Abstract
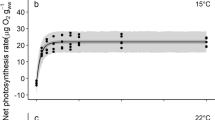
We determined the chronic effects of dehydration on the photochemical efficiency of a cultivated brown alga, Undaria pinnatifida (Alariaceae, Laminariales), in young sporophytes as cultivated sporelings. The effective quantum yields of photosystem II (ΔF/Fm′) at 50% humidity decreased markedly after 20 min of emersion and dropped almost zero after 60 min of emersion; the values did not restore even after subsequent 1-day immersion. The decreasing values coincided with a decrease in absolute water content of less than 40%. However, under 99% humidity up to 5-day emersion, the ΔF/Fm′ well remained and the last state exhibited a similar level to the initial value after a 48-h emersion at 20 °C and after 72 h of emersion at 10 °C, suggesting that the thalli were not truly dehydrated under saturated humidity and that photosynthetic activity was maintained for several days even without immersion in seawater. In addition, the subsequent growth of young sporophytes exposed to transportation storage stress featuring (1) immersed in seawater with aeration (ST1), (2) those without aeration (ST2), and (3) wrapped in paper towels moistened with seawater (ST3) revealed that the sporophytes exposed at lower temperature exhibited a higher subsequent growth than those at a higher temperature. The subsequent growth of ST3 was lower than ST1; however, the values of ST3 were higher than those of ST2 more than 12 °C, associating with poor seawater quality without aeration. These results indicated that the maintenance of moisture in the alga at low temperatures might be essential for the transportation of the sporelings.




Similar content being viewed by others
Data availability
The datasets generated in the present study are available from the corresponding author on reasonable request.
References
Apoya M, Ogawa H, Nanba N (2002) Alginate content of farmed Undaria pinnatifida (Harvey) Suringar from the three bays of Iwate, Japan during harvest period. Bot Mar 45:445–452
Aquino RS, Grativol C, Mourão PAS (2011) Rising from the se: correlations between sulfated polysaccharides and salinity in plants. PloS One 6: e18862
Bollen M, Battershill CN, Pilditch CA, Bischof K (2017) Desiccation tolerance of different life stages of the invasive marine kelp Undaria pinnatifida: potential for overland transport as invasion vector. J Exp Mar Biol Ecol 496:1–8
Borlongan IA, Nishihara GN, Shimada S, Terada R (2017) Photosynthetic performance of the red alga Solieria pacifica (Solieriaceae) from two different depths in the sublittoral waters of Kagoshima, Japan. J Appl Phycol 29:3077–3088
Borlongan IA, Arita R, Nishihara GN, Terada R (2020) The effects of temperature and irradiance on the photosynthesis of two heteromorphic life history stages of Saccharina japonica (Laminariales) from Japan. J Appl Phycol 32:4175–4187
Borlongan IA, Matsumoto K, Nakazaki Y, Shimada N, Kozono J, Nishihara GN, Shimada S, Watanabe Y, Terada R (2018) Photosynthetic activity of two life history stages of Costaria costata (Laminariales, Phaeophyceae) in response to PAR and temperature gradient. Phycologia 57:159–168
Borlongan IA, Nishihara GN, Shimada S, Terada R (2019) Assessment of photosynthetic performance in the two life history stages of Alaria crassifolia (Laminariales, Phaeophyceae). Phycol Res 67:28–38
Bürkner PC (2018) Advanced Bayesian multilevel modeling with the R package brms. R J 10:395–411
Campbell SJ, Bite JS, Burridge TR (1999) Seasonal patterns in the photosynthetic capacity, tissue pigment and nutrient content of different development stages of Undaria pinnatifida (Phaeophyta: Laminariales) in Port Phillip Bay, southern-eastern Australia. Bot Mar 42:231–241
Contreras-Porcia L, Thomas D, Flores V, Correa JA (2011) Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J Exp Bot 62:1815–1829
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, BorowitzkaMA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Springer, Dordrecht, pp 1–17
Dan A, Ohno M, Matsuoka M (2015) Changes of the research and development on the resources of Undaria and Laminaria in the culture ground of Tokushima coasts. Bull Tokushima Pref Fish Res Inst 10:25–48
Dean PR, Hurd CL (2007) Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J Phycol 43:1138–1148
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Duarte CM, Wu Jiaping, Xiao X, Bruhn A, Krause-Jensen D (2017) Can seaweed farming play a role in climate change mitigation and adaptation? Front Mar Sci 12.
Forbord S, Steinhovden KB, Solvang T, Handå A, Skjermo J (2020) Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J Appl Phycol 32:2201–2212
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2018) The photosynthetic responses to PAR and temperature including chilling-light stress on the heteromorphic life history stages of a brown alga, Cladosiphon okamuranus (Chordariaceae) from Ryukyu Islands, Japan. Phycol Res 66:209–217
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2019) Effect of photosynthetically active radiation and temperature on the photosynthesis of two heteromorphic life history stages of a temperate edible brown alga, Cladosiphon umezakii (Chordariaceae, Ectocarpales), from Japan. J Appl Phycol 31:1259–1270
Gao X, Endo H, Taniguchi K, Agatsuma Y (2013a) Genetic differentiation of high-temperature tolerance in the kelp Undaria pinnatifida sporophytes from geographically separated populations along the Pacific coast of Japan. J Appl Phycol 25:567–574
Gao S, Niu J, ChenW WG, Xie X, Pan G, GuW ZhuD (2013b) The physiological links of the increased photosystem II activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and rehydration. Photosynth Res 116:45–54
Gao S, Shen S, Wang G, Niu J, Lin A, Pan G (2011) PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol 52:885–893
Gao S, Wang G (2012) The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J Exp Bot 63:4349–4358
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interaction between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–300
Hanelt D (1998) Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar Biol 131:361–369
Ito T, Borlongan IA, Nishihara GN, Endo H, Terada R (2021) The effects of irradiance, temperature, and desiccation on the photosynthesis of a brown alga, Sargassum muticum (Fucales), from a native distributional range in Japan. J Appl Phycol 33:1777–1791
Japan Fisheries Cooperative (2020) Annual report of Wakame in the Sanriku region. URL: https://www.zengyoren.or.jp/
Ji Y, Tanaka J (2002) Effect of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycol Res 50:145–153
Kameyama R, Nishihara GN, Kawagoe C, Terada R (2021) The effects of four stressors, irradiance, temperature, desiccation, and salinity on the photosynthesis of a red alga, Agarophyton vermiculophyllum (Gracilariales) from a native distributional range in Japan. J Appl Phycol. 33:2561–2575
Kanagasabhapathy M, Sasaki H, Haldar S, Yamasaki S, Nagata S (2006) Antibacterial activities of marine epibiotic bacteria isolated from brown algae of Japan. Ann Microbiol 56:167–173
Kerrison PD, Stanley MS, Hughes AD (2018) Textile substrate seeding of Saccharina latissima sporophytes using a binder: an effective method for the aquaculture of kelp. Algal Res 33:352–357
Kim KY, Garbary DJ (2007) Photosynthesis in Codium fragile (Chlorophyta) from a Nova Scotia estuary: responses to desiccation and hyposalinity. Mar Biol 151:99–107
Kim WJ, Kim SM, Lee YH, Kim HG et al (2008) Isolation and characterization of marine bacterial strain degrading fucoidan from Korean Undaria pinnatifida sporophylls. J Microbiol Biotechnol 18:616–623
Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21:234–244
Kokubu S, Nishihara GN, Watanabe Y, Tsuchiya Y, Amano Y, Terada R (2015) The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 54:235–247
Kubanek J, Lester SE, Fenical W, Hay ME (2004) Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar Ecol Prog Ser 277:79–93
Küpper FC, Kloareg B, Guern J, Potin P (2001) Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiol 125:278–291
Lee YK, Jung HJ, Lee HK (2006) Marine bacteria associated with the Korean brown alga, Undaria pinnatifida. J Microbiol 44:694–698
Lüning K (1990) Seaweeds: their environment, biogeography, and ecophysiology. Wiley, New York
Migita S (1964) Freeze-preservation of Porphyra thalli in viable state - I, viability of Porphyra tenera preserved at low temperature after freezing in the sea water and freezing under half-dried condition. Bull Fac Fish Nagasaki Univ 17:44–54 (in Japanese with English abstract)
Migita S, Abe N (1966) Formation of spores in Conchocelis of Porphyra. Bull Fac Fish Nagasaki Univ 20:1–13 (in Japanese with English abstract)
Pavia H, Toth G (2000) Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology 81:3212–3225
Pedersen EJ, Miller DL, Simpson GL, Ross N (2019) Hierarchical Generalized Additive Models in Ecology: an Introduction with Mgcv. PeerJ 7:e6876
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview. Algal Res 15:9–23
R Development Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org (acessed on 10 June 2021)
Saito Y (1958) Cultivation of Undaria pinnatifida. Aquacult Sci 5: 16–20 (in Japanese)
Sakai K (2012) Damage to the fisheries industry in Miyagi Prefecture and efforts for reconstruction. Nippon Suisan Gakkaishi 78:285–287 (in Japanese)
Sato Y, Endo H, Oikawa H, Kanematsu K, Naka H, Mogamiya M, Kawano S, Kazama Y (2020a) Sexual difference in the optimum environmental conditions for growth and maturation of the brown alga Undaria pinnatifida in the gametophyte stage. Genes 11:944
Sato Y, Hirano T, Ichida H, Murakami M, Fukunishi N, Abe T, Kawano S (2017a) Morphological and physiological differences among cultivation lines of Undaria pinnatifida in a common garden experiment using a tank culture system. J Appl Phycol 29:2287–2295
Sato Y, Hirano T, Niwa K, Suzuki T, Fukunishi N, Abe T, Kawano S (2016) Phenotypic differentiation in the morphology and nutrient uptake kinetics among Undaria pinnatifida thalli cultivated at six sites in Japan. J Appl Phycol 28:3447–3458
Sato Y, Kozono J, Nishihara GN, Terada R (2020b) Effect of light and temperature on photosynthesis of a cultivated brown alga, Saccharina sculpera (Laminariales), from Japan. Phycologia 59:375–384
Sato Y, Nagoe H, Nishihara GN, Terada R (2021) The photosynthetic response of cultivated juvenile and mature Undaria pinnatifida (Laminariales) sporophytes to light and temperature. J Appl Phycol 33:3437–3448
Sato Y, Yamaguchi M, Hirano T, Fukunishi N, Abe T, Kawano S (2017b) Effect of water velocity on Undaria pinnatifida and Saccharina japonica growth in a novel tank system designed for macroalgae cultivation. J Appl Phycol 29:1429–1436
Skriptsova A, Khomenko V, Isakov V (2004) Seasonal changes in growth rate, morphology and alginate content in Undaria pinnatifida at the northern limit in the Sea of Japan (Russia). J Appl Phycol 16:17–21
Suárez-Jiménez R, Hepburn CD, Hyndes GA, McLeod RJ, Taylor RB, Hurd CL (2017) Importance of the invasive macroalga Undaria pinnatifida as trophic subsidy for a beach consumer. Mar Biol 164:113
Tatewaki M (1966) Formation of a crustose sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62–66
Terada R, Abe T, Kawaguchi S (2010) Reproductive phenology of three species of Gracilaria: G. blodgettii Harvey, G. vermiculophylla (Ohmi) Papenfuss and G. salicornia (C. Agardh) Dawson (Gracilariales, Rhodophyta) from Okinawa, Ryukyu Islands. Japan Coast Mar Sci 34:129–134
Terada R, Kimura M, Yamamoto H (2000) Growth and maturation of Gracilaria vermiculophylla (Ohmi) Papenfuss from Hakodate, Hokkaido, Japan. Jpn J Phycol 48:203–209 (in Japanese with English abstract)
Terada R, Nishihara GN, Arimura K, Watanabe Y, Mine T, Morikawa T (2021a) Photosynthetic response of a cultivated red alga, Neopyropia yezoensis f. narawaensis (=Pyropia yezoensis f. narawaensis; Bangiales, Rhodophyta) to dehydration stress differs with between two heteromorphic life-history stages. Algal Res 55:102262
Terada R, Takaesu M, Borlongan IA, Nishihara GN (2021b) The photosynthetic performance of a cultivated Japanese green alga Caulerpa lentillifera in response to three different stressors, temperature, irradiance, and desiccation. J Appl Phycol 33:2547–2559
Terada R, Yuge T, Watanabe Y, Mine T, Morikawa T, Nishihara GN (2020) Chronic effects of three different stressors, irradiance, temperature, and desiccation, on the PSII photochemical efficiency in the heteromorphic life-history stages of cultivated Pyropia yezoensis f. narawaensis (Bangiales) from Japan. J Appl Phycol 32:3273–3284
Terada R, Vo TD, Nishihara GN, Shioya K, Shimada S, Kawaguchi S (2016) The effect of irradiance and temperature on the photosynthesis and growth of a cultivated red alga Kappaphycus alvarezii (Solieriaceae) from Vietnam, based on in situ and in vitro measurements. J Appl Phycol 28:457–467
Torde TA, Marcus SE, Jam M, Tonon T, Blackburn RS, Herve C, Knox JP (2015) Monoclonal antibodies directed to fucoidan preparations from brown algae. PloS One 10: e0118366
Van Alstyne KL (1988) Herbivory grazing increases polyphenolic defenses in the intertidal brown alga Fucus distichus. Ecology 69:655–663
Vehtari A, Gelman A, Gabry J (2017) Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput 27:1413–1432
Wang WJ, Wang FJ, Zhu JY, Sun XT, Yao CY, Xu P (2011) Freezing tolerance of Porphyra yezoensis (Bangiales, Rhodophyta) gametophyte assessed by chlorophyll fluorescence. J Appl Phycol 23:1017–1022
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014) The effect of irradiance and temperature responses and the phenology of a native alga, Undaria pinnatifida (Laminariales), at the southern limit of its natural distribution in Japan. J Appl Phycol 26:2405–2415
Watanabe Y, Morikawa T, Mine T, Kawamura Y, Nishihara GN, Terada R (2017) Chronological change and the potential of recovery on the photosynthetic efficiency of Pyropia yezoensis f. narawaensis (Bangiales) during the sporelings frozen storage treatment in the Japanese Nori cultivation. Phycol Res 65:265–271
Weinberger F, Friedlander M, Hoppe HG (1999) Oligoagars elicit an oxidative burst in Gracilaria conferta (Rhodophyta). J Phycol 35:747–755
Wiltens J, Schreiber U, Vidaver W (1978) Chlorophyll fluorescence induction: an indicator of photosynthetic activity in marine algae understanding desiccation. Can J Bot 56:2787–2794
Wood SN (2003) Thin plate regression splines. J R Stat Soc B 65:95–114
Yamanaka R, Akiyama K (1993) Cultivation and utilization of Undaria pinnatifida (wakame) as food. J Appl Phycol 5:249–253
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho, Tokyo (in Japanese)
Acknowledgements
We thank Osamu Hatakeyama and Hiroshi Akama for performing the experiments and providing information regarding the transfer method of Undaria sporelings. We also thank Drs. Teruko Konishi and Michihiro Ito of University of the Ryukyus for their suggestions and comments, which improved our manuscript. We thank Hikari Nagoe for supporting the data analysis. All authors have provided consent. YS, GNN, and RT also thank two anonymous reviewers for their constructive comments during the review process.
Funding
This research was funded in part by a Grant-in-Aid for Scientific Research (category B, #20H03076) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and the Japan Society for the Promotion of Science (to YS, DS, GNN, and RT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10811_2021_2616_MOESM1_ESM.pptx
Supplemental Figure 1. Total weight variations (Upper) in sporelings cultured on ropes with young sporophytes of Undaria pinnatifida pre-incubated at 18 °C, 100 µmol photons m−2 s−1 (12/12 h light/dark cycle) in 1.25% PESI medium with aeration after exposure to three storage conditions: immersed in sterilized seawater with aeration (ST1), immersed in sterilized seawater without aeration (ST2), and wrapped in paper towels moistened with sterilized seawater (ST3); samples were maintained under dark conditions for 1, 3, 6, 12, 24, 48, 72, and 96 h, and at four temperature levels (4, 12, 18, and 24 °C). All weight data were transformed using the natural logarithm (Bottom), and the slopes for each individual at every experimental condition were calculated as their daily growth rates and their values were used to generate Fig. 4.
Supplemental Figure 2. Variations in photochemical efficiency (ΔF/Fm') in sporelings cultured on ropes with young sporophytes of Undaria pinnatifida for cultivation in Miyagi, Japan. Sporophytes were pre-incubated at 18 °C, 100 µmol photons m−2 s−1 (12/12 h light/dark cycle) in 1.25% PESI medium with aeration after exposure to three storage conditions: immersed in seawater with aeration (left), immersed in seawater without aeration (middle), and wrapped in paper towels moistened with sterilized seawater (right) and maintained under dark conditions for 1, 3, 6, 12, 24, 48, 72, and 96 h. The symbols indicate the average of actual values measured (n = 3), and bars represent standard deviation.
Supplementary file1 (PPTX 818 kb)
Rights and permissions
About this article
Cite this article
Sato, Y., Saito, D., Nishihara, G.N. et al. Effects of different stressors on the PSII photochemical efficiency and application to sporeling transportation in cultured young sporophytes of Undaria pinnatifida. J Appl Phycol 34, 551–563 (2022). https://doi.org/10.1007/s10811-021-02616-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02616-8