Abstract
This study investigates glio-vascular interactions in human fetal brain at midgestation, specifically examining the expression and immunolocalization of the CXCL12/CXCR4/CXCR7 ligand-receptor axis and its possible role in the vascular patterning of the developing brain. At midgestation, the telencephalic vesicles are characterized by well developed radial glia cells (RGCs), the first differentiated astrocytes and a basic vascular network mainly built of radial vessels. RGCs have been recognized to contribute to cerebral cortex neuro-vascular architecture and have also been demonstrated to act as a significant source of neural cells (Rakic, Brain Res 33:471–476, 1971; Malatesta et al, Development 127:5253–5263, 2000). According to our hypothesis CXCL12, a potent migration and differentiation chemokine released by RGCs, may act as a linking factor coordinating neuroblast migration with vessel growth and patterning through the activation of different ligand/receptor axes. The obtained results support this hypothesis showing that together with CXCR4/CXCR7-reactive neuroblasts, which migrate in close association with CXCL12 RGCs, layer-specific subsets of CXCL12 RGCs and astrocytes specifically contact the microvessel wall. Moreover, the CXCL12/CXCR4/CXCR7 system appears to be directly involved in microvessel growth, its members being differentially expressed in angiogenically activated microvessels and vascular sprouts.









Similar content being viewed by others
References
Bär T (1980) The vascular system of the cerebral cortex. Adv Anat Embryo Cell Biol, vol 59. Springer, Berlin
Diotel N, Vaillant C, Gueguen M-M, Mironov S, Anglade I, Servili A et al (2010) CXCR4 and CXCL12 expression in radial glial cells of the brain of adult zebrafish. J Comp Neurol 518:4855–4876
Engelhardt B (2003) Development of the blood–brain barrier. Cell Tissue Res 314:119–129
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C (2004) Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231(3):503–509
Graham GJ, Locati M, Mantovani A, Rot A, Thelen M (2012) The biochemistry and biology of the atypical chemokine receptors. Immunol Lett 145:30–38
Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A et al (2012) CXC Chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS One 7(8):e42814
Hinton CV, Avraham S, Karsenty Avraham HK (2010) Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis 27:97–105
Kollmar O, Rupertus K, Scheuer C, Nichels RM, Haberl GCY, Tilton B et al (2010) CXCR4 and CXCR7 regulate angiogenesis and CT26.WT tumor growth indipendent from SDF-1. Int J Cancer 126:1302–1315
Kramp BK, Sarabi A, Koenen RR, Weber C (2011) Heterophilic chemokine receptor interactions in chemokine signaling and biology. Exp Cell Res 317:655–663
Li M, Ransohoff RM (2008) Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol 84(2):116–131
Lin J, Lemke C, Redies C, Yan X, Mix E, Rolfs A et al (2011) ADAM17 overexpression promotes angiogenesis by increasing blood vessel sprouting and pericyte number during brain microvessel development. Int J Dev Biol 55(10–12):961–968
Liu KK, Dorovini-Zis K (2009) Regulation of CXCL12 and CXCR4 expression by human brain endothelial cells and their role in CD4+ and CD8+ T cell adhesion and transendothelial migration. J Neuroimmunol 215(1–2):49–64
Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T et al (1998) Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 95:9448–9453
Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127:5253–5263
Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A et al (2007) CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. PNAS 104(40):15735–15740
Milaire J (1992) Histochemical aspects of organogenesis in vertebrates. Part II, the central nervous system. In: Graumann W (ed) Handbuch der Histochemie. Fischer, Stuttgart, pp 163–172
Na I-K, Scheibenbogen C, Adam C, Stroux A, Ghadjar P, Thiel E et al (2008) Nuclear expression of CXCR4 in tumor cells of non–small cell lung cancer is correlated with lymph node metastasis. Hum Pathol 39:1751–1755
Norman MG, O’Kushy JR (1986) The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol 45:222–232
O’Rahilly R, Müller F (1994) The embryonic human brain. An atlas of developmental stages. Wiley-Liss, New York
Patel JR, McCandless EE, Dorsey D, Klein RS (2010) CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. PNAS 107(24):11062–11067
Peng H, Kolb R, Kennedy JE (2007) Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. J Neuroimmun Pharmacol 2(3):251–258
Rakic P (1971) Guidance of neurons migrating to the fetal monkey neocortex. Brain Res 33:471–476
Risau W (1991) Vasculogenesis, angiogenesis and endothelial cell differenziation during embryonic development. In: Feinberg RN, Sherer GK, Auerbach R (eds) The development of the vascular system, vol 14. Karger, Basel, pp 58–68
Risau W (1993) Development of vascular system of organs and tissue. In: Shaper W, Shaper J (eds) Collateral circulation. Kluwer, Norwell, pp 17–28
Roncali L, Errede M, Girolamo F, Virgintino D (2008) Vascular cell roles during early human brain angiogenesis. In: Ribatti D, Nico B (eds) Recent advances in angiogenesis in central nervous system. Transworld Research Network, Kerala, pp 67–78
Rubie C, Frick VO, Ghadjar P, Wagner M, Justinger C, Faust SK et al (2011) CXC receptor-4 mRNA silencing abrogates CXCL12-induced migration of colorectal cancer cells. J Translat Med 9:22–35
Salmaggi A, Maderna E, Calatozzolo C, Gaviani P, Canazza A, Milanesi I et al (2009) CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol Ther 8:1608–1614
Schulte A, Günther HS, Phillips HS, Kemming D, Martens T, Kharbanda S et al (2011) A distinct subset of glioma cell lines with stem-cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia 59:590–602
Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X et al (2009) Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1A/CXCR4 axis. Cancer Res 69(15):6057–6064
Strasser GA, Kaminker JS, Tessier-Lavigne M (2010) Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115:5102–5110
Stumm R, Höllt V (2007) CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol 38:377–382
Terasaki M, Sugita Y, Arakawa F, Okada Y, Ohshima K, Shigemori M (2011) CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target. Brain Tumor Pathol 2:89–97
Thelen M, Thelen S (2008) CXCR7, CxCR4 and CXCL12: an accentric trio? J Neuroimmunol 198:9–13
Tiveron M-C, Cremer H (2008) CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol 18:237–244
Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J et al (2006) Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci 26(51):13273–13278
Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ (2007) Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol 500:1007–1033
Unoki N, Murakami T, Nishijima K, Ogino K, van Rooijen N, Yoshimura N (2010) SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Investig Ophthalmol Vis Sci 51(7):3362–3371
van der Meer P, Ulrich AM, Gonzales-Scarano F, Lavi E (2000) Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol 69:192–201
Virgintino D, Robertson D, Benagiano V, Errede M, Bertossi M, Ambrosi G et al (2000) Immunogold cytochemistry of the blood–brain barrier glucose transporter GLUT1 and endogenous albumin in the developing human brain. Brain Res Dev Brain Res 123:95–101
Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A et al (2004) Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol 122:51–59
Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB et al (2007) An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 10:35–45
Virgintino D, Errede M, Girolamo F, Capobianco C, Robertson D, Vimercati A et al (2008) Fetal blood–brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol 67:50–61
Würth R, Barbieri F, Bajetto A, Pattarozzi A, Gatti M (2001) Expression of CXCR7 chemokine receptor in human meningioma cells and in intratumoral microvasculature. J Neuroimmunol 234:115–123
Yoshitake N, Fukui H, Yamagishi H, Sekikawa A, Fujii S, Tomita S et al (2008) Expression of SDF-1a and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer 98(10):1682–1689
Zheng K, Li H-Y, Su X-L, Wang X-Y, Tian T, Li F et al (2010) Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res 29:31–44
Zlotnik A, Yoshie O, Nomiyama H (2006) The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 7:243
Acknowledgments
M.V.C. Pragnell is gratefully acknowledged for language help.
Details of funding
This work has been funded by grants from Fondazione Cassa di Risparmio di Puglia (FCRP 2010) and from the Italian Ministry of the University and Scientific Research (MIUR), project PRIN 2008.
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Maurizio Scarpa
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
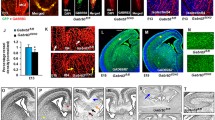
A gallery of steps in human forebrain vascularization, from a preplate stage (A) to the cortex pre-lamination stage at midgestation (I). Microvessels are revealed by immunostaining for vessel basal lamina components, collagen IV (A, B, D, E, G, I) and fibronectin (C, F, H). The first penetrating microvessels (A, D, G), a capillary loop (B), radial stem vessels (C). E and F, details of stage G and C, respectively. Initial (H) and late (I) formation of H-shaped collaterals. MZ, marginal zone; CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. Immunolabelling with rabbit anti-collagen type IV (1:100 dilution; Acris Antibodies; Hiddenhausen, Germany) and rabbit anti-fibronectin (1:500 dilution; Millipore, Billerica, MA, USA), nuclear counterstaining with propidium iodide (Molecular Probes). (JPEG 386 kb)
Supplementary material 2
A schematic outline of cerebral cortex, radial astrocytes depicted in Fig. 6. In the cortex, radial astrocytes appear strongly labelled by both GFAP and CXCL12, with complete overlapping of the two markers. In type 1 and 2 the nucleus is positioned on the top, close to the short, basal processes and to the origin of recurrent processes (arrowhead), while radial astrocytes type 3 and 4 have an upside-down position. (JPEG 60 kb)
Supplementary material 3
A short series of single optical planes showing GFAP/CXCL12high RGC and astrocyte perivascular contacts (projection image in Fig. 5f). (AVI 153605 kb)
Supplementary material 4
Triple immunostaining with GFAP, CXCL12, and collagen type-IV. A cortex microvessel (asterisk) showing a CXCL12-reactive endothelial cell (arrow) and perivascular GFAP/CXCL12high astrocyte endfeet (arrowhead). (JPEG 111 kb)
Rights and permissions
About this article
Cite this article
Virgintino, D., Errede, M., Rizzi, M. et al. The CXCL12/CXCR4/CXCR7 ligand-receptor system regulates neuro-glio-vascular interactions and vessel growth during human brain development. J Inherit Metab Dis 36, 455–466 (2013). https://doi.org/10.1007/s10545-012-9574-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9574-y