Abstract
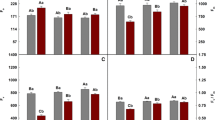
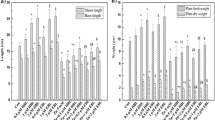
Limited information is available on the role of brassinosteroids (BRs) in response of plants to nutrient deficiency. To understand the functions of BRs in response to iron deficiency, we investigated the effect of 24-epibrassinolide (EBR) on activities of ferric-chelate reductase (FCR), H+-ATPase, Ca2+-ATPase, nitrate reductase (NR), antioxidant enzymes, Fe and other minerals content and distribution, chlorophylls, soluble protein, free proline, reactive oxygen species, and malondialdehyde in peanut (Arachis hypogea L.) plants subjected to Fe deficiency (10−5 M Fe(III)-EDTA) with foliar application of EBR (0, 10−8, 5.0×10−8, 10−7, 5.0×10−7, and10−6 M). Results show that EBR increased Fe translocation from roots to shoots and increased Fe content in cell organelles. Activities of antioxidant enzymes increased and so the ability of resistance to oxidative stress was enhanced. As result of enhancement of H+-ATPase and Ca2+-ATPase activities, the inhibition of Fe, Ca, Mg, and Zn uptake and distribution was ameliorated. Chlorophyll, soluble protein, and free proline content also increased and consequently, chlorosis induced by Fe deficiency was alleviated. The results demonstrate that EBR had a positive role in regulating peanut growth and development under Fe deficiency and an optimal concentration appeared to be 10−7 M.
Similar content being viewed by others
Abbreviations
- ALA:
-
aminolevulinic acid
- BRs:
-
brassinosteroids
- Car:
-
carotenoids
- CAT:
-
catalase
- Chl:
-
chlorophyll
- CK1:
-
control
- CK2:
-
low Fe treatment
- EBR:
-
24-epibrassinolide
- EBR1-5:
-
low Fe treatment combined with foliar application of various concentrations of EBR
- FCR:
-
ferric-chelate reductase
- MDA:
-
malondialdehyde
- NR:
-
nitrate reductase
- O2 •− :
-
superoxide anion
- PM:
-
plasma membrane
- POD:
-
peroxidase
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
References
Abadía, J., Morales, F., Abadía, A.: Photosystem II efficiency in low chlorophyll, iron-deficient leaves. — Plant Soil 215: 183–192, 1999.
Abadía, J., Vázquez, S., Rellán-Álvarez, R., El-Jendoubi, H., Alvarez-Fernández, A., López-Millán, A.F.: Towards a knowledge-based correction of iron chlorosis. — Plant Physiol. Biochem. 49: 471–482, 2011.
Alcaraz, C.F., Martinez-Sánchez, F., Sevilla, F., Hellin, E.: Influence of ferredoxin levels on nitrate reductase activity in iron deficient lemon leaves. — J. Plant Nutr. 9: 1405–1413, 1986.
Alex, A.R., Martin, R.M., Jane, E.T., Alistair, M.H.: Calcium ions as intracellular second messengers in higher plants. — Adv. bot. Res. 22: 45–96, 1996.
Ali, B., Hasan, S.A., Hayat, S., Hayat, Q., Yadav, S., Fariduddin, Q., Ahmad, A.: A role of brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L.) Wilczek. — Environ. exp. Bot. 62: 153–159, 2008.
Ali, B., Hayat, S., Ahmad, A.: 28-Homobrassinolide ameliorates the salt stress in chickpea (Cicer arietinum L.). - Environ. exp. Bot. 59: 217–223, 2007.
Arora, P., Bhardwaj, R., Kanwar, M.K.: Effect of 24-epibrassinolide on growth, protein content and antioxidative defense system of Brassica juncea L. subjected to cobalt ion toxicity. — Acta Physiol. Plant. 34: 2007–2017, 2012.
Arora, N., Bhardwaj, R., Sharma, P., Arora, H.K.: Effect of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. — Acta Physiol. Plant. 30: 833–839, 2008.
Bacaicoa, E., Ángel, M.Z., Diane, L., Roberto, B.: Relationship between the hormonal balance and the regulation of iron deficiency stress responses in cucumber. — J. amer. Soc. hort. Sci. 134: 589–601, 2009.
Bajguz, A.: Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. — Plant Physiol. Biochem. 38: 209–215, 2000.
Bajguz, A., Tretyn, A.: The chemical characteristic and distribution of brassinosteroids in plants. — Phytochemistry 62: 1027–1046, 2003.
Bandurska, H.: Does proline accumulated in the leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. — Acta Physiol. Plant. 23: 483–490, 2001.
Bartwal, A., Mall, R., Lohani, P., Guru, S.K., Arora, S.: Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. — J. Plant Growth Regul. 32: 216–232, 2013.
Bates, L.S., Walden, R.T., Tearse, I.D.: Rapid determination of free proline for water stress studies. — Plant Soil 39: 205–207, 1973.
Bradford, M.M.: A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. — Anal. Biochem. 72: 248–254, 1976.
Briskin, D.P., Leonard, R.T., Hodges, T.K.: Isolation of the plasma membrane: markers and general principles. — Method. Enzymol. 148: 542–558, 1987.
Cakmak, I., Kirkby, E.: Role of magnesium in carbon partitioning and alleviating photoxidative damage. — Physiol. Plant. 133: 692–704, 2008.
Cerana, R., Bonetti, A., Marre, M.T., Romani, G., Lado, P.: Effects of a brassinosteroid on growth and electrogenic proton extrusion in Azuki bean epicotyls (Vigna angularis). — Physiol. Plant. 59: 23–27, 1983.
Chen, W.W., Yang, J.L., Qin, C., Jin, C.W., Mo, J.H., Ye, T., Zheng, S.J.: Nitric oxide acts downstream of auxin to trigger root ferric- chelate reductase activity in responses to iron deficiency in Arabidopsis. — Plant. Physiol. 154: 810–819, 2010.
Curie, C., Briat, J.-F.: Iron transport and signaling in plants. — Annu. Rev. Plant Biol. 54: 183–206, 2003.
Fariduddin, Q., Khalil, R.R.A.E., Mir, B.A., Yusuf, M., Ahmad, A.: 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. - Environ. Monitor. Assess. 185: 7845–7856, 2013.
Fariduddin, Q., Khanam, S., Hasan, S.A., Ali, B., Hayat, S.A., Ahmad, A.: Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. — Acta. Physiol. Plant. 31: 889–897, 2009.
Fariduddin, Q., Yusuf, M., Ahmad I., Ahmad, A.: Brassinosteroids and their role in response of plants to abiotic stresses. — Biol. Plant. 58: 9–17, 2014.
Frédéric, G., Duby, G., Stedingk, E.V., Zhao, R.M., Morsomme, P., Boutry, M.: Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. — Plant Physiol. 144: 173–177, 2007.
Gao, L., Shi, Y.X.: Genetic differences in resistance to iron deficiency chlorosis in peanut. — J Plant Nutr. 30: 37–52, 2007.
Gille, G., Sigler, K.: Oxidative stress in living cells. — Folia microbiol. 2: 131–152, 1995.
Gonzalo, M.J., Lucena, J.J., Hernández-Apaolaza, L.: Effect of silicon addition on soybean (Glycine max) and cucumber (Cucumis sativus) plants grown under iron deficiency. — Plant Physiol. Biochem. 70: 455–461, 2013.
Graziano, M., Beligni, M.V., Lamattina, L.: Nitric oxide improves internal iron availability in plants. — Plant Physiol. 130: 1852–1859, 2002.
Graziano, M., Lamattina, L.: Nitric oxide and iron in plants: an emerging and converging story. — Trends Plant Sci. 10: 4–8, 2005.
Graziano, M., Lamattina, L.: Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. — Plant J. 52: 949–960, 2007.
Grotz, N., Guerinot, M.L.: Molecular aspects of Cu, Fe and Zn homeostasis in plants. — Biochim. biophys. Acta 1763: 595–608, 2006.
Hajlaoui, H., Denden, M., Ayeb, N.E.: Changes in fatty acids composition, hydrogen peroxide generation and lipid peroxidation of salt-stressed corn (Zea mays L.) roots. — Acta Physiol. Plant. 31: 787–796, 2009.
Hakan, C.A., Vahap, K.: Some parameters in relation to iron nutrition status of peach orchards. — J. Biol. environ. Sci. 1: 111–115, 2007.
Hasan SA, Hayat S, Ali B, Ahmad A.: 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. - Environ. Pollut. 151: 60–66, 2008.
Hartzendorf, T., Rolletschek, H.: Effect of NaCl salinity on amino acid and carbohydrate contents of Phragmites australis. — Aquat. Bot. 69: 195–208, 2001.
Hayat, S., Hasan, S.A., Yusuf, M., Hayat, Q., Ahmad, A.: Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. — Environ. exp. Bot. 69: 105–112, 2010.
Hayat, S., Yadav, S., Wani, A., Irfan, M., Ahmad, A.: Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the growth, carbonic anhydrase activity and photosynthetic efficiency of Lycopersicum esculentum. — Photosynthetica 49: 397–404, 2011.
Heath, R.L., Packer, L.: Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. — Arch. Biochem. Biophys. 125: 189–198, 1968.
Hell, R., Stephan, U.W.: Iron uptake, trafficking and homeostasis in plants. — Planta. 216: 541–551, 2003.
Hoagland, D.R., Arnon, D.I.: The water-culture method for growing plants without soil. — Calif. Agr. Exp. Sta. 347: 1–32, 1950.
Ishimaru, Y., Suzuki, M., Tsukamoto, T., Suzuki, K., Nakazono, M., Kobayashi, T., Wada, Y., Watanabe, S., Matsuhashi, S., Takahashi, M., Nakanishi, H., Nishizawa, N.K.: Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. — Plant J. 45: 335–346, 2006.
Iturbe-Ormaetxe, I., Moran, J. F., Arrese-Igor, C., Gogorcena, Y., Klucas, S. R. V., Becana, M.: Activated oxygen and antioxidant defences in iron-deficient pea plants. — Plant Cell Environ. 18: 421–129, 1995.
Jaleel, C.A., Riadh, K., Gopi, R., Manivannan, P., Inès, J., Al-Juburi, H.J., Zhao, C.X., Shao, H.B., Panneerselvam, R.: Antioxidant defence responses: physiological plasticity in higher plants under abiotic constraints. — Acta Physiol. Plant. 31: 427–436, 2009.
Jaworski, E.G.: Nitrate reductase assay in intact plant tissues. — Biochem. biophys. Res. Co. 43: 1274–1279, 1971.
Jin, C.W., He, X.Y., Wu, P., Zheng, S.J.: Mechanisms of microbially enhanced Fe acquisition in red clover (Trifolium pretense L.). — Plant Cell Environ. 29: 888–897, 2006.
Jin, C.W., You, G.Y., He, Y.F., Tang, C.X., Wu, P., Zheng, S.J.: Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. — Plant Physiol. 144: 278–285, 2007.
Kagale, S., Divi, U.K., Kronchko, J.E., Keller, W.A., Krishna, P.: Brassinosteroid conifers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. — Planta 225: 353–364, 2007.
Kanwar, M.K., Bhardwaj, R., Chowdhary, S.P., Arora, P., Sharma, P., Kumar, S.: Isolation and characterization of 24-epibrassinolide from Brassica juncea L. and its effects on growth, Ni ion uptake, antioxidant defense of Brassica plants and in vitro cytotoxicity. — Acta Physiol. Plant. 35: 1351–1362, 2013.
Khripach, V., Zhabinskii, V., Groot, A.: Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. — Ann. Bot. 86: 441–447, 2000.
Kim, S.A., Punshon, T., Lanzirotti A., Li, L., Alonso, J.M., Ecker, J.R., Kaplan, J.: Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. — Science 314: 1295–1298, 2006.
Knudson, L.L., Tibbitts, T.W., Edwards, G.E.: Measurement of ozone injury by determination of leaf chlorophyll concentration. — Plant Physiol. 60: 606–608, 1977.
Kobayashi, T., Yoshihara, T., Jiang, T., Goto, F., Nakanishi, H., Mori, S., Nishizawa, N.K.: Combined deficiency of iron and other divalent cations mitigates the symptoms of iron deficiency in tobacco plants. — Physiol. Plant. 119: 400–408, 2003.
Kong, J., Dong, Y.J., Xu, L.L., Liu, S., Bai, X.Y.: Effects of exogenous salicylic acid on alleviating chlorosis induced by iron deficiency in peanut seedlings (Arachis hypogaea L.). — J. Plant Growth Regul. 33: 715–729, 2014a.
Kong, J., Dong, Y.J., Xu, L.L., Liu, S., Bai, X.Y.: Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. — Bot. Stud. 55: 9, 2014b.
Krishna, P.: Brassinoteroid-mediated stress responses. — J. Plant Growth Regul. 22: 289–297, 2003.
Larcher, W.: Physiological Plant Ecology - Ecophysiology and Stress Physiology of Functional Groups. 3rd Ed. - Springer, Tokyo 1995.
Legay, S., Guignard, C., Ziebel, J., Evers, D.: Iron uptake and homeostasis related genes in potato cultivated in vitro under iron deficiency and overload. — Plant Physiol. Biochem. 60: 180–189, 2012.
Mori, S.: Iron acquisition by plants. — Curr. Opin. Plant Biol. 2: 250–253, 1999.
Marschner, H.: Mineral Nutrition of Higher Plants. - Academic Press, Cambridge 1995.
Marschner, H., Römheld, V.: Strategies of plants for acquisition of iron. — Plant Soil 165: 261–274, 1994.
Matysik, J., Alia, A., Bhalu, B., Mohanty, P.: Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. — Curr. Sci. 82: 525–532, 2002.
Mittler, R.: Oxidative stress, antioxidants and stress tolerance. — Trends Plant Sci. 7: 405–410, 2002.
Muller, M., Schmidt, W.: Environmentally induced plasticity of root hair development in Arabidopsis. — Plant Physiol. 134: 409–419, 2004.
Nickel, K.S., Cunningham, B.A.: Improved peroxidase assay method using leuco-2,3,6-trichloroindophenol and application to comparative measurements of peroxidase catalysis. — Anal. Biochem. 27: 292–299, 1969.
Ogweno, J.O., Song, X.S., Shi, K., Hu, W.H., Mao, W.H., Zhou, Y.H., Yu, J.Q., Nogués, S.: Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicum esculentum. — J. Plant Growth Regul. 27: 49–57, 2008.
Ohinishi, T., Gall, R.S., Mayer, M.L.: An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. — Anal. Biochem. 69: 261–267, 1975.
Palmgren, M.G.: Plant plasma membrane H+-ATPase: powerhouses for nutrient uptake. — Annu Rev Plant Biol. 52: 817–845, 2001.
Patra, H.L., Kar, M., Mishre, D.: Catalase activity in leaves and cotyledons during plant development and senescence. — Biochem. Pharmacol. 172: 385–390, 1978.
Piñol, R., Simón, E.: Effect of 24-epibrassinolide on chlorophyll fluorescence and photosynthetic CO2 assimilation in Vicia faba plants treated with the photosynthesis-inhibiting herbicide Terbutryn. — J. Plant Growth Regul. 28: 97–105, 2009.
Pushnik, J.C., Miller, G.W.: Iron regulation of chloroplast photosynthetic function: mediation of PS I development. — J. Plant Nutr. 12: 407–421, 1989.
Rodríguez-Lucena, P., Hernández-Apaolaza, L., Lucena, J.J.: Comparison of iron chelates and complexes supplied as foliar sprays and in nutrient solution to correct iron chlorosis of soybean. — J. Plant Nutr. Soil Sci. 173: 120–126, 2010.
Sairam, R.K.: Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress conditions of two wheat varieties. — Plant Growth Regul. 14: 173–18, 1994.
Sasse, J.M.: Physiological actions of brassinosteroids: an update. — J. Plant Growth Regul. 22: 276–288, 2003.
Schmidt, W.: Mechanisms and regulation of reduction-based iron uptake in plants. — New Phytol. 141: 1–26, 1999.
Shenker, M., Chen, Y.: Increasing iron availability to crops: fertilizers, organo-fertilizers, and biological approaches. — Soil Sci Plant Nutr. 51: 1–17, 2005.
Sevilla, F., Del Rio, L.A., Hellin, E.: Superoxide dismutases from a citrus plant: presence of two iron-containing isoenzymes in leaves of lemon trees (Citrus limonum L.). — J. Plant Physiol. 116: 381–387, 1984.
Shi, G.R., Cai, Q.S., Liu, Q.Q., Wu, L.: Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. — Acta Physiol. Plant. 31: 969–977, 2009.
Shi, Q.H., Zhu, Z.J.: Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. — Environ. exp. Bot. 63: 317–326, 2008.
Simaei, M., Khavarinejad, R.A., Saadatmand, S., Bernard, F., Fahimi, H.: Interactive effects of salicylic acid and nitric oxide on soybean plants under NaCl salinity. — Russ. J. Plant Physiol. 58: 783–790, 2011.
Sirhindi, G., Kumar, S., Bhardwaj, R., Kumar, M.: Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. — Physiol. mol. Biol. Plants 15: 335–341, 2009.
Stewart, R.C., Bewley, J.D.: Lipid peroxidation associated with accelerated aging of soybean axes. — Plant Physiol. 65: 245–248, 1980.
Su, Y., Liu, J.L., Lu, Z.W., Wang, X.M., Zhang, Z., Shi, G.G.: Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. — Environ. exp. Bot. 97: 40–48, 2014.
Takker, P.N., Kaur. N.P.: HCl method for Fe2+ estimation to resolve iron chlorosis in plants. — J. Plant Nutr. 7: 81–90, 1984.
Tewari, R.K., Kumar, P., Neetu, Sharma, P.N.: Signs of oxidative stress in the chlorotic leaves of iron starved plants. — Plant Sci. 169: 1037–1045, 2005.
Thomine, S., Lelièvre, F., Debarbieux, E., Schroeder, J.I., Barbier-Brygoo, H.: AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. — Plant J. 34: 685–695, 2003.
Vert, G., Grotz, N., Dedaldechamp, F., Gaymard, F., Guerinot, M.L., Briat, J.F., Curie, C.: IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. — Plant Cell 14: 1223–1233, 2002.
Wang, B., Li, Y.S., Zhang, W.H.: Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. — Ann. Bot. 110: 681–688, 2012.
Wilen, R.W., Sacco, M., Gusta, L.V., Krishna, P.: Effects of 24-epibrassinolide on freezing and thermotolerance of bomegrass (Bromus inermis) cell cultures. — Physiol. Plant. 95: 195–202, 1995.
Zhang, X.W., Dong, Y.J., Qiu, X.K., Hu, G.Q., Wang, Y.H., Wang, Q.H.: Exogenous nitric oxide alleviates irondeficiency chlorosis in peanut growing on calcareous soil. — Plant Soil Environ. 58: 111–120, 2012.
Zhao, Y,J,, Chen, J,: Studies on physiological action and application of 24-epibrassinolide in agriculture. - In: Hayat S, Ahmad A (ed.): Brassinosteroids: Bioactivity and Crops Productivity. Pp. 159–170. Kluwer Academic Publishers, Dordrecht 2003.
Zuo, Y.M., Zhang, F.S.: Soil and crop management strategies to prevent iron deficiency in crops. — Plant Soil. 339: 83–95, 2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: The authors thank Pingping Yang (the College of Animal Science and Technology, the Shandong Agricultural University, China) for her supplying instruments and patient guidance. This research was financially supported by the Shandong Provincial Natural Science Foundation of China (ZR2013CM003) and the Shandong Province Higher Educational Science and Technology Program (J14LF08).
Rights and permissions
About this article
Cite this article
Song, Y.L., Dong, Y.J., Tian, X.Y. et al. Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. Biol Plant 60, 329–342 (2016). https://doi.org/10.1007/s10535-016-0596-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-016-0596-4