Abstract
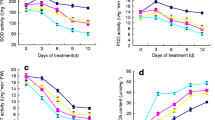
To investigate the effects of exogenously applied brassinosteroids on the thermotolerance of plants, leaf CO2 assimilation, chlorophyll fluorescence parameters, and antioxidant enzyme metabolism were examined in tomato (Lycopersicon esculentum Mill. cv. 9021) plants with or without 24-epibrassinolide (EBR) application. Tomato plants were exposed to 40/30°C for 8 days and then returned to optimal conditions for 4 days. High temperature significantly decreased the net photosynthetic rate (P n), stomatal conductance (G s), and maximum carboxylation rate of Rubisco (V cmax), the maximum potential rate of electron transport contributed to ribulose-1,5-bisphosphate (RuBP), as well as the relative quantum efficiency of PSII photochemistry (ФPSII), photochemical quenching (q P), and increased nonphotochemical quenching (NPQ). However, only slight reversible photoinhibition occurred during heat stress. Interestingly, EBR pretreatment significantly alleviated high-temperature-induced inhibition of photosynthesis. The activities of antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPOD), and catalase (CAT) increased during heat treatments, and these increases proved to be more significant in EBR-treated plants. EBR application also reduced total hydrogen peroxide (H2O2) and malonaldehyde (MDA) contents, while significantly increasing shoot weight following heat stress. It was concluded that EBR could alleviate the detrimental effects of high temperatures on plant growth by increasing carboxylation efficiency and enhancing antioxidant enzyme systems in leaves.




Similar content being viewed by others
References
Allen DJ, Mckee IF, Farage PK, Baker NR (1997) Analysis of limitation to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ 20:633–640
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: Relationships in green cells. Physiol Plant 100:224–233
Anderson JA (2002) Catalase activity, hydrogen peroxide content and thermotolerance of pepper leaves. Sci Hort 95:277–284
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Ann Rev Plant Biol 55:373–399
Baker NR, Nogués S, Allen DJ (1997) Plants and UV-B; response to environmental change. In: Lumsden PJ (ed) Photosynthesis and photoinhibition. Cambridge University Press, Cambridge pp 95–111
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature inhigher plants. Ann Rev Plant Physiol 31:491–543
Bilger W, Björkman O (1990) Role of the xanthophylls cycle in photo protection elucidated by measurements of light induced absorbance changes, fluorescence and photosynthesis in Hedera canariensis. Photosyn Res 25:173–185
Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. PlantCell 14:S97–S110
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein - dye binding. Anal Biochem 72:248–254
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of super oxide dismutase ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B (2005) Loss of function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123:57–66
Clouse SD (1996) Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J 10:1–8
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Ann Rev Plant Physiol Plant Mol Biol 49:427–451
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Nat Acad Sci U S A 97:13430–13435
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize to heat stress. Plant Physiol 129:73–1780
Dhaubhadel S, Chaundhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Ershova A, Khripach V (1996) Effects of epibrassinolide on lipid peroxidation in Pisum sativum at normal aeration and under oxygen deficiency. Russ J Plant Physiol 43:750–752
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153
Garcia-Ferris C, Moreno J (1994) Oxidative modification and breakdown of ribulose 1,5-bisphosphate carboxylase/oxygenase induced in Euglena gracilis by nitrogen starvation. Planta 193:208–215
Genty B, Briatais JM, Baker NR (1989) The relationships between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Giannopolitis CN, Ries SK (1977) Superoxide dismutase I. Occurrences in higher plants. Plant Physiol 59:309–414
Gilmore AM (1997) Mechanistic aspects of xanthophylls cycle dependent photoprotection in higher plant chloroplast and leaves. Physiol Plant 99:197–209
Haldimann P, Feller U (2005) Growth at moderately elevated temperature alters the physiological response of the photosynthetic apparatus to heat stress in pea (Pisum sativum L.) leaves. Plant Cell Environ 28:302–317
Haubrick LL, Assman SM (2006) Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ 29:446–457
Havaux M (1993) Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci 94:19–33
Heckathorn SA, Downs CA, Sharkey TD, Coleman JS (1998) The small, methionine-rich chloroplast heat shock proteins protect photosystem II electron transport during heat stress. Plant Physiol 116: 439–444
Hirotsu N, Makino A, Ushio A, Mae T (2004) Changes in the thermal dissipation and the electron flow in the water–water cycle in rice grown under conditions of physiologically low temperature. Plant Cell Physiol 45:635–644
Horton P, Ruban AV, Walters RG (1996 Regulation of light harvesting in green plants. Ann Rev Plant Physiol Plant Mol Biol 47:655–684
Irihimovitch V, Shapira M (2000) Glutathione redox potential modulated by reactive oxygen species regulates translation of Rubisco large subunit in the chloroplast. J Biol Chem 275:16289–16295
Ishida H, Shimizu S, Makino A, Mae T (1998) Light dependent fragmentation of the large subunit of ribulose -5,1 bisphosphate carboxylase/oxygenase in chloroplast isolated from wheat leaves. Planta 204:305–309
Khripach V, Zhabinskii V, De Groot A (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Kulaeva ON, Burkhanova EA, Fedina AB, Khokhlova VA, Bokebayeva GA, Vorbrodt HM, Adam G (1991) Effects of brassinosteroids on protein synthesis and plant cell ultrastructure under stress conditions. In: Cutler HG, Yokota T, Adam G (eds), Brassinosteroids: Chemistry, Bioactivity, and Applications, Am Chem Soc Symp Ser 474. Washington, DC: American Chemical Society, pp 141–155
Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Physiol 120:173–181
Leakey ADB, Press MC, Scholes JD (2003) High temperature inhibition of photosynthesis is greater under sun flecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ 26:1681–1690
Lefebvre S, Lawson T, Fryer M, Zalchleniuk OK, Lloyd JC, Raines CA (2005) Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138:451–460
Mandava NB (1988) Plant growth-promoting brassinosteroids. Ann Rev Plant Physiol Plant Mol Biol 39:23–25
Mittler R (2002) Oxidative stress antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Montoya T, Nomura T, Yokota T, Farrar K, Harrison K, Jones JGD, Kaneta T, Kamiya W, Szekeres M, Bishop GR (2005) Patterns of dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. Plant J 42:262–269
Mazzora LM, Núñez M, Hechararria M, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzyme activity in tomato under different temperatures. Biol Plant 45:593–596
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:676–690
Nakashita H, Yasuda M, Nitta T, Asami T, Fujikoa S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroids functions in a broad range of disease resistance in tobacco and rice. Plant J 33:887–898
Nogués S, Baker NR (2000) Effect of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Núñez M, Mazzafera P, Mazzora LM, Sigueira WJ, Zullo MAT (2003) Influence of a brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47:67–70
Ölcer H, Llyod JC, Raines CA (2001) Photosynthetic capacity is differentially affected by reduction in sedoheptulose-1,7-bisphosphatase activity during leaf development in transgenic tobacco plants. Plant Physiol 125:982–989
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis. Curr Opin Plant Biol 5:193–198
Ozdemir F, Bor M, Demiral T, Turkan I (2004) Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L) under salinity stress. Plant Growth Regul 42:203–211
Patterson BD, Mackae EA, Mackae I (1984) Estimation of hydrogen peroxide in plants extracts using Titanium (ıv). Anal Biochem 139:487–492
Pfannschmidt T (2003) Chloroplast redox signals how photosynthesis controls its own genes. Trends Plant Sci 8:33–41
Quinn PJ, Williams WP (1985) Environmental induced changes in chloroplast membranes and their effects on photosynthetic function. In: Baker NR, Barberand J (eds), Photosynthetic mechanisms and the environment. Amsterdam: Elsevier, pp. 1–47
Rivero RM, Ruiz JM, Romero L (2004) Oxidative metabolism in tomato plants subjected to heat stress. J Hort Sci Biotechnol 79:560–564
Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470
Scandalios JG (2002) Oxidative stress responses—What have genome-scale studies taught us? Genome Biol 3:10191–10196
Sharkey TD (2006) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29:1659–1670
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Steber CM, McCourt P (2001) A role for brassinosteroid in germination in Arabidopsis. Plant Physiol 125:763–769
Takahama U, Oniki T (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33:379–387
van Kooten O, Snel J (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Res 25:147–150
Verhoeven AS, Demmig-Adams B, Adams WWIII (1997) Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol 113:817–824
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wilen RW, Sacco M, Gusta LV, Krishna P (1995) Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol Plant 95:195–202
Willenkens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I (2006) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29:1659–1670
Yordanov I, Dilova S, Petkova R, Pangelova T, Goltsev VKH (1986) Mechanisms of the temperature damage and acclimation of the photosynthetic apparatus. Photobiochem Photobiophys 12:147–155
Yu JQ, Matsui Y (1997) Effects of root exudates and allelochemicals on ion uptake by cucumber seedlings. J Chem Ecol 23:17–827
Yu JQ, Zhou YH, Ye SF, Huang LF (2002) 24-epibrassinolide and abscisic acid protect cucumber seedlings from chilling injury. J Hort Sci Biotechnol 77:430–473
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role forbrassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Zhou YH, Yu JQ, Huang LF, Nogués S (2004) The relationship between CO2 assimilation, photosynthetic electron transport and water-water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant Cell Environ 27:1503–1514
Zhou YH, Yu JQ, Mao WH, Huang LF, Song XS, Nogués S (2006) Genotypic variation on Rubisco expression, photosynthetic electron flow and antioxidant metabolism in the chloroplasts of chill-exposed cucumber plants. Plant Cell Physiol 47:192–199
Acknowledgments
This work was supported by the National Natural Science Foundation of China (3050344, 30671428) and the National Outstanding Youth Scientist Foundation (30235029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogweno, J.O., Song, X.S., Shi, K. et al. Brassinosteroids Alleviate Heat-Induced Inhibition of Photosynthesis by Increasing Carboxylation Efficiency and Enhancing Antioxidant Systems in Lycopersicon esculentum . J Plant Growth Regul 27, 49–57 (2008). https://doi.org/10.1007/s00344-007-9030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-007-9030-7