Abstract
Objectives
Cerebrotendinous xanthomatosis (CTX) is a rare inherited neurodegenerative disorder in bile acid synthesis. The natural history of neurological abnormalities in CTX is not well understood. The object of this study was to determine neurological progression in CTX.
Methods
A literature search on PubMed for “cerebrotendinous xanthomatosis” yielded 91 publications that reported cases of CTX patients. Two independent reviewers abstracted information about the presence and age of onset of neurological abnormalities in published CTX cases. For each neurological abnormality, we estimated the probability of its onset at any given age using cumulative incidence function analysis. We also present our own case series, in which five CTX patients were evaluated.
Results
The literature search yielded 194 CTX cases (ages ranging from newborn to 67 years old). The most common neurological abnormalities were corticospinal tract abnormalities including weakness, hyperreflexia, spasticity, Babinski sign (59.8%), ataxia (58.8%), cognitive decline (46.4%), and gait difficulty (38.1%); 68 (35.0%) had baseline cognitive problems. Cumulative incidence function analysis revealed that ataxia, gait difficulties, and corticospinal tract abnormalities developed throughout life, while cognitive decline tended to develop later in life. Of the less common neurological abnormalities, seizures, psychiatric changes and speech changes developed throughout life, while parkinsonism and sensory changes tended to develop later in life. Our case series corroborated this temporal pattern of neurological abnormalities.
Conclusion
We provide estimates for the neurological progression of CTX, categorizing neurological abnormalities according to time and probability of development. Our approach may be applicable to other rare disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebrotendinous xanthomatosis (CTX) is a rare inherited neurodegenerative disorder in bile acid synthesis associated with mutations in the CYP27A1 gene and elevation in cholestanol levels (Moghadasian et al 2002; Degos et al 2016). Systemic abnormalities in CTX include tendon xanthomas, juvenile cataracts, and chronic diarrhea (Schimschock et al 1968; Moghadasian et al 2002). Neurological symptoms are typically progressive, including cognitive or psychiatric changes, ataxia, spasticity, weakness, sensory impairment, and speech changes, or more rarely, parkinsonism or seizures (Schimschock et al 1968; Moghadasian et al 2002). While treatment with chenodeoxycholic acid (CDCA) can lower cholestanol levels, some CTX patients experience progressive symptoms even on CDCA (Berginer et al 1984; Moghadasian et al 2002). It is unclear whether this is due to late initiation of CDCA or due to symptom progression recalcitrant to CDCA, but in any case, this highlights the importance of development of novel therapies for CTX.
Future development of any treatment for CTX requires understanding the progression of neurological symptoms in CTX, as any potential therapy should demonstrate modulation of neurological progression. While the symptom constellation of tendon xanthomas, cataracts, diarrhea, and neurological abnormalities have been documented in the past, little attention has been given to the progression of neurological abnormalities over time. The goal of this study is thus to analyze the neurological progression of CTX. Given that CTX is a relatively rare disease, we present an approach to evaluate the temporal progression of neurological abnormalities based on analysis of published cases of CTX in the current literature. We also present a new case series of CTX to illustrate the temporal progression of neurological abnormalities.
Methods
Literature review
In the literature review, we reviewed published case series or cohorts of CTX patients. A search performed in January 2016 using PubMed for “cerebrotendinous xanthomatosis” yielded 590 publications. We excluded papers not published in English, papers not about human patients, review papers, and papers without clinical case descriptions of CTX. Two independent reviewers (J.C.W., K.W.) analyzed the CTX cases reported in these publications. For each case reported, we documented the presence and age of onset (if available) of the following neurological abnormalities: 1) cognitive decline, 2) ataxia, 3) corticospinal tract abnormalities (CST, including weakness, hyperreflexia, spasticity, Babinski sign), 4) sensory loss, 5) speech changes, 6) gait difficulty, 7) psychiatric changes, 8) seizure, and 9) parkinsonism. We also documented the presence and age of onset of the following non-neurological abnormalities: 1) diarrhea, 2) xanthomas, and 3) cataracts. If a patient was first reported to have an abnormality at or by a certain age, then that age would be considered the age of onset. CDCA treatment and age of treatment initiation if applicable were also documented. CDCA treatment was considered an independent event from neurological abnormalities.
Some reports provided qualitative information about the timing of onset of neurological or non-neurological abnormalities without stating the exact ages of onset. We estimated the ages of onset in these cases using the following rules: if the decade of onset (such as “30s”) of onset was known, then the decade (i.e., “30 years old”) would be noted as age of onset. If a patient had the abnormality since infancy, age of onset would be noted as “1 year old”. If a patient had the abnormality since childhood or “school age” or “early age”, age of onset would be noted as “10 years old”. If patient had the abnormality since teenage years, age of onset would be noted as “15 years old”.
Data analysis
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc.). For each abnormality, we estimated the proportion of patients with onset of that abnormality at any given age using the cumulative incidence function (CIF) (Putter et al 2007; Varadhan et al 2010). This estimate correctly accounts for patients who never had the abnormality prior to loss to follow up or never had the abnormality prior to death. Briefly, the CIF is the probability of experiencing an event within a time interval (Putter et al 2007; Varadhan et al 2010). At a given time point, the cumulative incidence function is the probability of experiencing the event before that time and before occurrence of a different type of event (Austin et al 2016). This method is preferred over the Kaplan-Meier estimate, which does not take into account the competing risk of death which precludes the primary event of interest (i.e., onset of the neurological abnormality) (Putter et al 2007; Varadhan et al 2010). The CIF accounts for the competing risk of death by taking into account the cause-specific hazard (instantaneous rate of experience an event at a specific time) of both the abnormality and death (Putter et al 2007; Zhang et al 2008; Varadhan et al 2010).
For some patients, the reports indicated presence of symptoms, but the age of onset was unknown. To roughly account for this missing data, we estimated two CIFs, one “worst case scenario” and one “best case scenario.” For the “worst” case CIF, we assumed that symptom onset occurred at the earliest age of onset in that domain observed within our cohort. For the “best” case CIF, we assumed that symptom onset occurred just prior to last follow up. These estimates should bracket the estimate we would obtain if we had no missing age of onset data.
Patients
In our case series, five patients with biochemically confirmed CTX were evaluated by neurologists (including F.S.E.) at Massachusetts General Hospital in a pediatric and adult neurology clinic. This retrospective medical record review was approved by the Partners HealthCare (Massachusetts General Hospital) institutional review board. Our CTX case series was not included in the CIF analysis, as above, in order to provide a comparison with CTX cases obtained from literature.
Results
Summary of literature review
Literature review included 91 unique publications reporting case series of CTX patients (Menkes et al 1968; Schimschock et al 1968; Stahl et al 1971; Salen and Polito 1972; Farpour and Mahloudji 1975; Schreiner et al 1975; Canelas et al 1983; Berginer et al 1988a, b; Chang et al 1992; Hokezu et al 1992; Mondelli et al 1992; Wevers et al 1992; Dotti et al 1994; Meiner et al 1994; Nakashima et al 1994; Dotti et al 1995; Soffer et al 1995; Kuwabara et al 1996; Watts et al 1996; Dormans et al 1997; Chen et al 1998; Kawabata et al 1998; Wakamatsu et al 1999; Barkhof et al 2000; Vanrietvelde et al 2000; Burnett et al 2001; Ohno et al 2001; Lee et al 2002; Rystedt et al 2002; Gaikwad et al 2003; Ito et al 2003; Kato et al 2003; Bartholdi et al 2004; Lange et al 2004; Valdivielso et al 2004; Clemen et al 2005; Lorincz et al 2005; Brodsky et al 2006; Siman-Tov et al 2006; Bhattacharyya et al 2007; Geraldes et al 2007; Gonzalez-Cuyar et al 2007; Hansson et al 2007; Okuma et al 2007; Price Evans et al 2007; Smithard et al 2007; Wang et al 2007; Mehta and Shmerling 2008; Pilo de la Fuente et al 2008; Szlago et al 2008; Berginer et al 2009; Sandeep et al 2009; Bonnot et al 2010; Bourkiza et al 2010; Cerqueira et al 2010; Chang et al 2010; Gallus et al 2010; Habaragamuwa and Bajekal 2010; Kamate et al 2010; Nozue et al 2010; Reichwaldt et al 2010; Schneider et al 2010; Su et al 2010; Bhojwani and Khot 2011; Chen et al 2011a, b; Huang et al 2011; Matysik et al 2011; Monson et al 2011; Posada and Ramos 2011; Rafiq et al 2011; Seidel et al 2011; Agrawal and Garg 2012; Androdias et al 2012; Chen et al 2012; Huijgen et al 2012; Kauffman et al 2012; Kottahachchi et al 2012; Koyama et al 2012; McKinnon and Bosch 2012; Mignarri et al 2012a, b; Pedroso et al 2012; Jain et al 2013; Khan et al 2013; Pudhiavan et al 2013; Kapas et al 2014; Luyckx et al 2014; Mandrile et al 2014; Uygunoglu et al 2014). Within these publications, there was clinical information on 194 CTX patients (83 female, 110 male, one unspecified) with ages ranging from newborn to 67 years old. In 134 (71.6%) of these cases, cholestanol level testing was performed. In 89 (45.9%) of these cases, genetic testing for mutations in the sterol 27-hydroxylase gene (CYP27A1) was performed. Eighty (41.2%) of these patients were reported to have received CDCA. Of these 194 CTX patients, 116 (59.8%) had CST abnormalities, 114 (58.8%) had ataxia, 90 (46.4%) had cognitive decline, 74 (38.1%) had gait difficulty, 41 (21.1%) had sensory loss, 37 (19.1%) had seizure, 36 (18.6%) had speech changes, 34 (17.5%) had psychiatric changes, and 19 (9.8%) had parkinsonism; 68 (35.0%) had baseline cognitive problems. Non-neurological symptoms were also documented: 147 (75.8%) had cataracts, 138 (71.1%) had xanthomas, and 42 (21.6%) had diarrhea. There were 14 (7.2%) CTX patients with reported death from any cause.
Temporal analysis of neurological abnormalities
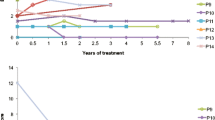
We used the cumulative incidence function (CIF) to estimate the probability of developing a neurological abnormality at a given age (Varadhan et al 2010). The CIF for each neurological abnormality is visualized, categorized by early, middle and late onset (Fig. 1). The number of cases that reported ages of onset for each abnormality is as follows: 53 for ataxia, 51 for CST abnormalities, 50 for cognitive decline, 61 for gait difficulty, 25 for sensory loss, 21 for psychiatric changes, 27 for speech changes, 23 for seizure, nine for parkinsonism, 33 for diarrhea, 66 for xanthomas, and 73 for cataracts. As discussed above, patients who had neurological abnormalities without reported ages of onset or estimated ages of onset were included in the CIF analyses using the “best” and “worst” case estimates. We also present the CIF for the initiation of CDCA (n = 66) as a comparison (Fig. 2) and death from any cause (n = 14 cases) (Fig. 3).
Cumulative incidence function (CIF) of neurological abnormalities in cerebrotendinous xanthomatosis patients from the literature review. For each neurological abnormality, CIF provides an estimate of the probability of developing a neurological abnormality at any given age, taking into account the competing risk of death. The red line is the “worst case” estimate, and the blue line is the “best case” estimate for CIF versus age. The figures illustrate CIF analysis results for: a neurological abnormalities that develop with a relatively higher probability (ataxia, corticospinal tract abnormalities, cognitive decline, gait difficulty), b neurological abnormalities that develop with a relatively lower probability (parkinsonism, seizures, speech changes, psychiatric changes, sensory loss)
Cumulative incidence function (CIF) of chenodeoxycholic acid (CDCA) initiation and non-neurological abnormalities in cerebrotendinous xanthomatosis patients from the literature review. The CIF provides an estimate of the probability of initiating CDCA treatment or developing a non-neurological abnormality (diarrhea, xanthomas, cataracts) at any given age, taking into account the competing risk of death. The red line is the “worst case” estimate, and the blue line is the “best case” estimate for CIF versus age
Practically, CIF graphs can be interpreted in the following way. For each abnormality, CIF is the probability that a CTX patient develops that symptom at any given age. For example, at age of 30 years old, there is an approximately 20% (best case estimate) to 40% (worst case estimate) probability of developing ataxia. While the various CIF analyses were largely descriptive of each neurological abnormality, some patterns in temporal progression of neurological symptoms were observed (Fig. 1a, b). Ataxia, CST abnormalities, and gait difficulty appeared to develop throughout life with relatively high probability of development. Seizures, psychiatric and speech changes also developed throughout life, but with relatively lower probability of development. Cognitive decline developed throughout life, but weighted later in life, with relatively high probability of development. Parkinsonism and sensory changes tended to occur later in life, but with relatively lower probability compared to cognitive decline. As for non-neurological abnormalities, cataracts tended to develop earlier and throughout life; xanthomas developed throughout life; and diarrhea developed throughout life with relatively lower probability (Fig. 2). Thus, the various neurological and non-neurological abnormalities occur throughout life, but can be categorized into those that tend to develop earlier, throughout or later in life, with higher or lower probability of development (Table 1).
There were not enough CTX cases who started CDCA prior to age 20 to determine the association between treatment initiation of CDCA and symptom development. CDCA treatment tended to be initiated throughout life (with a pattern that matched the progression of symptoms such as ataxia, CST, and gait abnormalities), which could be due to confounding by indication (i.e., CDCA was likely initiated with worsening symptoms) or delay in diagnosis of CTX (i.e., average diagnostic delay is 15–20 years from symptom onset) (Pilo-de-la-Fuente et al 2011; Mignarri et al 2014; Sekijima et al 2018) (Fig. 2).
Summary of case series
In our case series of five CTX patients, average age at diagnosis was 34.2 ± 6.8 years old (yo). Table 2 summarizes ages of onset of the most common neurological abnormalities. Baseline cognitive difficulty was common. Cataracts occurred relatively early in the course of disease. Ataxia, CST abnormalities, gait difficulties, speech changes, and psychiatric changes presented in mid-course. Parkinsonism presented later in one patient. One patient died. Patients received CDCA at an average age of 35.0 ± 7.4 yo.
Case 1
This 35-year-old man with normal developmental history was diagnosed with CTX at 28 yo. He had chronic diarrhea at 14 yo, required bilateral cataract removal by 27 yo, and had declining memory. On initial exam at 29 yo, he had dysarthria, fine beating nystagmus on leftward gaze, mild end target tremor, hyperreflexia, bilateral Babinski signs, decreased sensation in lower extremities, and leftward drift of gait. He had elevated cholestanol level (25.3 μg/mL), diffuse slowing on EEG, and bilateral dentate nuclei encephalomalacia and deep white matter hyperintensities on MRI brain. He was started on CDCA 250 mg TID, and after 6 months, he had improved gait, memory and tremor, with normalization of cholestanol levels. However, at 30 yo, he had worsening cognition and control of temper. Due to increased ALT and AST levels, CDCA dose was reduced to once daily. At 34 yo, he had worsened gait and speech, and increased hyperactivity. MRI brain showed worsening cerebellar lesions. Coenzyme Q10 was added. At 35 yo, he had worsened abulia, paucity of speech, impulsiveness, agitation, tremor, and erratic and shuffling gait. CDCA dose was doubled, but he continued to decline. Notably, however, he never developed tendon xanthomas.
Case 2
This 33-year-old man was diagnosed with CTX at 27 yo. Since infancy, he had chronic diarrhea, and since school age, he had learning difficulties. He developed bilateral Achilles tendon xanthomas at 13 yo. At 27 yo, elevated cholestanol confirmed CTX diagnosis and he was started on CDCA 250 mg BID. After 7 months of CDCA, there was improved mood and gait, and decreased cholestanol levels. At 29 yo, he only had mild ataxia when walking. At 30 yo, he remained neurologically stable, but had worsened Achilles tendon xanthomas and urinary bile acid levels. CDCA was increased to 500 mg BID, and simvastatin was added. By 31 yo, cholestanol level had normalized. He remained neurologically stable, but had continued xanthoma growth. CTX gene sequence testing confirmed heterozygosity for two mutations in CYP27A1 (p.R127Q:c.380G>A and p.R395C:c.11830T) and a novel variant (p.Y99C:c.296A>G).
Case 3
This 36-year-old woman was diagnosed with CTX at 34 yo. Since early childhood, she had chronic diarrhea, painless swelling of Achilles tendons, mild developmental delay and mildly wide-based gait. She required bilateral cataract removal at 9 yo. At 31 yo, she developed progressive weakness and falls. On initial exam at 34 yo, she was wheelchair-bound, dysarthric, dysphagic, and had head bobbing. Exam was notable for nystagmus, ataxia, and distal peripheral neuropathy. MRI brain with T2 FLAIR hyperintensity with cerebellum and pons with mild atrophy, elevated cholestanol level and homozygous CYP27A1 mutation (p.R474W: c.1420C>TW) confirmed diagnosis of CTX. She was started on CDCA 250 mg TID. She fractured her ankle multiple times that year, after which she was no longer able to walk. She developed worsened head shaking movements, dysphagia, dysarthria, and mood instability. Since starting CDCA, her chronic diarrhea resolved, but neurological symptoms progressed. By 36 yo, she was unable to ambulate, non-verbal, severely spastic, and dependent in activities of daily living.
Case 4
This 68-year-old man was diagnosed with CTX at 41 yo, with first symptoms of Achilles tendon xanthomas, ataxia, and cataracts. He also had chronic urinary incontinence. CDCA was started at 44 yo, and cholestanol level normalized at 55 yo. At 57 yo, he remained stable on CDCA. Examination was notable for mild dysarthria and shuffling, wide-based, slow gait. Two years later, he was stable, except for slower gait. MRI brain at 65 yo showed mild cerebellar abnormalities. At 66 yo, over 6 months, he became much slower, had decreased mobility, and required aid for ambulation. Exam was notable for parkinsonism, including decreased blink rate, cogwheeling at the right wrist, and stooped posture.
Case 5
This 40-year-old man presented with elevated urinary bile alcohols, Achilles tendon xanthomas, and recent cataract surgery. He had no history of developmental delay, but required special tutoring in school. His history included bile duct obstruction at 10 weeks old, and seizure at 24 yo. On initial exam, he had tremor of outstretched hands, decreased distal vibration sensation in legs, and ataxia with poor coordination and tandem gait. CDCA 250 mg TID was initiated at 41 yo without improvement. MRI brain showed bilateral cerebellar lesions. EMG/NCS revealed evidence of peripheral neuropathy. By 42 yo, he developed dysarthria. At 43 yo, he developed worsening speech with word-finding difficulty, increased ataxia and ambulatory difficulty, dysphagia, increased tone in extremities, and a more withdrawn personality. He was started on lovastatin 20 mg QPM, but speech, swallowing and gait continued to decline. Exam at 45 yo showed worsened dysarthria, increased tone in extremities, slow and clumsy fine finger movements, bilateral Babinski signs, and a very wide-based ataxic gait. He continued to deteriorate, and died at 46 yo.
Discussion
We evaluated the temporal progression of neurological abnormalities in CTX, which is a neurodegenerative, potentially disabling disorder. As CTX is a relatively rare disorder, there are limited numbers of cases at any particular medical center, so the ability to analyze information about this disorder from existing published cases is essential. Using the cumulative incidence function (CIF) to analyze information abstracted from these published cases, we visualized the probability of developing various neurological abnormalities at any given age. These estimations provide a baseline for comparison with CTX patients in the clinical setting.
Based on the CIF analysis, we categorized neurological and non-neurological abnormalities based on whether they tend to develop earlier, throughout or later in life, and based on whether they have a higher or lower probability of development (Table 1). In summary, baseline cognitive problems were seen in about one third of cases. Of the most common abnormalities, gait difficulty, ataxia, CST abnormalities, and xanthomas developed throughout life, while cognitive decline tended to develop later in life. Of the less common abnormalities, seizures, psychiatric changes, speech changes, and diarrhea developed throughout life, while parkinsonism and sensory changes tended to develop later in life. Similarly, in our case series, we observed that ataxia, CST abnormalities, gait difficulty, speech changes, and psychiatric changes tended to occur throughout the course of life, while cataracts were relatively early and parkinsonism was relatively late in the course of disease. In our case series and literature review, some cases had stabilization or improvement of neurological abnormalities with CDCA, but other cases reported continued progression of neurological abnormalities with CDCA.
Comparing with previous studies, in a retrospective study of 13 CTX cases, there were early cognitive difficulties, and then development of gait difficulty, peripheral neuropathy, and psychiatric symptoms (Degos et al 2016). A retrospective review of 55 CTX cases reported intellectual disability and epilepsy with earlier onset, and spasticity, ataxia, polyneuropathy, and parkinsonism with later onset (Mignarri et al 2014). Parkinsonism can be seen in CTX cases, and usually occurs later in life (Su et al 2010; Mignarri et al 2012b).
The neurological abnormalities in CTX correlate with the known widespread abnormalities in the peripheral and central nervous systems, including cerebellum, corticospinal tract, lateral and posterior spinal cord, and basal ganglia. In one of the earliest reports of CTX, there was atrophy in the cerebellum including the dentate nuclei; demyelination and degeneration of the pyramids, atrophy of cerebral peduncles, and gliosis of the internal capsule; demyelination in the lateral and posterior columns of the spinal cord; neuronal loss in the olives; and some basal ganglia involvement (Van Bogaert et al 1937; Schimschock et al 1968). Peripheral nerves could be normal (Philippart and Van Bogaert 1969), or abnormal with loss of myelinated fibers and elevated cholestanol levels (Katz et al 1985; Soffer et al 1995). Another neuropathological study of CTX likewise reported degeneration of the cerebellum especially in the dentate nuclei; neuronal loss in olives and pontine nuclei; abnormalities in the cerebral peduncles, middle cerebellar peduncles, pontine tegmentum and pyramids; lesions in the lateral and posterior spinal cord; and optic tract nerve fiber loss (Soffer et al 1995). Interestingly, in our study, despite the relatively high probability of cognitive decline later in life, there remained a relatively low probability of seizures and psychiatric changes, suggesting that cognitive decline could not be attributed to seizures or psychiatric changes alone. Future studies are needed to examine the relationship between neuropathological features (such as white matter lesions, or cerebellar degeneration) and cognitive decline in CTX. While it is known that there are abnormal lipid deposits in the central nervous system in CTX, future studies are also needed to elucidate the mechanisms through which abnormally elevated bile precursors (i.e., cholestanol and bile alcohols) are related to neuropathological abnormalities in CTX (Soffer et al 1995; Gonzalez-Cuyar et al 2007; Nie et al 2014).
Our study had several limitations. CTX cases published in the literature might not be fully representative of all CTX cases as they were not randomly sampled. For neurological or non-neurological abnormalities that occurred prior to first clinical assessment, reporting might be subject to recall bias. A symptom that was not reported was not necessarily absent. Furthermore, only a minority of cases reported ages of onset of neurological abnormalities, and ages of onset in some of these cases were estimated from qualitative information. Ages of onset of these neurological abnormalities were more likely overestimated than underestimated, as there could have been symptom onset prior to the ages when they were first noted in clinical assessments. It is unclear from our analysis whether CDCA altered the natural history of decline in CTX, so this question would best be answered with a prospective study. An important limitation to consider in our case series is that cases seen in a subspecialty pediatric or adult neurology clinic could be neurologically more severely affected than the general patient population.
We analyzed the progression of neurological abnormalities in CTX, categorizing neurological and non-neurological abnormalities according to time and probability of development. While a prospective study is ideal to study clinical progression in a disease, it is often not feasible for rare diseases such as CTX. Our approach takes advantage of abundant information in the literature about a rare neurological disorder for which there is often limited clinical information, and may be applied to other rare disorders. Knowledge of neurological progression in CTX is important both for clinical prognostication and management, and for development of efficacious therapies for CTX. Future studies will involve continued prospective follow-up of CTX patients, and evaluation of any changes in neurological progression of CTX with novel therapies.
References
Agrawal NK, Garg S (2012) Cerebrotendinous xanthomatosis: a rare disorder with a rare presentation. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2012-006202
Androdias G, Vukusic S, Gignoux L et al (2012) Leukodystrophy with a cerebellar cystic aspect and intracranial atherosclerosis: an atypical presentation of cerebrotendinous xanthomatosis. J Neurol 259:364–366
Austin PC, Lee DS, Fine JP (2016) Introduction to the analysis of survival data in the presence of competing risks. Circulation 133:601–609
Barkhof F, Verrips A, Wesseling P et al (2000) Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology 217:869–876
Bartholdi D, Zumsteg D, Verrips A et al (2004) Spinal phenotype of cerebrotendinous xanthomatosis--a pitfall in the diagnosis of multiple sclerosis. J Neurol 251:105–107
Berginer VM, Salen G, Shefer S (1984) Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med 311:1649–1652
Berginer VM, Carmi R, Salen G (1988a) Pregnancy in women with cerebrotendinous xanthomatosis (CTX): high risk condition for fetus and newborn infant? Am J Med Genet 31:11–16
Berginer VM, Foster NL, Sadowsky M, Townsend JA 3rd, Siegel GJ, Salen G (1988b) Psychiatric disorders in patients with cerebrotendinous xanthomatosis. Am J Psychiatry 145:354–357
Berginer VM, Gross B, Morad K et al (2009) Chronic diarrhea and juvenile cataracts: think cerebrotendinous xanthomatosis and treat. Pediatrics 123:143–147
Bhattacharyya AK, Lin DS, Connor WE (2007) Cholestanol metabolism in patients with cerebrotendinous xanthomatosis: absorption, turnover, and tissue deposition. J Lipid Res 48:185–192
Bhojwani RA, Khot R (2011) Cerebrotendinous xanthomatosis: a rare genetic disorder. BMJ Case Rep. http://dx.doi.org/10.1136/bcr.08.2011.4582
Bonnot O, Fraidakis MJ, Lucanto R et al (2010) Cerebrotendinous xanthomatosis presenting with severe externalized disorder: improvement after one year of treatment with chenodeoxycholic acid. CNS Spectr 15:231–236
Bourkiza R, Joyce S, Patel H et al (2010) Cerebrotendinous xanthomatosis (CTX): an association of pulverulent cataracts and pseudo-dominant developmental delay in a family with a splice site mutation in CYP27A1—a case report. Ophthalmic Genet 31:73–76
Brodsky JW, Beischer AD, Anat D et al (2006) Cerebrotendinous xanthomatosis: a rare cause of bilateral Achilles tendon swelling and ataxia. A case report. J Bone Joint Surg Am 88:1340–1344
Burnett JR, Moses EA, Croft KD et al (2001) Clinical and biochemical features, molecular diagnosis and long-term management of a case of cerebrotendinous xanthomatosis. Clin Chim Acta 306:63–69
Canelas HM, Quintao EC, Scaff M, Vasconcelos KS, Brotto MW (1983) Cerebrotendinous xanthomatosis: clinical and laboratory study of 2 cases. Acta Neurol Scand 67:305–311
Cerqueira AC, Nardi AE, Bezerra JM (2010) Cerebrotendinous xanthomatosis: a treatable hereditary neuro-metabolic disease. Clinics (Sao Paulo) 65:1217–1218
Chang CC, Lui CC, Wang JJ et al (2010) Multi-parametric neuroimaging evaluation of cerebrotendinous xanthomatosis and its correlation with neuropsychological presentations. BMC Neurol 10:59
Chang WN, Kuriyama M, Lui CC, Jeng SF, Chen WJ, Chee EC (1992) Cerebrotendinous xanthomatosis in three siblings from a Taiwanese family. J Formos Med Assoc 91:1190–1194
Chen W, Kubota S, Teramoto T et al (1998) Genetic analysis enables definite and rapid diagnosis of cerebrotendinous xanthomatosis. Neurology 51:865–867
Chen SF, Tsai NW, Chang CC et al (2011a) Neuromuscular abnormality and autonomic dysfunction in patients with cerebrotendinous xanthomatosis. BMC Neurol 11:63
Chen WC, Wu KC, Hu CH, Chern TC, Jou IM (2011b) A compound heterozygous mutation of CYP27A1 gene in a Taiwanese patient with cerebrotendinous xanthomatosis. J Orthop Sci 16:825–827
Chen Q, Liu W, Jiang B, Yu R, Li X, Li H (2012) Fluoxetine-responsive depression in a Chinese cerebrotendinous xanthomatosis. Gen Hosp Psychiatry 34(578):e571–e574
Clemen CS, Spottke EA, Lutjohann D et al (2005) Cerebrotendinous xanthomatosis: a treatable ataxia. Neurology 64:1476
Degos B, Nadjar Y, Amador Mdel M et al (2016) Natural history of cerebrotendinous xanthomatosis: a paediatric disease diagnosed in adulthood. Orphanet J Rare Dis 11:41
Dormans TP, Verrips A, Bulten J, Cox N (1997) Pulmonary lymphangioleiomyomatosis and cerebrotendinous xanthomatosis: is there a link? Chest 112:273–274
Dotti MT, Federico A, Signorini E et al (1994) Cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease): CT and MR findings. AJNR Am J Neuroradiol 15:1721–1726
Dotti MT, Manneschi L, Federico A (1995) Mitochondrial enzyme deficiency in cerebrotendinous xanthomatosis. J Neurol Sci 129:106–108
Farpour H, Mahloudji M (1975) Familial cerebrotendinous xanthomatosis. Report of a new family and review of the literature. Arch Neurol 32:223–225
Gaikwad SB, Garg A, Mishra NK, Gupta V, Srivastava A, Sarkar C (2003) Cerebrotendinous xanthomatosis: neuroimaging findings in two siblings from an Indian family. Neurol India 51:401–403
Gallus GN, Dotti MT, Mignarri A et al (2010) Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur J Neurol 17:1259–1262
Geraldes R, Santos-Bento M, de Carvalho M (2007) Cerebrotendinous xanthomatosis: no involvement of the autonomic nervous system in a case with severe neuropathy. Neurophysiol Clin 37:47–49
Gonzalez-Cuyar LF, Hunter B, Harris PL, Perry G, Smith MA, Castellani RJ (2007) Cerebrotendinous xanthomatosis: case report with evidence of oxidative stress. Redox Rep 12:119–124
Habaragamuwa BW, Bajekal R (2010) Cerebrotendinous xanthomatosis and anaesthesia. Br J Anaesth 105:237–238
Hansson M, Olin M, Floren CH et al (2007) Unique patient with cerebrotendinous xanthomatosis. Evidence for presence of a defect in a gene that is not identical to sterol 27-hydroxylase. J Intern Med 261:504–510
Hokezu Y, Kuriyama M, Kubota R, Nakagawa M, Fujiyama J, Osame M (1992) Cerebrotendinous xanthomatosis: cranial CT and MRI studies in eight patients. Neuroradiology 34:308–312
Huang L, Miao XD, Yang DS, Tao HM (2011) Bilateral Achilles tendon enlargement. Orthopedics 34:e960–e964
Huijgen R, Stork AD, Defesche JC et al (2012) Extreme xanthomatosis in patients with both familial hypercholesterolemia and cerebrotendinous xanthomatosis. Clin Genet 81:24–28
Ito S, Kuwabara S, Sakakibara R et al (2003) Combined treatment with LDL-apheresis, chenodeoxycholic acid and HMG-CoA reductase inhibitor for cerebrotendinous xanthomatosis. J Neurol Sci 216:179–182
Jain RS, Sannegowda RB, Agrawal A, Hemrajani D, Jain R, Mathur T (2013) ‘Hot cross bun’ sign in a case of cerebrotendinous xanthomatosis: a rare neuroimaging observation. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2012-006641
Kamate M, Chetal V, Hattiholi V (2010) Cerebrotendinous xanthomatosis. Indian J Pediatr 77:697–698
Kapas I, Katko M, Harangi M et al (2014) Cerebrotendinous xanthomatosis with the c.379C>T (p.R127W) mutation in the CYP27A1 gene associated with premature age-associated limbic tauopathy. Neuropathol Appl Neurobiol 40:345–350
Kato H, Koyabu S, Aoki S et al (2003) An autopsy case of gallbladder cancer developing in a Japanese man with cerebrotendinous xanthomatosis: genetic analysis of the sterol 27-hydroxylase and p53 genes. Pathology 35:141–144
Katz DA, Scheinberg L, Horoupian DS, Salen G (1985) Peripheral neuropathy in cerebrotendinous xanthomatosis. Arch Neurol 42:1008–1010
Kauffman MA, Gonzalez-Moron D, Consalvo D, Kochen S (2012) Cerebrotendinous xanthomatosis revealed in drug-resistant epilepsy diagnostic workup. Am J Med Sci 343:332–333
Kawabata M, Kuriyama M, Mori S, Sakashita I, Osame M (1998) Pulmonary manifestations in cerebrotendinous xanthomatosis. Intern Med 37:922–926
Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS (2013) Juvenile cataract morphology in 3 siblings not yet diagnosed with cerebrotendinous xanthomatosis. Ophthalmology 120:956–960
Kottahachchi DC, Balasooriya BL, Panangala L, Ranawaka UK (2012) Two siblings with cerebrotendinous xanthomatosis. Ceylon Med J 57:128–129
Koyama S, Kawanami T, Tanji H et al (2012) A case of cerebrotendinous xanthomatosis presenting with epilepsy as an initial symptom with a novel V413D mutation in the CYP27A1 gene. Clin Neurol Neurosurg 114:1021–1023
Kuwabara K, Hitoshi S, Nukina N et al (1996) PET analysis of a case of cerebrotendinous xanthomatosis presenting hemiparkinsonism. J Neurol Sci 138:145–149
Lange MC, Zetola VF, Teive HA et al (2004) Cerebrotendinous xanthomatosis: report of two Brazilian brothers. Arq Neuropsiquiatr 62:1085–1089
Lee Y, Lin PY, Chiu NM, Chang WN, Wen JK (2002) Cerebrotendinous xanthomatosis with psychiatric disorders: report of three siblings and literature review. Chang Gung Med J 25:334–340
Lorincz MT, Rainier S, Thomas D, Fink JK (2005) Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol 62:1459–1463
Luyckx E, Eyskens F, Simons A, Beckx K, Van West D, Dhar M (2014) Long-term follow-up on the effect of combined therapy of bile acids and statins in the treatment of cerebrotendinous xanthomatosis: a case report. Clin Neurol Neurosurg 118:9–11
Mandrile G, Gallus GN, Mura G et al (2014) Cerebrotendinous xanthomatosis: recurrence of the CYP27A1 mutation p.Arg479Cys in Sardinia. Neurol Sci 35:1303–1305
Matysik S, Orso E, Black A, Ahrens N, Schmitz G (2011) Monitoring of 7alpha-hydroxy-4-cholesten-3-one during therapy of cerebrotendinous xanthomatosis: a case report. Chem Phys Lipids 164:530–534
McKinnon JH, Bosch EP (2012) Clinical reasoning: a case of treatable spastic paraparesis. Neurology 79:e50–e53
Mehta BP, Shmerling RH (2008) Teaching neuroimage: cerebrotendinous xanthomatosis. Neurology 71:e4
Meiner V, Meiner Z, Reshef A, Bjorkhem I, Leitersdorf E (1994) Cerebrotendinous xanthomatosis: molecular diagnosis enables presymptomatic detection of a treatable disease. Neurology 44:288–290
Menkes JH, Schimschock JR, Swanson PD (1968) Cerebrotendinous xanthomatosis. The storage of cholestanol within the nervous system. Arch Neurol 19:47–53
Mignarri A, Dotti MT, Del Puppo M et al (2012a) Cerebrotendinous xanthomatosis with progressive cerebellar vacuolation : six-year MRI follow-up. Neuroradiology 54:649–651
Mignarri A, Falcini M, Vella A et al (2012b) Parkinsonism as neurological presentation of late-onset cerebrotendinous xanthomatosis. Parkinsonism Relat Disord 18:99–101
Mignarri A, Gallus GN, Dotti MT, Federico A (2014) A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis 37:421–429
Moghadasian MH, Salen G, Frohlich JJ, Scudamore CH (2002) Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol 59:527–529
Mondelli M, Rossi A, Scarpini C, Dotti MT, Federico A (1992) Evoked potentials in cerebrotendinous xanthomatosis and effect induced by chenodeoxycholic acid. Arch Neurol 49:469–475
Monson DM, DeBarber AE, Bock CJ et al (2011) Cerebrotendinous xanthomatosis: a treatable disease with juvenile cataracts as a presenting sign. Arch Ophthalmol 129:1087–1088
Nakashima N, Sakai Y, Sakai H et al (1994) A point mutation in the bile acid biosynthetic enzyme sterol 27-hydroxylase in a family with cerebrotendinous xanthomatosis. J Lipid Res 35:663–668
Nie S, Chen G, Cao X, Zhang Y (2014) Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J Rare Dis 9:179
Nozue T, Higashikata T, Inazu A et al (2010) Identification of a novel missense mutation in the sterol 27-hydroxylase gene in two Japanese patients with cerebrotendinous xanthomatosis. Intern Med 49:1127–1131
Ohno T, Kobayashi S, Hayashi M, Sakurai M, Kanazawa I (2001) Diphenylpyraline-responsive parkinsonism in cerebrotendinous xanthomatosis: long-term follow up of three patients. J Neurol Sci 182:95–97
Okuma H, Kitagawa Y, Tokuoka K, Takagi S (2007) Cerebrotendinous xanthomatosis with cerebellar ataxia as the chief symptom. Intern Med 46:1259–1261
Pedroso JL, Pinto WB, Souza PV et al (2012) Early-onset epilepsy as the main neurological manifestation of cerebrotendinous xanthomatosis. Epilepsy Behav 24:380–381
Philippart M, Van Bogaert L (1969) Cholestanolosis (cerebrotendinous xanthomatosis). A follow-up study on the original family. Arch Neurol 21:603–610
Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR et al (2011) Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol 18:1203–1211
Pilo de la Fuente B, Ruiz I, Lopez de Munain A, Jimenez-Escrig A (2008) Cerebrotendinous xanthomatosis: neuropathological findings. J Neurol 255:839–842
Posada IJ, Ramos A (2011) Botulinum toxin-responsive oromandibular dystonia in cerebrotendinous xanthomatosis. Parkinsonism Relat Disord 17:570–572
Price Evans DA, Salah KA, Mobrad MA, Mitchell WD, Olin M, Eggertsen G (2007) Cerebrotendinous xanthomatosis in a Saudi Arabian family-genotyping and long-term follow-up. Saudi Med J 28:1113–1118
Pudhiavan A, Agrawal A, Chaudhari S, Shukla A (2013) Cerebrotendinous xanthomatosis--the spectrum of imaging findings. J Radiol Case Rep 7:1–9
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430
Rafiq M, Sharrack N, Shaw PJ, Hadjivassiliou M (2011) A neurological rarity not to be missed: cerebrotendinous xanthomatosis. Pract Neurol 11:296–300
Reichwaldt I, Zustin J, Wenke K, Ridderbusch I (2010) Differential diagnosis of tendon tumors: xanthomas caused by hyperlipidemia in children. J Pediatr Surg 45:e9–12
Rystedt E, Olin M, Seyama Y et al (2002) Cerebrotendinous xanthomatosis: molecular characterization of two Scandinavian sisters. J Intern Med 252:259–264
Salen G, Polito A (1972) Biosynthesis of 5 -cholestan-3 -ol in cerebrotendinous xanthomatosis. J Clin Invest 51:134–140
Sandeep P, Jayakrishnan C, Sadanan S, Sreekumar S, Thulasidharan NK (2009) Cerebrotendinous xanthomatosis: a treatable neurodegenerative disease. J Assoc Physicians India 57:716–717
Schimschock JR, Alvord EC Jr, Swanson PD (1968) Cerebrotendinous xanthomatosis. Clinical and pathological studies. Arch Neurol 18:688–698
Schneider H, Lingesleben A, Vogel HP, Garuti R, Calandra S (2010) A novel mutation in the sterol 27-hydroxylase gene of a woman with autosomal recessive cerebrotendinous xanthomatosis. Orphanet J Rare Dis 5:27
Schreiner A, Hopen G, Skrede S (1975) Cerebrotendinous xanthomatosis (cholestanolosis). Investigations on two sisters and their family. Acta Neurol Scand 51:405–416
Seidel S, Kasprian G, Prayer D, Krssak M, Sycha T, Auff E (2011) Visualisation of treatment response in a case of cerebrotendinous xanthomatosis. J Neurol Neurosurg Psychiatry 82:703–704
Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y (2018) Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet. http://dx.doi.org/10.1038/s10038-017-0389-4
Siman-Tov T, Meiner V, Gadoth N (2006) Could steroids mask the diagnosis of cerebrotendinous xanthomatosis? J Neurol Sci 243:83–86
Smithard A, Lamyman MJ, McCarthy CL, Gibbons CL, Cooke PJ, Athanasou N (2007) Cerebrotendinous xanthomatosis presenting with bilateral Achilles tendon xanthomata. Skelet Radiol 36:171–175
Soffer D, Benharroch D, Berginer V (1995) The neuropathology of cerebrotendinous xanthomatosis revisited: a case report and review of the literature. Acta Neuropathol 90:213–220
Stahl WL, Sumi SM, Swanson PD (1971) Subcellular distribution of cerebral cholestanol in cerebrotendinous xanthomatosis. J Neurochem 18:403–413
Su CS, Chang WN, Huang SH et al (2010) Cerebrotendinous xanthomatosis patients with and without parkinsonism: clinical characteristics and neuroimaging findings. Mov Disord 25:452–458
Szlago M, Gallus GN, Schenone A et al (2008) The first cerebrotendinous xanthomatosis family from Argentina: a new mutation in CYP27A1 gene. Neurology 70:402–404
Uygunoglu U, Gunduz A, Menku SF et al (2014) Cerebrotendinous xanthomatosis: the effectiveness of high-dose piracetam for the treatment of cerebellar and sensorial ataxia. Cerebellum 13:787–790
Valdivielso P, Calandra S, Duran JC, Garuti R, Herrera E, Gonzalez P (2004) Coronary heart disease in a patient with cerebrotendinous xanthomatosis. J Intern Med 255:680–683
Van Bogaert L, Scherer HJ, Epstein E (1937) Une forme cerebrale de la cholesterinose generalisee. Masson & Cie, Paris
Vanrietvelde F, Lemmerling M, Mespreuve M, Crevits L, De Reuck J, Kunnen M (2000) MRI of the brain in cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease). Eur Radiol 10:576–578
Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C (2010) Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care 48:S96–105
Wakamatsu N, Hayashi M, Kawai H et al (1999) Mutations producing premature termination of translation and an amino acid substitution in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis associated with parkinsonism. J Neurol Neurosurg Psychiatry 67:195–198
Wang Z, Yuan Y, Zhang W, Zhang Y, Feng L (2007) Cerebrotendinous xanthomatosis with a compound heterozygote mutation and severe polyneuropathy. Neuropathology 27:62–66
Watts GF, Mitchell WD, Bending JJ, Reshef A, Leitersdorf E (1996) Cerebrotendinous xanthomatosis: a family study of sterol 27-hydroxylase mutations and pharmacotherapy. QJM 89:55–63
Wevers RA, Cruysberg JR, Van Heijst AF et al (1992) Paediatric cerebrotendinous xanthomatosis. J Inherit Metab Dis 15:374–376
Zhang MJ, Zhang X, Scheike TH (2008) Modeling cumulative incidence function for competing risks data. Expert Rev Clin Pharmacol 1:391–400
Acknowledgments
The authors also thank Dr. Katherine Sims for her contributions to patient care for several CTX patients included in the case series.
Funding
The authors acknowledge support from the NINDS Research Education Program (R25) (J.C.W.) and industry support from Retrophin for data mining (K.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helskinski Declaration of 1975, as revised in 2000. The retrospective medical records review was approved by the Partners HealthCare institutional review board (IRB). The requirement for signed consent form was waived by the Partners Healthcare IRB on the basis that the research presented no more than minimal risk of harm to subjects and involved no procedures for which written consent is normally required outside of the research context.
Animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Communicated by: Robert Steiner
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wong, J.C., Walsh, K., Hayden, D. et al. Natural history of neurological abnormalities in cerebrotendinous xanthomatosis. J Inherit Metab Dis 41, 647–656 (2018). https://doi.org/10.1007/s10545-018-0152-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-018-0152-9