUV), which represents the radioactivity of tissue for given time, mass, and initial tracer injection. The maximum uptake value (SUVmax) was used to characterize the tumor metabolic activities for a given region of interest, which was verified by biopsy/surgical location obtained from clinical pathology reports. SUV > 2.5 was used to define the tumor-associated metabolic volume, SUVvol [13, 20]. The mean uptake value within the metabolic volume is defined as SUVmean.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were selected to include representative sections of carcinoma and adjacent normal breast tissue. All IHC stains were performed using a Polymer and/or SA-HRP Detection System. Estrogen (ER) and progesterone receptor (PR) status, Ki-67, P53, and HER2/neu labeling index were determined with the SP1, 1E2, K-2, DO7, and 4B5 antibodies (Ventana Medical Systems, Tucson, AZ, USA), respectively. ER, PR, Ki-67, and P53 immunoexpressions were evaluated as the percentage of cells exhibiting definite nuclear staining. The threshold for the definition of TNBC was a lack (<1 % positivity) of any ER and PR immunoreactivity and a score of 0 or 1+ for HER2/neu immunoexpression and absence of amplification by fluorescent in situ hybridization (FISH). The PathVysion HER2 DNA probe kits (Abbott Molecular, Chicago, IL, USA) were used to specify the ratio of HER2/neu over the chromosome 17. A ratio less than 1.8 is negative for HER2 amplification and a ratio greater than 2.2 is positive for amplification, whereas a ratio of 1.8 to 2.2 is considered as equivocal for amplification. Immunostaining for CK 5/6 and EGFR was performed using monoclonal antibodies D5/16 B4 for CK5/6 (Dako, Glostrup, Denmark) and 2-18C9 for EGFR (Dako, Glostrup, Denmark). Results were recorded as the percentage of invasive carcinoma cells showing cytoplasmic and/or cytoplasmic membrane immunoreactivity for the corresponding antigen and the intensity of staining. The basal-like phenotype is defined as triple negative (ER, PR, and HER2 negative) and EGFR or CK5/6 positive [21].
Statistical analyses
Patient’s disease-free survival (DFS) was defined from the date of the primary treatment to the date of first local recurrence or distant metastasis or death [22]. Statistical analysis was performed using R software 3.1.1 (The R foundation for statistical computing, Vienna, Austria) and SPSS 17.0 statistical software (SPSS Inc, Chicago, IL, USA).
Prognostic values for DFS were examined along with clinicopathological factors such as age, tumor size, modified Bloom-Richardson (MBR) score, nuclear grade, tubule formation, mitosis, pathologic tumor stage (TNM-pT), lymph node stage (pN), metastasis (pM), ER, PR, HER2/neu, P53, Ki-67, CK5/6, and EGFR. The association of these variables with survival was analyzed using univariate and multivariate Cox-proportional hazard regression analysis. The independence of individual clinicopathological variables was examined by correlation. The cutoff values of biomarkers were estimated by the time-dependent survival ROC curves (survival ROC library in R), which were able to identify the threshold value by taking into account the number of months until censoring or recurrence from disease [23–26]. The resultant cutoff values were further examined by the Kaplan-Meier curves and log-rank tests [27, 28].
For the patients with pre-treatment PET/CT images, prognostic values of image features, SUVmax, SUVmean, and SUVvol were evaluated. The significant clinicopathological factors identified in the full cohort data were also re-evaluated in the imaging subgroup. The statistical difference between subgroup patients with and without PET/CT images was evaluated by Wilcoxon rank sum tests. A p value less than 0.05 was considered significant.
Results
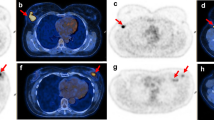
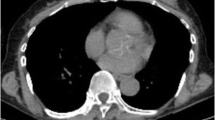
Patient and tumor characteristics are summarized in Table 1. All patients in the full cohort were women with a median age of 57 years (range, 28–92 years) and a median disease-free survival of 11.5 months. No significant difference was observed between patients with and without PET/CT images. The imaging subgroup had a median age of 57 years (range, 28–88 years) and a median disease-free survival of 11.4 months. 70 % patients were CK5/6 positive, and 91 % patients were EGFR positive. A total of 73 (92 %) patients were identified as having basal-like TNBC (any EGFR and/or CK5/6 positivity). 67 patients were treated with chemotherapy and/or radiotherapy, and 12 patients were treated with neither chemotherapy nor radiotherapy. Figure 1 shows two TNBC cases of pre-treatment PET/CT images with the different SUVmax and prognosis: patient 1, diagnosed with low SUVmax (=1.5) and low expressions of basal marker (EGFR = 10 %, CK5/6 = 10 %), is still event free after 24 months; patient 2, diagnosed with high SUVmax (=19.7) and high expressions of EGFR (=70 %) and CK5/6 (=95 %), had lung metastasis after 21 months event free.
Two TNBC cases of pre-treatment PET/CT images with the different SUVmax and prognosis. PET images were fused with CT images, and the locations of tumor were pointed by yellow arrows. a SUVmax = 1.5 for a 77-yr-old patient with EGFR = 10 %, CK5/6 = 10 %, Ki-67 = 39 %, who was undergone lumpectomy and is still event free after 24 months. b SUVmax = 19.7 for a 75-yr-old patient with EGFR = 70 %, CK5/6 = 95 %, Ki-67 = 57 %, SUVmean = 7.5, SUVvol = 52.1 cc, who was undergone lumpectomy, and had lung metastasis after 21 months event free
Prognosis of PET Image features
All image features (SUVmax, SUVmean, SUVvol) significantly correlated with proliferation marker Ki-67 (p < 0.010) and significantly correlated with each other (all with p < 0.001). Additionally, SUVmax significantly correlated with tumor MBR grade (p = 0.034) and tumor mitotic index (p = 0.019), whereas SUVmean also significantly correlated with tumor MBR grade (p = 0.025), mitotic index (p = 0.032), and CK5/6 (p = 0.031). SUVvol significantly correlated with tumor size (p ≪ 0.001), pathological T stage (p = 0.023).
The prognostic values of image features were examined by univariate Cox analysis, as shown in Table 2. Maximum FDG uptake (SUVmax) was significant in both continuous (p = 0.021) and discrete status (≤3.5 vs. >3.5) with p = 0.006. The cutoff value of SUVmax was estimated using time-dependent survival ROC analysis. As a result, cutoff 3.5 maximized both sensitivity and specificity of SUVmax for the disease-free survival with AUC = 0.654. Figure 2b shows the ROC of SUVmax, where the cutoff point is marked by the red circle. The resultant cutoff percentile was verified by examining the prognostic value of SUVmax using log-rank test (p = 0.002) and Cox regression analysis (p = 0.006). As shown in Fig. 2a, patients were stratified into two risk groups with SUVmax (≤3.5 vs. >3.5), and the patients in high SUVmax groups had worse survival outcome.
Kaplan-Meier curves of disease-free survival of PET image features for TNBC patients with pre-treatment PET/CT, a SUVmax at cutoff level 3.5 with log-rank p = 0.002, b SUVmax cutoff value (red circle) is determined by time-dependent survival ROC curve with AUC = 0.654, c SUVmean at cutoff level 2.9 with log-rank p < 0.001, and d SUVvol at cutoff level 10 ml with log-rank p < 0.001
Mean FDG uptake (SUVmean) was also significant in stratifying patient risks with optimal cutoff 2.9. The prognostic difference between two patient risk groups was illustrated by Kaplan-Meier curves (log-rank p = 0.005) shown in Fig. 2c. Metabolic uptake volume (SUVvol) has the similar results shown in Fig. 2d, where patients with large metabolic volume had worse outcome than those with small volume (≤10 ml vs. 10 ml, p = 0.001). Due to the strong correlation (p ≪ 0.001) between SUVvol and pathological volume derived by the tumor size, we compared the difference between these two volumes using Wilcoxon rank sum test. The results show that PET-defined tumor volume was not significantly different from the pathology-derived tumor volume (p > 0.10). This suggests that PET-defined volume can be used to estimate tumor volume prior to surgery or other treatment.
Prognostic value of basal biomarkers
Basal biomarkers EGFR and CK5/6 were evaluated by univariate Cox analysis in both continuous and discrete status. The optimal cutoff values were estimated using time-dependent survival ROC analysis. As a result, an EGFR cutoff of 15 % maximized both sensitivity and specificity for the outcome with AUC = 0.788, and while for CK5/6, 50 % is the optimal cutoff point with AUC = 0.611. As shown in Table 2, EGFR and CK5/6 were both significant in univariate Cox analysis with p = 0.001 and 0.011, respectively. The stratification of patient risks is illustrated by Kaplan-Meier curves (Fig. 3), with log-ranks p = 0.0008 for EGFR and 0.011 for CK5/6, respectively. To remove the bias due to sample size, the same tests were also conducted in full cohort patients (n = 200), and produced the same results (p = 0.004 for EGFR, p = 0.018 for CK5/6) shown in Table 2.
Integrating prognosis of PET imaging and basal biomarkers
Multivariate Cox analysis in Table 2 identifies that SUVmax and EGFR were significant prognostic factors for disease-free survival (p = 0.031, and 0.004, respectively). To integrate prognosis of biological and imaging markers, patients were first stratified by EGFR into low (≤15 %) and high (>15 %) risk groups. Further, SUVmax was used as a variable to stratify the two EGFR groups. In the high EGFR group, patients with high FDG uptake (SUVmax > 3.5) had worse survival outcome (median DFS = 7.6 months) than those patients with low FDG uptake (SUVmax ≤ 3.5, median DFS = 11.6 months). In the low EGFR group, high SUVmax also indicated worse survival outcome (17.2 months) than low SUVmax (22.8 months). Figure 4 shows that the risk stratification with integrative EGFR and PET was statistically significant with log-rank p ≪ 0.001. The results of patient risk groups stratified by SUVmax and EGFR are listed in Table 3. The majority of TNBC patients (47 %) were in group 4 with high SUVmax and high EGFR expression. Compared to low-risk groups, group 4 has the shortest median DFS (7.6 months), highest median SUVmax (11.3), SUVmean (5.2), SUVvol (10.9 ml), EGFR (60 %), CK5/6 (20 %), and Ki-67 (60 %). Contrarily, the low-risk group 1 has all favorable characteristics: long DFS (22.8 months), and low expression EGFR (5 %), and SUVmax (2.0).
Kaplan-Meier curves of disease-free survival of risk groups for TNBC patients with pre-treatment PET/CT. The patients were classified into four risk groups: group 1 (EGFR ≤ 15, SUVmax ≤ 3.5, n = 12), group 2 (EGFR ≤ 15, SUVmax > 3.5, n = 15), group 3 (EGFR > 15, SUVmax ≤ 3.5, n = 13), and group 4 (EGFR > 15, SUVmax > 3.5, n = 37), with log-rank p < 0.0001
Discussion
TNBC is a highly diversity group of cancers with character of the absence of expression of ER, PR, and HER2 [1, 2]. Recent gene expression microarray studies have revealed at least six subtypes, with luminal androgen receptor or molecular apocrine cancers forming a distinct group within triple-negative disease [3, 4]. This finding suggests that the prognosis of TNBC subtypes would be different due to diversity of genomic expression. However, the selection of gene expression subtypes for a single cancer is challenging in the clinics [8]. Recent clinical trials on EGFR suggest that it is unlikely that a targeted therapy will have substantial effective activity in unselected TNBCs [29, 30]. An efficient approach would use biomarker or other methods to screen for the potentially sensitive subset of patients [4, 11]. Considering cost and efficiency, it is also highly desirable for the screening to be completed during regular clinical procedures.
IHC and PET/CT are commonly performed before primary treatment starts. Both methods have their advantages and disadvantages in stratification of breast cancer. IHC biomarkers CK5/6 and EGFR have been used to identify basal-like breast cancer in clinical routine. Expression of EGFR and/or CK5/6 has been used to predict the basal phenotype associated with poor survival [22]. Recent studies [31–33] suggested that the overexpression level of basal markers may affect the stratification of TNBC patients. Viale et al. [31] showed that immunoreactivity of EGFR may have different prognostic values at different cutoff points, and low expression of basal biomarkers may not necessarily lead to poor survival. Zhang et al. [32] reported that EGFR had more significant prognostic value than CK5/6 in the study of 136 TNBC patients. Thike et al. [33] showed that basal cytokeratins had significant prognostic values, whereas EGFR did not in their patient cohort. These suggest that a single biomarker only indicates a specific signaling pathway and thus may not be reliable to stratify TNBC patients. It is desirable to exploit a statistical model to integrate information from multiple biomarkers [33, 34]. In addition, because examined tissue usually is obtained from a single location, it may not represent the heterogeneity of a tumor.
18F-FDG-PET/CT has been used in initial staging and stratification of the phenotypes of breast cancer. Specifically, PET/CT is used to identify occult distant metastases and involvement of extra-axillary lymph node, which serve as critical indices in initial staging of breast cancer [14, 35]. A study on a cohort of 142 patients [15] showed that PET/CT provided stronger prognostic stratification in the primary staging of large breast cancer than conventional imaging (such as mammogram, breast ultrasound, bone scan, X-rays, and/or CT). Although PET imaging is able to provide global metabolic uptake related to tumorous transformation (e.g., metabolism and proliferation), the relationship between tumor heterogeneity and image features is still ambiguous. Several efforts [17, 18, 36, 37] have been made to correlate metabolic uptakes with immunoexpression status of tissue biomarkers. Osborne et al. [36] showed that metabolic activities of ER-negative tumors were significantly higher than those of ER-positive tumors. Aogi et al. [37] reported that the maximum metabolic uptake predicted survival outcome for patients with the luminal-type breast cancer. Recent study [15] also suggested that FDG uptake of TNBC tumor typically had higher metabolic activities than those of other phenotype breast cancers, and it could be potentially used to predict the outcome of neoadjuvant chemotherapy [17, 18]. To the best of our knowledge, no PET imaging study has been reported on the stratification of prognosis among TNBC patients. Our method, integration of basal biomarkers and PET provides a new avenue to identify TNBC patients who potential have relative good prognosis and may be spared from overtreatment and those who may need targeted therapy.
Our results indicate that basal marker EGFR was a significant biomarker for the large patient cohort (n = 200) and for a smaller subgroup (n = 79). Not all basal marker positive breast cancer was necessarily associated with poor prognosis, and the patients with low expression (e.g. EGFR < 15 %) had better survival outcome than those with EGFR > 15 %. Similarly, pre-treatment PET also had significant prognostic value to identify low- and high-risk patients. The integration of these two prognostic markers further stratified TNBC patients into four subgroups. The patients with high EGFR expression and high FDG uptake had much worse survival outcome than those patients with low EGFR expression and low FDG uptake. The results also suggest that non-EGFR pathways play critical roles in those EGFR positive cases. This might be one of the reasons for the failure of EGFR clinical trials [29, 30]. Since both biomarkers can be obtained before treatment, these low-risk patients may be selected as candidates for further screening with targetable genes. Our prognostic analysis would help clinicians identify low- and high-risk patients early, so they could then select appropriate treatment strategies for the individual TNBC patients. Further study or analysis may help determine whether study of EGFR + PET-guided neoadjuvant therapy warrants a full-scale clinical trial for TNBC patients.
Conclusions
This work indicates that pre-treatment 18F-FDG-PET/CT imaging has significant prognostic value for predicting survival outcome of TNBC patients. Integrated with prognostic basal biomarkers, especially EGFR, PET imaging can further stratify TNBC patients into different risk groups. Given the limited sample size here, new clinical studies are needed to assess the specificity and sensitivity of PET imaging in identification of non-basal-like TNBC defined by expression gene profiling analysis. Further prospective validation will help define prognostic and predictive value of the PET imaging as a pre-treatment screening approach to identify patients with sensitive targetable genes for clinical trials, and also help identify patients at higher risk who may benefit from aggressive treatment strategies.
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- 18F-FDG:
-
18-fluoro-2-deoxy-d-glucose
- SUV:
-
Standard uptake value
- SUVmax:
-
Maximum value of SUV of a defined volume
- SUVmean:
-
Mean value of SUV of a defined volume
- SUVvol:
-
Volume of interested segmented by a threshold (SUV > 2.5 in this study)
- IHC:
-
Immunohistochemistry
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- EGFR:
-
Epidermal growth factor receptor
- CK5/6:
-
Cytokeratin 5/6
References
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees C et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T et al (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167. doi:10.1200/JCO.2008.18.1370
Prat A, Adamo B, Cheang MCU, Anders CK, Carey LA, Perou CM (2013) Molecular characterization of basal-like and non-basal-like triple-negative breast cancers. Oncologist 18(2):122–133
Turner NC, Reis-Filho JS (2013) Tackling the diversity of triple-negative breast cancer. Clin Cancer Res 19(23):6380–6388. doi:10.1158/1078-0432.CCR-13-0915
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig. doi:10.1172/JCI45014DS1
Pistelli M, Caramanti M, Biscotti T, Santinelli A, Pagliacci A, De Lisa M et al (2014) Androgen receptor expression in early triple-negative breast cancer: clinical significance and prognostic associations. Cancers 6(3):1351–1362. doi:10.3390/cancers6031351
Gasparini P, Fassan M, Cascione L, Guler G, Balci S, Irkkan C et al (2014) Androgen receptor status is a prognostic marker in non-basal triple negative breast cancers and determines novel therapeutic options. PLoS ONE 9(2):e88525. doi:10.1371/journal.pone.0088525
Chen X, Li J, Gray WH, Lehmann BD, Bauer JA, Shyr Y et al (2012) TNBC type: a subtyping tool for triple-negative breast cancer. Cancer Inform 11:147–156
Weigelt B, Pusztai L, Ashworth A, Reis-Filho JS (2012) Challenges translating breast cancer gene signatures into the clinic. Nature Rev. Clin Oncol 9(1):58–64. doi:10.1038/nrclinonc.2011.125
Mackay A, Weigelt B, Grigoriadis A, Kreike B, Natrajan R, A’Hern R et al (2011) Microarray-based class discovery for molecular classification of breast cancer: analysis of interobserver agreement. J Natl Cancer Inst 103(8):662–673. doi:10.1093/jnci/djr071
Lehmann BD, Pietenpol JA (2013) Identification and use of biomarkers in treatment strategies for triple negative breast cancer subtypes. J Pathol 232:142–150
Wahl RL, Cody RL, Hutchins GD, Mudgett EE (1991) Primary and metastatic breast carcinoma: initial clinical evalua-tion with PET and the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiol 179:765–770
Avril N, Rose CA, Schelling M et al (2000) Breast imaging with positron emission tomography and fluorine-18 fluorode-oxyglucose: use and limitations. J Clin Oncol 18:3495–3502
Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C et al (2013) 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nuc Med 54(1):5–11. doi:10.2967/jnumed.112.106864
Cochet A, Dygai-Cochet I, Riedinger J-M, Humbert O, Berriolo-Riedinger A, Toubeau M et al (2014) 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging 41(3):428–437. doi:10.1007/s00259-013-2595-4
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B et al (2008) Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for d. Cancer 112(5):995–1000. doi:10.1002/cncr.23226
Groheux D, Hindié E, Giacchetti S, Delord M, Hamy A-S, de Roquancourt A et al (2012) Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nuc Med 53(2):249–254. doi:10.2967/jnumed.111.094045
Groheux D, Giacchetti S, Delord M, de Roquancourt A, Merlet P, Hamy AS et al (2014) Prognostic impact of (18)F-FDG PET/CT staging and of pathological response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. doi:10.1007/s00259-014-2941-1
Groheux D (2014) Predicting pathological complete response in breast cancer early. Lancet Oncol 15(13):1415–1416. doi:10.1016/S1470-2045(14)71020-4
Avril N, Dose J, Jdnicke F, Bense S et al (1996) Metabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose. J Clin Oncol 14:1848–1857
Cheang MCU, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14(5):1368–1376. doi:10.1158/1078-0432.CCR-07-1658
Hudis C, Barlow W, Costantino J et al (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25:2127–2132
Adams H, Tzankov A, Lugli A, Zlobec I (2009) New time-dependent approach to analyse the prognostic significance of immunohistochemical biomarkers in colon cancer and diffuse large B-cell lymphoma. J Clin Pathol 62:986–997
Keam B, Im S-A, Lee K-H, Han S-W, Oh D-Y, Kim JH et al (2011) Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. doi:10.1186/bcr2834
Blanche P, Dartigues J-F, Jacqmin-Gadda H (2013) Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 32(30):5381–5397. doi:10.1002/sim.5958
Kang MH, Go S-I, Song H-N, Lee A, Kim S-H, Kang J-H et al (2014) The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer 111(3):452–460. doi:10.1038/bjc.2014.317
Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A (2007) Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 60(10):1112–1116. doi:10.1136/jcp.2006.044537
Takahashi H, Kurishima K, Ishikawa H (2010) Optimal Cutoff Points of CYFRA21-1 for Survival Prediction in Non-small Cell Lung Cancer Patients Based on Running Statistical. Analysis 3838:3833–3837
Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX et al (2012) TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 30(21):2615–2623. doi:10.1200/JCO.2010.34.5579
Baselga J, Stemmer S, Pego A, Chan A, Goeminne J-C, Graas M-P et al (2010) Cetuximab + cisplatin in estrogen receptor-negative, progesterone receptor-negative, HER2-negative (triple-negative) metastatic breast cancer: results of the randomized phase II BALI-1 trial. Cancer Res 70:95s
Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A et al (2009) Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat 116(2):317–328. doi:10.1007/s10549-008-0206-z
Zhang M, Zhang X, Zhao S, Wang Y, Di W, Zhao G et al (2013) Prognostic value of survivin and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Target Oncol. doi:10.1007/s11523-013-0300-y
Thike AA, Iqbal J, Cheok PY, Chong APY, Tse GM-K, Tan B et al (2010) Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol 34(7):956–964. doi:10.1097/PAS.0b013e3181e02f45
Kutomi G (2012) Clinicopathological characteristics of basal type breast cancer in triple-negative breast cancer. J Cancer Ther 03(05):836–840. doi:10.4236/jct.2012.325106
Groheux D, Moretti JL, Baillet G et al (2008) Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys 71(3):695–704
Osborne JR, Port E, Gonen M et al (2010) 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med 51(4):543–550
Aogi K, Kadoya T, Sugawara Y et al (2015) Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat 150(1):209–217
Acknowledgments
We thank Trista Leong for her support using the cancer registry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author's contributions
YY carried out the design of study, data collection, imaging processing, participated in statistical analysis and drafted the manuscript. XC participated in design of study and participated in drafting of manuscript. BS carried out the pathology IHC results. WA participated in coordination of study and verification of clinical information. XZ participated in statistical analysis. BF participated in coordination of study, helped data analysis and manuscript preparation. All authors read and approved the final manuscript.
Competing interests
Yue is supported by Martz Translational Breast Cancer Research Fund, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center. Cui is supported by National Institutes of Health (CA151610), the Avon Foundation (02-2014-063), David Salomon Translational Breast Cancer Research Fund, the Fashion Footwear Charitable Foundation of New York, Inc., Associates for Breast and Prostate Cancer Studies, and the Margie and Robert E. Petersen Foundation. None of authors have any non-financial competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yue, Y., Cui, X., Bose, S. et al. Stratifying triple-negative breast cancer prognosis using 18F-FDG-PET/CT imaging. Breast Cancer Res Treat 153, 607–616 (2015). https://doi.org/10.1007/s10549-015-3558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3558-1