Abstract
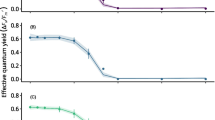
Thalli of Xanthoparmelia somloensis with natural content of polyols (control) and polyol-free thalli (acetone-rinsed) were used to study ribitol effects at low temperatures. Thalli segments were cultivated in ribitol concentration of 32 or 50 mM for 168 h at temperatures +5, 0, and −5 °C. The chlorophyll fluorescence parameters (potential yield of photochemical reactions in PS 2 (variable to maximum fluorescence ratio, Fv/Fm), effective quantum yield of photochemical reactions in PS 2 (ΦPS2), and non-photochemical quenching (NPQ) were monitored in 24-h intervals using an imaging system. The effect of 32 mM ribitol on Fv/Fm and ΦPS2 was apparent only at −5 °C, however, the effect was seen throughout the whole exposure. Surprisingly, 50 mM ribitol concentration treatment led to a decrease in Fv/Fm and ΦPS2 and to an increase in NPQ values at −5 °C, while no change was observed at 0 °C and +5 °C. Acetone-rinsing caused decrease of Fv/Fm, ΦPS2 and NPQ.
Similar content being viewed by others
Abbreviations
- Car:
-
carotenoids
- Chl:
-
chlorophyll
- Fv/Fm :
-
variable to maximum fluorescence ratio (potential yield of photochemical reactions in PS 2)
- NPQ:
-
non-photochemical quenching of chlorophyll fluorescence
- ΦPS2 :
-
effective quantum yield of photochemical reactions in PS 2
References
Abebe, T., Guenzi, A.C., Martin, B., Cushman, J.C.: Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity.— Plant Physiol. 131: 1748–1755, 2003.
Armstrong, R.A., Smith, S.N.: The levels of ribitol, arabitol and mannitol in individual lobes of the lichen Parmelia conspersa (Ehrh ex Ach) Ach. — Environ. exp. Bot. 34: 253–260, 1994.
Armstrong, R.A., Smith, S.N.: Does radial growth of the lichen Parmelia conspersa depend exclusively on growth processes at the lobe tip? — Environ. exp. Bot. 39: 263–269, 1998.
Aubert, S., Juge, C., Boisson, A.M., Gout, E., Bligny, R.: Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. — Planta 226: 1287–1297, 2007.
Barták, M., Gloser, J., Hájek, J.: Visualized photosynthetic characteristics of the lichen Xanthoria elegans related to daily courses of light, temperature and hydration: a field study from Galindez Island, maritime Antarctica. — Lichenologist 37: 433–443, 2005.
Barták, M., Váczi, P., Hájek, J., Smykla, J.: Low-temperature limitation of primary photosynthetic processes in Antarctic lichens Umbilicaria antarctica and Xanthoria elegans. — Polar Biol. 31: 47–51, 2007.
Barták, M., Vráblíková, H., Hájek, J.: Sensitivity of photosystem 2 of Antarctic lichens to high irradiance stress: Fluorometric study of fruticose (Usnea antarctica) and foliose (Umbilicaria decussata) species. — Photosynthetica 41: 497–504, 2003.
Brunauer, G., Hager, A., Grube, M., Türk, R., Stocker-Wörgötter, E.: Alterations in secondary metabolism of aposymbiotically grown mycobionts of Xanthoria elegans and cultured resynthesis stages. — Plant Physiol. Biochem. 45:146–151, 2007.
Candan, M., Yilmaz, M., Tay, T., Kivanc, M., Turk, H.: Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. — Z. Naturforsch. C. 61: 319–323, 2006.
Chang, H.L., Chao, G.R., Chen, C.C., Mau, J.L.: Non-volatile taste components of Agaricus blazei, Antrodia camphorata and Cordyceps militaris mycelia. — Food Chem. 74: 203–207, 2001.
Chapman, B.E., Roser, D.J., Seppelt, R.D.: C-13 NMR analysis of antarctic cryptogam extracts. — Antarct. Sci. 6: 295–305, 1994.
Coxson, D.S., Coyle, M.: Niche partitioning and photosynthetic response of alectorioid lichens from subalpine spruce-fir forest in north-central British Columbia, Canada: the role of canopy microclimate gradients. — Lichenologist 35: 157–175, 2003.
Da Silva, M.D.C., Iacomini, M., Jablonski, E., Gorin, P.A.J.: Carbohydrate, glycopeptide and protein components of the lichen Sticta sp. and effect of storage. — Phytochemistry 33: 547–552, 1993.
Dahlman, L., Persson, J., Nasholm, T., Palmqvist, K.: Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. — Planta 217: 41–48, 2003.
Dudley, S.A., Lechowicz, M.J.: Losses of polyol through leaching in Sub-Arctic lichens. — Plant Physiol. 83: 813–815, 1987.
Elix, J.A., Wardlaw, J.H.: Lusitanic acid, peristictic acid and verrucigeric acid. Three new beta-orcinol depsidones from the lichens Relicina sydneyensis and Xanthoparmelia verrucigera. — Aust. J. Chem. 53: 815–818, 2000.
Farrar, J.F.: Ecological physiology of the lichen Hypogymnia physodes. II. Effects of wetting and drying cycles and the concept of ‘physiological buffering’. — New Phytol. 77: 105–113, 1976.
Feige, G.B.: Probleme der Flechtenphysiologie. — Nova Hedwigia 30: 725–774, 1978.
Feige, G.B., Jensen, M.: Basic carbon and nitrogen metabolism of lichens. — In: Reisser, W. (ed.): Algae and Symbioses: Plants, Animals, Fungi, Viruses, Interactions Explored. Pp. 277–299. Biopress, Bristol 1992.
Fontaniella, B., Vicente, C., Legaz, M.E.: The cryoprotective role of polyols in lichens: effects on the redistribution of RNAse in Evernia prunastri thallus during freezing. — Plant Physiol. Biochem. 38: 621–627, 2000.
Friedmann, E.I., Sun, H.J.: Communities adjust their temperature optima by shifting producer-to-consumer ratio, shown in lichens as models: I. Hypothesis. — Microbiol. Ecol. 49: 523–527, 2005.
Genty, B., Briantais, J.M., Baker, N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. biophys. Acta 990: 87–92, 1989.
Hager, A., Brunauer, G., Turk, R., Stocker-Woergoetter, E.: Production and bioactivity of common lichen metabolites as exemplified by Heterodea muelleri (Hampe) Nyl. — J. chem. Ecol. 34: 113–120, 2008.
Hájek, J., Barták, M., Gloser, J.: Effects of thallus temperature and hydration on photosynthetic parameters of Cetraria islandica from contrasting habitats. — Photosynthetica 39: 427–435, 2001.
Hájek, J., Váczi, P., Barták, M.: Photosynthetic electron transport at low temperatures in the green algal foliose lichens Lasallia pustulata and Umbilicaria hirsuta affected by manipulated levels of ribitol. — Photosynthetica 47: 199–205, 2009.
Hamada, N., Okazaki, K., Shinozaki, M.: Accumulation of monosaccharides in lichen mycobionts cultured under osmotic conditions. — Bryologist 97: 176–179, 1994.
Kappen, L., Schroeter, B., Hestmark, G., Winkler, J.B.: Field measurements of photosynthesis of umbilicarious lichens in winter. — Bot. Acta 109: 292–298, 1996.
Lange, O.L.: Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation II. Diel and seasonal patterns of net photosynthesis and respiration. — Flora 198: 55–70, 2003.
Legaz, M.E., Avalos, A., De Torres, M., Escribano, M.I., Gonzáles, A., Martin-Falquina, A., Pérezurria, E., Vicente, C.: Annual variations in arginine metabolism and phenolic content of Evernia prunastri. — Environ. exp. Bot. 26: 385–396, 1986.
Lines, C.E., Ratcliffe, R.G., Rees, T.A.V., Southon, T.E.: A 13C NMR study of photosynthate transport and metabolism in the lichen Xanthoria catcicola Oxner. — New Phytol. 111: 447–456, 1989.
McEvoy M., Nybakken L., Solhaug K.A., Gauslaa, Y.: UV triggers the synthesis of the widely distributed secondary lichen compound usnic acid. — Mycol. Progr. 5: 221–229, 2006.
Palmqvist, K.: Carbon economy in lichens. — New Phytol. 148: 11–36, 2000.
Pannewitz, S., Green, T.G.A., Maysek, K., Schlensog, M., Seppelt, R., Sancho, L.G., Turk, R., Schroeter, B.: Photosynthetic responses of three common mosses from continental Antarctica. — Antarct. Sci. 17: 341–352, 2005.
Rankovic, B., Misic, M., Sukdolak, S.: Evaluation of antimicrobial activity of the lichens Lasallia pustulata, Parmelia sulcata, Umbilicaria crustulosa, and Umbilicaria cylindrica. — Mikrobiology 76: 723–727, 2007.
Reiter, R., Hoftberger, M., Green, T.G.A., Turk, R.: Photosynthesis of lichens from lichen-dominated communities in the alpine/nival belt of the Alps — II: Laboratory and field measurements of CO2 exchange and water relations. — Flora 203: 34–46, 2008.
Richardson, D.H.S., Smith, D.C.: Lichen physiology. IX. Carbohydrate movement from the Trebouxia symbiont of Xanthoria aureola to the fungus. — New Phytol. 67: 61–68, 1968.
Roach, J.A.G., Musser, S.M., Morehouse, K., Woo, J.Y.J.: Determination of usnic acid in lichen toxic to elk by liquid chromatography with ultraviolet and tandem mass spectrometry detection. — J. Agr. Food Chem. 54: 2484–2490, 2006.
Roser, D.J., Melick, D.R., Ling, H.U., Seppelt, R.D.: Polyol and sugar content of terrestrial plants from continental Antarctica. — Antarct. Sci. 4: 413–420, 1992a.
Roser, D.J., Melick, D.R., Seppelt, R.D.: Reductions in the polyhydric alcohol content of lichens as an indicator of environmental pollution. — Antarct. Sci. 4: 185–188, 1992b.
Schlensog, M., Schroeter, B.: A new method for the accurate in situ monitoring of chlorophyll a fluorescence in lichens and bryophytes. — Lichenologist 33: 443–452, 2001.
Schreiber, U., Bilger, W., Neubauer, C.: Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. — In: Schulze, E.D., Caldwell, M.M. (ed.): Ecophysiology of Photosynthesis. Pp. 49–70. Springer-Verlag, Berlin — Heidelberg — New York 1995.
Solhaug, K.A., Gauslaa, Y.: Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. — Plant Cell Environ. 27: 167–176, 2004.
Stoop, J.M.H., Williamson, J.D., Pharr, D.M.: Mannitol metabolism in plants: a method for coping with stress. — Trends Plant Sci. 1: 139–144, 1996.
Sturgeon, R.J.: Biosynthesis and utilization of storage sugars in algae, fungie, and lichens. — Physiol. vég. 23: 95–106, 1985.
Takahagi, T., Ikezawa, N., Endo, T., Ifuku, K., Yamamoto, Y., Kinoshita, Y., Takeshita, S., Sato, F.: Inhibition of PSII in atrazine-tolerant tobacco cells by barbatic acid, a lichenderived depside. — Biosci. Biotechnol. Biochem. 70: 266–268, 2006.
Tsai, S.Y., Tsai, H.L., Mau, J.L.: Non-volatile taste components of Agaricus blazei, Agrocybe cylindracea and Boletus edulis. — Food Chem. 107: 977–983, 2008.
Van Kooten, O., Snel, J.F.H.: The use of chlorophyll fluorescence nomenclature in plant stress physiology. — Photosynth. Res. 25: 147–150, 1990.
Vojtíšková, L., Munzarová, E., Votrubová, O., Čížková, H., Lipavská, H.: The influence of nitrogen nutrition on the carbohydrate and nitrogen status of emergent macrophyte Acorus calamus L. — Hydrobiologia 563: 73–85, 2006.
Wellburn, A.R.: The spectral determination of chlorophyll a and chlorophhyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. — J. Plant Physiol. 144: 307–313, 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: The study reported in this paper was supported by the KJB601630808 project funded by the Grant Agency of the Czech Academy of Science (GAAV), Czech Republic.
Rights and permissions
About this article
Cite this article
Hájek, J., Váczi, P., Barták, M. et al. Cryoproective role of ribitol in Xanthoparmelia somloensis . Biol Plant 53, 677–684 (2009). https://doi.org/10.1007/s10535-009-0122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-009-0122-z