Abstract
Purpose
To determine whether 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtype and is able to predict molecular subtypes.
Methods
This retrospective study involved 306 patients with 308 mass-type invasive breast cancers (mean size 2.65 cm, range 1.0–15.0 cm) who underwent 18F-FDG PET/CT before therapy. The correlations between primary tumour 18F-FDG uptake on PET/CT, expressed as SUVmax, and clinicopathological findings and molecular subtype, i.e. luminal A, luminal B (HER2-negative), luminal B (HER2-positive), HER2-positive and triple-negative, were analysed. The predictors of these subtypes were investigated.
Results
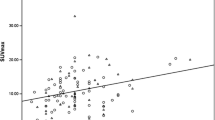
The mean SUVmax of the 308 tumours was 5.33 ± 3.63 (range 1.15–19.01). Among the subtypes of the 308 tumours, 87 (28.2 %) were luminal A, 111 (36.0 %) were luminal B (HER2-negative), 31 (10.1 %) were luminal B (HER2-positive), 26 (8.4 %) were HER2-positive and 53 (17.2 %) were triple-negative, and the corresponding mean SUVmax were 3.41 ± 2.07 (range 1.18–14.30), 5.17 ± 3.52 (range 1.35–19.01), 6.57 ± 3.84 (range 1.42–15.58), 7.55 ± 3.63 (range 2.30–13.60) and 6.97 ± 4.17 (range 1.15–16.06), respectively. A cut-off value of 3.60 yielded 70.1 % sensitivity and 66.1 % specificity with an area under the receiver operating characteristics curve (AUC) of 0.734 for predicting that a tumour was of the luminal A subtype. A cut-off value of 6.75 yielded 65.4 % sensitivity and 75.2 % specificity with an AUC of 0.704 for predicting a HER2-positive subtype.
Conclusion
SUVmax, a metabolic semiquantitative parameter, shows a significant correlation with the molecular subtype of breast cancer, and is useful for predicting the luminal A or HER2-positive subtype.



Similar content being viewed by others
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
van’t Veer LJ, Paik S, Hayes DF. Gene expression profiling of breast cancer: a new tumor marker. J Clin Oncol. 2005;23:1631–5.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50.
Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Dubsky P, Filipits M, Jakesz R, Rudas M, Singer CF, Greil R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24:640–7.
Niemiec J, Adamczyk A, Malecki K, Ambicka A, Rys J. Tumor grade and matrix metalloproteinase 2 expression in stromal fibroblasts help to stratify the high-risk group of patients with early breast cancer identified on the basis of St Gallen recommendations. Clin Breast Cancer. 2013;13:119–28.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50.
Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Wang CL, MacDonald LR, Rogers JV, Aravkin A, Haseley DR, Beatty JD. Positron emission mammography: correlation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status and 18F-FDG. AJR Am J Roentgenol. 2011;197:W247–55.
Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–8.
Koo HR, Park JS, Kang KW, Cho N, Chang JM, Bae MS, et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24:610–8.
García Vicente AM, Soriano Castrejón Á, León Martín A, Chacón López-Muñiz I, Muñoz Madero V, Muñoz Sánchez Mdel M, et al. Molecular subtypes of breast cancer: metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11.
Miyake KK, Nakamoto Y, Kanao S, Tanaka S, Sugie T, Mikami Y, et al. Diagnostic value of 18F-FDG PET/CT and MRI in predicting the clinicopathologic subtypes of invasive breast cancer. AJR Am J Roentgenol. 2014;203:272–9.
Murase K, Yanai A, Saito M, Imamura M, Miyagawa Y, Takatsuka Y, et al. Biological characteristics of luminal subtypes in pre- and postmenopausal estrogen receptor-positive and HER2-negative breast cancers. Breast Cancer. 2014;21:52–7.
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–44.
Caudle AS, Yu TK, Tucker SL, Bedrosian I, Bedrosian I, Litton JK, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83.
Compliance with ethical standards
Conflicts of interest
None.
Human and animal rights and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This was a retrospective study, for which formal consent is not required. This article does not describe any studies with animals performed by any of the authors. This retrospective study was approved by the institutional review board, and the need for patient informed consent was waived.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitajima, K., Fukushima, K., Miyoshi, Y. et al. Association between 18F-FDG uptake and molecular subtype of breast cancer. Eur J Nucl Med Mol Imaging 42, 1371–1377 (2015). https://doi.org/10.1007/s00259-015-3070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3070-1