Abstract
Postmenopausal women with advanced breast cancer recurring/progressing on or after initial (adjuvant or first-line) endocrine therapy may be treated multiple times with one of several endocrine or combinatorial targeted treatment options before initiating chemotherapy. In the absence of direct head-to-head comparisons of these treatment options, an indirect comparison can inform treatment choice. This network meta-analysis compared the efficacy of everolimus plus exemestane with that of fulvestrant 250 and 500 mg in the advanced breast cancer setting following adjuvant or first-line endocrine therapy. The reported hazard ratios (HRs) for progression-free survival (PFS) or time to progression from six studies that formed a network to compare everolimus plus exemestane (BOLERO-2 trial) with fulvestrant were analyzed by means of a Bayesian network meta-analysis. In the primary comparison (PFS analysis based on the local review of disease progression from BOLERO-2 with the data from the other studies), everolimus plus exemestane appeared to be more efficacious than both fulvestrant 250 mg (HR = 0.47; 95 % credible interval [CrI] 0.38–0.58) and 500 mg (HR = 0.59; 95 % CrI 0.45–0.77). Similar results were obtained in an alternate comparison based on central review of disease progression from BOLERO-2 with the data from the other studies (HR = 0.40; 95 % CrI 0.31–0.51 and HR = 0.50; 95 % CrI 0.37–0.67, respectively), and in a subgroup analysis of patients who had received prior aromatase inhibitor therapy (HR = 0.47; 95 % CrI 0.38–0.58 and HR = 0.55; 95 % CrI 0.40–0.76, respectively). These results suggest that everolimus plus exemestane may be more efficacious than fulvestrant in patients with advanced breast cancer who progress on or after adjuvant or first-line therapy with a nonsteroidal aromatase inhibitor.
Similar content being viewed by others
Introduction
For patients with hormone-receptor-positive advanced breast cancer, endocrine therapy is the recommended initial treatment, and aromatase inhibitors (AIs) are the preferred option for postmenopausal women because they elicit prolonged disease control compared with tamoxifen [1–3]. Treatment options for patients who experience disease progression after nonsteroidal AI therapy are limited. Recently, the combination regimen of everolimus, an mTOR inhibitor, and exemestane, a steroidal AI, was approved for use in this setting based on significant improvement of progression-free survival (PFS) versus exemestane alone [4]. Other options include exemestane alone, fulvestrant (an estrogen-receptor down-regulator), megestrol acetate, and chemotherapy [1–3]. Current guidelines recommend continued endocrine therapy for responsive disease; however, clinical questions regarding optimal sequencing of therapy remain [1–3].
Exemestane has shown activity in patients with advanced breast cancer as a second- and third-line agent following nonsteroidal AI treatment [5]. In patients who had disease progression after first-line letrozole or anastrozole (n = 249), exemestane provided a clinical benefit rate of 27 % with a median PFS of 3.4 months [5]. Even after two prior endocrine treatments in the advanced breast cancer setting (n = 60), exemestane demonstrated a clinical benefit rate of 38 % [6].
Robust clinical evidence favoring the use of everolimus plus exemestane rather than exemestane alone was demonstrated in BOLERO-2, a phase 3 study in postmenopausal women with hormone-receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer progressing/recurring on/after nonsteroidal AI treatment [4, 7]. In this study, median PFS was more than doubled in the everolimus plus exemestane group (7.8 months vs 3.2 for exemestane alone, by local assessment; hazard ratio [HR] = 0.45; P < 0.0001). Central assessment of median PFS confirmed the clinical benefit with everolimus plus exemestane (11.0 months vs 4.1 for exemestane alone; HR = 0.38; P < 0.0001). In addition, PFS benefits were consistent across patient subgroups defined by age, race, presence of visceral metastases, and prior chemotherapy.
A clinical trial of fulvestrant 250 mg in postmenopausal women with hormone-receptor-positive advanced breast cancer progressing on nonsteroidal AI treatment showed a similar duration of disease control as with exemestane (median time to progression [TTP] = 3.7 months in both treatment groups; HR = 0.963; P = 0.6531) [8]. However, a clinical trial of fulvestrant 250 mg versus fulvestrant 500 mg in patients progressing after endocrine treatment demonstrated a significant, albeit modest, improvement in median PFS in the fulvestrant 500-mg group (5.5 vs 6.5 months, respectively; HR = 0.80; P = 0.006) [9]. In patients previously treated with an AI, the magnitude of median PFS benefit with 500 versus 250 mg fulvestrant was less than in patients previously treated with an antiestrogen.
The currently available clinical evidence data support the use of both everolimus plus exemestane and fulvestrant in postmenopausal women with advanced breast cancer progressing on or after nonsteroidal AI therapy. However, the limited clinical data on direct comparisons of these agents present a challenge to optimization of disease management. Although the apparent magnitude of PFS benefit with everolimus plus exemestane exceeds that with fulvestrant, such empirical cross-trial comparisons have limited validity. In such cases, treatment decisions may be guided by an indirect comparison, which can estimate relative efficacy parameters of different treatment regimens across clinical studies. For example, a recent indirect treatment comparison for the efficacy of bevacizumab plus interferon-alpha-2a versus tyrosine kinase inhibitors as first-line treatment in metastatic renal cell carcinoma showed that the two treatment regimens were similar with regard to PFS [10]. Guidelines for conducting and interpreting indirect treatment comparisons exist to standardize and support this area of research [11–13]. This network analysis was conducted to compare the efficacy of everolimus plus exemestane versus fulvestrant in patients with advanced breast cancer who are eligible for further endocrine therapies.
Methods
Evidence base: search strategy
As everolimus (plus exemestane) and fulvestrant 250 mg have both been compared with exemestane monotherapy, an indirect comparison was possible through the common exemestane arms in the studies. However, comparisons involving fulvestrant 500 mg, and everolimus plus tamoxifen, required the identification of additional studies with common treatment/comparator arms to complete the network. Studies connecting everolimus and fulvestrant were identified through systematic reviews of treatments for advanced or metastatic breast cancer found from a database search of the Cochrane Library (CDSR, DARE, and HTA, 2010–2012), EMBASE, and MEDLINE. Search terms included breast or mammary and disease descriptors (cancer, neoplasm, oncology, tumor, malignancy, carcinoma, adenocarcinoma, or sarcoma) as well as metastasis, advanced, secondary, recurrent, inoperable, disseminated, or incurable. Search terms for treatments included everolimus or Afinitor as well as SDZ-RAD, rad001, or 159351-69-6. Recent studies were identified through Web site searches (National Horizon Scanning Centre, NICE, FDA, EMA, ASCO, ESMO, ISPOR, ECCO, EBCC, and SABCS) conducted March 8–9, 2012.
Eligible studies were assessed for quality based on seven items: appropriate randomization; adequate concealment of treatment allocation; groups similar at the onset of the study in terms of prognostic factors, care providers, participants, and outcome assessors blind to treatment allocation; unexpected imbalances in dropouts between groups; evidence to suggest that more outcomes were measured than reported; intent-to-treat analysis; and appropriate methods used to account for missing data.
Quality assessments and assumptions
Data extracted from each trial included design, methodology, statistical methods, patient characteristics, and outcome measures. Subgroup data, whenever possible, were analyzed for relevance. The primary analysis was for relative efficacy (PFS/TTP) between everolimus and fulvestrant in the full patient population of the studies included in the network, using the local assessment of PFS from BOLERO-2. Secondary analyses included a subgroup analysis by prior AI treatment and an additional analysis for everolimus plus exemestane versus fulvestrant using the BOLERO-2 central assessment of PFS. Using the connector studies, exploratory analyses of the relative efficacy of everolimus plus tamoxifen versus fulvestrant were conducted. Additionally, the network meta-analysis of the full patient population was performed for the relative efficacy of everolimus plus tamoxifen versus fulvestrant using the Howell study [14] in place of the Paridaens study (which was a comparison of tamoxifen versus exemestane and needed the SoFEA/EFECT studies to complete a network for comparison versus fulvestrant 250 mg) as an additional sensitivity analysis [15]. The Howell study was identified following the systematic review, and therefore did not have data extracted in as much detail as the other studies and was not assessed for quality.
In conducting these analyses, several assumptions were made regarding the data from the individual studies. For the primary analysis, PFS and TTP were assumed to be similar because disease-specific death events were included in each endpoint in the studies analyzed. Moreover, as the survival time in advanced breast cancer is shorter than in early stage disease [9, 16], the majority of deaths were assumed to be disease-specific.
Statistical analysis
All treatments evaluated in the primary analysis were connected by one or more studies. Data consisted of the HRs for PFS/TTP and the 95 % confidence intervals (CI) for each of the trials as extracted from published reports. The log HRs and their precision (reciprocal of the variance) were calculated and input into the model. For the CONFIRM study, the HRs for the subgroup with prior AI therapy were calculated from a digitized copy of the published forest plot [9]. Accuracy was checked against calculations for known results (all patients); resulting differences on the HR scale were 0.005 or less. As not all studies included outcome information on patients with prior AI therapy, the only comparisons available were for everolimus plus exemestane, exemestane alone, and fulvestrant 250 and 500 mg.
The indirect treatment comparison for relative efficacy between everolimus (with exemestane or tamoxifen) and fulvestrant used a Bayesian approach as described by Welton et al. [17]. Specifically, a Bayesian fixed-effect model was used to describe the log HRs. In this type of comparison, evidence is synthesized from aggregate data arising from randomized, controlled studies. Exemestane was adopted as the reference treatment because it is used in clinical practice and is the treatment with the most information in the network; basic parameters of the model are the log HRs with respect to exemestane. The log HR from each study was assumed to be normally distributed, with the log HR mean equaling the true log HR observed in each study and the variance equaling the observed variability in each study. The Markov chain Monte Carlo (MCMC) software WinBUGS (version 1.4.3) was used to estimate the log HRs for each possible pairwise comparison. For each outcome measure, the analysis was performed with three chains, each run for a series of 20,000 burn-in simulations to allow convergence. Convergence was checked using the Gelman–Rubin statistic, and a visual inspection demonstrated satisfactory convergence by 20,000 iterations. A further 20,000 updates were run for each chain, and estimates were obtained from those updates. Median HRs and the 95 % credible interval (CrI) are presented for the HRs. If the 95 % CrI does not include 1, this suggests that there is evidence of a difference between the treatments.
Results
Network analysis evidence base
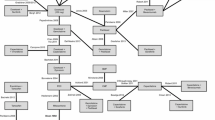
A total of seven studies were identified that could be used in a network analysis. Of these, only six were used to form the basis of a network analysis, with the seventh [14] used as an alternative for an additional sensitivity analysis. The evidence network with the connections between the comparators included in the primary analyses is shown in Fig. 1. Details of the individual study designs are provided in Table 1 [4, 5, 7–9, 14, 15, 18]. All patients in the 7 trials were postmenopausal and had locally advanced or metastatic disease. All trials reported on PFS/TTP outcomes. The TAMRAD study reviewed disease progression data locally, whereas the BOLERO-2 study included both local and central review. The other studies did not specifically state that a central review was performed. In addition, all but the open-label Paridaens and TAMRAD trials were double-blind.
In general, the overall patient demographics and disease characteristics were similar (Table 2) [4, 5, 8, 9, 14, 15, 18]. There were slight differences in the CONFIRM population versus the other primary studies regarding prior treatments before study entry. Although all patients in CONFIRM had prior endocrine therapy, prior AI therapy was not an inclusion criterion, whereas the other primary studies (except the Paridaens and Howell studies) had this constraint. However, information from the subgroup of patients who had prior AI treatment was available for analysis from the CONFIRM study (as noted in the statistical analysis), and was used in a comparison with the other studies’ patient populations who had prior AI therapy. The Paridaens and Howell studies also differed in other prior treatment eligibility requirements. The Paridaens study permitted prior chemotherapy but not endocrine therapy as first-line treatment in the advanced setting, whereas in the Howell study, first-line treatment for metastatic disease was not allowed and information on patients with prior AI therapy was not available. Hazard ratios for PFS/TTP in each study used in the analyses are shown in Table 3 [4, 5, 8, 9, 14, 15, 18, 19].
Analysis of efficacy
In the primary analysis, the results suggest that everolimus plus exemestane is more efficacious for PFS/TTP than both fulvestrant 250 (HR = 0.47; 95 % CrI 0.38–0.58) and 500 mg (HR = 0.59; 95 % CrI 0.45–0.77) (Table 4). The exploratory analysis of the other pairwise comparisons (using the Paridaens study) found no evidence of a difference between everolimus plus tamoxifen and fulvestrant 250 or 500 mg (Table 4). The analysis using the BOLERO-2 PFS data based on central review suggested that everolimus plus exemestane remained more efficacious for PFS/TTP than fulvestrant 250 mg and fulvestrant 500 mg (HR = 0.40; 95 % CrI 0.31–0.51 and HR = 0.50; 95 % CrI 0.37–0.67, respectively; Table 4).
The results of a sensitivity analysis based on an alternative network, which substituted the Howell study for the Paridaens study, were generally consistent with the analysis based on the original network, although this exploratory analysis suggested that everolimus plus tamoxifen is more efficacious for PFS/TTP than fulvestrant 250 mg (HR = 0.46; 95 % CrI 0.29–0.71) and 500 mg (HR = 0.57; 95 % CrI 0.35–0.92).
Prior aromatase inhibitor therapy
In patients who received prior AI therapy, based on local assessment of PFS from BOLERO-2, everolimus plus exemestane was more efficacious for PFS/TTP than fulvestrant 250 and 500 mg (HR = 0.47; 95 % CrI 0.38–0.58 and HR = 0.55; 95 % CrI 0.40–0.76, respectively; Table 5). Using the PFS data from the centrally reviewed BOLERO-2 study did not substantially change the results using the locally reviewed PFS data; everolimus plus exemestane remained more efficacious for PFS/TTP than fulvestrant 250 and 500 mg (Table 5).
Discussion
Direct comparative clinical evidence for optimal treatments following a nonsteroidal AI in postmenopausal women with hormone-receptor-positive advanced breast cancer is limited [8, 20]. In this setting, clinical studies of endocrine monotherapy or combinations of endocrine agents have demonstrated limited efficacy [8, 20], and the optimal sequence of endocrine treatment remains unclear. In contrast, combining endocrine agents with newer targeted agents (e.g., everolimus) to block cell signaling pathways known to interact with the estrogen receptor provided increased clinical benefits versus endocrine monotherapy [4, 7]. In the absence of head-to-head clinical studies, an indirect comparison of clinical evidence can be used to provide estimates of relative efficacy and inform treatment decisions.
In this network meta-analysis, everolimus plus exemestane was shown to provide an improved PFS/TTP compared with fulvestrant alone at either 250 or 500 mg. Everolimus plus exemestane also provided greater clinical benefit versus fulvestrant 250 and 500 mg in the subgroup of patients who had prior AI therapy. The results from this network analysis are supported, in part, by the clinical evidence from an earlier network meta-analysis, which reported that fulvestrant 500 mg was expected to be at least as efficacious as exemestane alone in postmenopausal women with advanced breast cancer who progressed on endocrine therapy [21], and everolimus plus exemestane was more efficacious than exemestane alone in this setting [4]. Furthermore, in this earlier meta-analysis, fulvestrant plus anastrozole did not increase clinical benefits (TTP and overall survival) versus anastrozole alone in patients with breast cancer at first relapse [22]. However, this patient population was dissimilar (premenopausal patients were included and very few patients received prior AI therapy) to the patients in the current analysis.
The Decision Support Unit of National Institute for Health and Clinical Excellence reports that network meta-analyses may suffer from problems of unobserved effect modifiers, as do pairwise meta-analyses [23]. However, provided the constituent trials are unbiased and were conducted in similar patient populations, both analyses should result in unbiased estimates of treatment effects and are superior to observational studies because they are based on randomized comparisons [23]. However, there are limitations inherent to these types of indirect comparisons. The small number of included studies precluded a quantitative exploration of heterogeneity among trial results. Heterogeneity is an important aspect in indirect analyses, and PFS (used in this analysis to assess comparative efficacy) is affected by patient prognosis at baseline. An important feature of this methodology is that no assumptions are made regarding trial-specific baselines and that between-trial heterogeneity is set to zero, thus assuming homogeneity of the underlying true treatment effects. The CONFIRM trial did have a different patient population than the other trials; however, the subgroup analysis of patients who had prior AI therapy ameliorated those differences. The patient populations from the Paridaens and Howell studies were also different in terms of prior endocrine therapies, which can bias the analysis, although they only affect the exploratory comparison of everolimus plus tamoxifen versus fulvestrant and do not affect the primary comparison of everolimus plus exemestane versus fulvestrant. In addition, the CONFIRM and EFECT studies did not have data on HER2 status. Inclusion of a sizable proportion of patients with HER2-positive disease may lower efficacy results compared with the other studies that had a low proportion of patients with HER2-positive disease.
Limitations specific to this network analysis include the use of both double-blind and open-label trials and a combination of two different outcome measures—PFS and TTP. Although PFS and TTP can have dissimilar results because deaths are not typically included in TTP, the two outcome measures had overlapping criteria for disease-specific deaths or deaths within 6 months of last tumor assessment as an inclusion event in the network studies. Survival time in advanced breast cancer is shorter than in early stage disease [9, 16]; therefore, the majority of on-study deaths were assumed to be disease-specific, and the analysis assumed that PFS and TTP would yield similar results.
In conclusion, this indirect treatment comparison suggests that everolimus in combination with exemestane may be more efficacious than fulvestrant 250 mg (HR = 0.47; 53 % risk reduction of PFS event) and 500 mg (HR = 0.59; 41 % risk reduction of PFS event) in postmenopausal women with hormone-receptor-positive, advanced breast cancer that has relapsed or progressed on/after a nonsteroidal AI. Future trials should evaluate a selective combination of these therapies to define an optimal treatment strategy.
References
Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N, Pagani O, Senkus E, Thomssen C, Aapro M, Bergh J, Di Leo A, El Saghir N, Ganz PA, Gelmon K, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Leadbeater M, Mayer M, Rodger A, Rugo H, Sacchini V, Sledge G, van’t Veer L, Viale G, Krop I, Winer E (2012) 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 21(3):242–252. doi:10.1016/j.breast.2012.03.003
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(suppl 7):vii11–vii19. doi:10.1093/annonc/mds232
National Comprehensive Cancer Network (2012) NCCN clinical practice guidelines in oncology: breast cancer. Version 3.2012. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 25 Oct 2013
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, Im YH, Braybrooke JP, Brunt AM, Cheung KL, Jyothirmayi R, Robinson A, Wardley AM, Wheatley D, Howell A, Coombes G, Sergenson N, Sin HJ, Folkerd E, Dowsett M, Bliss JM, SoFEA investigators (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998. doi:10.1016/S1470-2045(13)70322-X
Gennatas C, Michalaki V, Carvounis E, Psychogios J, Poulakaki N, Katsiamis G, Voros D, Kouloulias V, Mouratidou D, Tsavaris N (2006) Third-line hormonal treatment with exemestane in postmenopausal patients with advanced breast cancer progressing on letrozole or anastrozole. A phase II trial conducted by the Hellenic Group of Oncology (HELGO). Tumori 92(1):13–17
Piccart M, Baselga J, Noguchi S, Burris HA, Gnant M, Hortobagyi GN, Mukhopadhyay P, Taran T, Sahmoud T, Rugo H (2012) Final progression-free survival analysis of BOLERO-2: a phase III trial of everolimus for postmenopausal women with advanced breast cancer. Presented at CTRC-AACR San Antonio Breast Cancer Symposium, San Antonio, TX, 4–8 December 2012. Poster P6-04-02
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26(10):1664–1670. doi:10.1200/JCO.2007.13.5822
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JP, Sapunar F, Martin M (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28(30):4594–4600. doi:10.1200/JCO.2010.28.8415
Mickisch GH, Schwander B, Escudier B, Bellmunt J, Maroto JP, Porta C, Walzer S, Siebert U (2011) Indirect treatment comparison of bevacizumab+interferon-alpha-2a vs tyrosine kinase inhibitors in first-line metastatic renal cell carcinoma therapy. Clinicoecon Outcomes Res 3:19–27. doi:10.2147/CEOR.S16118
Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, Boersma C, Thompson D, Larholt KM, Diaz M, Barrett A (2011) Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 2. Value Health 14(4):429–437. doi:10.1016/j.jval.2011.01.011
Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 1. Value Health 14(4):417–428. doi:10.1016/j.jval.2011.04.002
National Institute for Health and Care Excellence (2013) A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials (TSD2). http://www.nicedsu.org.uk/Evidence-Synthesis-TSD-series(2391675).htm. Accessed 25 Oct 2013
Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, Watanabe T, Morris C, Webster A, Dimery I, Osborne CK (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 22(9):1605–1613. doi:10.1200/JCO.2004.02.112
Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, Piccart MJ, Bogaerts J, Therasse P (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 26(30):4883–4890. doi:10.1200/JCO.2007.14.4659
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Lang I, Wardley A, Rabaglio M, Price KN, Gelber RD, Coates AS, Thurlimann B, BIG Collaborative Group, International Breast Cancer Study Group (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol 12(12):1101–1108. doi:10.1016/S1470-2045(11)70270-4
Welton NJ, Sutton AJ, Cooper NJ, Abrams KR, Ades AE (2012) Evidence synthesis for decision making in healthcare. Wiley, Chichester. doi: 10.1002/9781119942986.ch1
Fulvestrant with or without anastrozole or exemestane alone in treating postmenopausal women with locally advanced or metastatic breast cancer (2013). http://www.clinicaltrials.gov/ct2/show/NCT00253422?term=sofea&rank=1. Accessed 25 Oct 2013
Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard JC, Debled M, Spaeth D, Legouffe E, Allouache D, El Kouri C, Pujade-Lauraine E (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30(22):2718–2724. doi:10.1200/JCO.2011.39.0708
Johnston S, Kilburn LS, Ellis P, Cameron D, Dodwell D, Howell A, Im YH, Coombes G, Dowsett M, Bliss JM (2012) Fulvestrant alone or with concomitant anastrozole vs exemestane following progression on non-steroidal aromatase inhibitor—first results of the SoFEa trial (CRUKE/03/021 & CRUK/09/007) (ISRCTN44195747). Eur J Cancer 48(suppl 3):S2 Abstract 2LBA
Cope S, Ouwens MJ, Jansen JP, Schmid P (2013) Progression-free survival with fulvestrant 500 mg and alternative endocrine therapies as second-line treatment for advanced breast cancer: a network meta-analysis with parametric survival models. Value Health 16(2):403–417. doi:10.1016/j.jval.2012.10.019
Bergh J, Jonsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattstrom D, Lindemann JP, Wiklund F, Henriksson R (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30(16):1919–1925. doi:10.1200/JCO.2011.38.1095
Dias S, Sutton AJ, Welton NJ, Ades AE (2011) NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment. Last updated April 2012. http://www.nicedsu.org.uk. Accessed 25 Oct 2013
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Tamalette Loh, PhD, ProEd Communications, Inc., for medical editorial assistance with this manuscript.
Conflict of interests
TB: Advisor for Novartis Pharmaceuticals Corporation, received research support and speaker honoraria from Novartis. RMcC: Received research support from Novartis Pharmaceuticals Corporation. SD: Received research support from Novartis Pharmaceuticals Corporation. JG: Received research support from Novartis Pharmaceuticals Corporation. DV: Received research support from Novartis Pharmaceuticals Corporation. KF: Received research support from Novartis Pharmaceuticals Corporation. JZ: Employee of Novartis Pharmaceuticals Corporation. GJ: Advisor for Novartis Pharmaceuticals Corporation, received research support and speaker honoraria from Novartis.
Source of Support
Research was funded by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bachelot, T., McCool, R., Duffy, S. et al. Comparative efficacy of everolimus plus exemestane versus fulvestrant for hormone-receptor-positive advanced breast cancer following progression/recurrence after endocrine therapy: a network meta-analysis. Breast Cancer Res Treat 143, 125–133 (2014). https://doi.org/10.1007/s10549-013-2778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2778-5