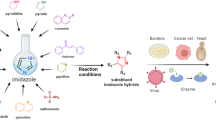
Abstract
Auranofin ([1-(thio-κS)-β-d-glucopyranose-2,3,4,6-tetraacetato](triethylphosphine)-gold) is a leading gold-based drug clinically used to treat arthritis. In the last years, it entered various drug reprofiling programs, and it has been found promising against various forms of tumor, including ovarian cancer. Evidence showed as its antiproliferative profile mainly depends on the inhibition of thioredoxin reductase (TrxR), being this mitochondrial system its main target. In this context, we report here the synthesis and biological evaluation of a novel complex designed as auranofin analogue obtained through the conjugation of a phenylindolylglyoxylamide ligand (which belongs to the so-called PIGA TSPO ligand family) with the auranofin-derived cationic fragment [Au(PEt3)]+. This complex is characterized by two parts. The phenylindolylglyoxylamide moiety, owing to its high affinity for TSPO (in the low nM range) should drive the compound to target mitochondria, whereas the [Au(PEt3)]+ cation is the actual anticancer-active molecular fragment. Overall, we wanted to offer the proof-of-concept that by coupling PIGA ligands to anticancer gold active moieties, it is possible to preserve and even improve anticancer effects, opening the avenue to a reliable approach for targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last years, gold complexes have attracted growing attention for medicinal applications (Nardon et al. 2014; Guarra et al. 2018; Balfourier et al. 2020; Lu et al. 2022). Although chrysotherapy has been used for centuries for various indications including skin infections and seizures (Wang et al. 2012), one of the first modern evidence, concerning the medicinal role of gold, can be dated back to 1890 when Robert Koch discovered that gold cyanide was effective against the tuberculosis bacillus in vitro (Balfourier et al. 2020). More recently, the approval of the Au(I) compound auranofin (AF hereafter) for the treatment of rheumatoid arthritis has contributed to stimulate increasing interest on the medicinal properties of gold-based molecules (Ott 2009; Nobili et al. 2010; Barry and Sadler 2013; Bertrand and Casini 2014; Massai et al. 2021; Cirri et al. 2022). Accordingly, several gold(I) and gold(III) compounds have been synthesized and tested for their anticancer, antimicrobial, antiviral and antiparasitic properties (Ott 2009; Massai et al. 2017; Yeo et al. 2018; Guarra et al. 2018; Bian et al. 2019; Marzo and Messori 2020; Maydaniuk et al. 2021; Chiaverini et al. 2022; Liu et al. 2022). Among them, particularly promising appear the analogues of AF bearing different ligands in place of the thiosugar. Several compounds belonging to this family resulted very promising for application against cancer thanks to their ability to tightly interact and coordinate the selenocysteine residue of the thioredoxin reductase (TrxR). This interaction impairs the key function of the enzyme, eventually inducing cell death (Marzo et al. 2019; Zoppi et al. 2020; Landini et al. 2020; Elie et al. 2020). AF derivatives are designed on the basis of the established concept that AF structure (Fig. 1) is characterized by the presence of a moiety acting as pharmacophore, the [Au(PEt3)]+ cation, and the thiosugar ligand linearly coordinated to the Au(I) center, that is not essential for the pharmacological activity. Rather, it is capable to improve the bioavailability profile upon oral administration (Sutton et al. 1972). In nice agreement with this view, previous studies showed that Et3PAuCl manifests biological properties similar to those of AF (Sutton et al. 1972). This implies that the chemical-physical features and the effects of AF can be conveniently tuned and modulated through the selective replacement of the thiosugar with ligands endowed with specific properties. This allows to control relevant parameters important for the desired pharmacological action (Marzo et al. 2017).
On the ground of these premises, we recently started to consider the chance to develop a complex in which the thiosugar is replaced with a ligand endowed with high affinity for the translocator protein (TSPO).
TSPO is a highly conserved nuclear-encoded 18 kDa protein, primarily localized in the outer mitochondrial membrane (Papadopoulos et al. 2006). Aberrant expression of TSPO has been linked to multiple diseases, including ovarian cancer (OC) (Trapani et al. 2013; Nutma et al. 2021).
Starting from 2004 (Primofiore et al. 2004; Da Settimo et al. 2008; Barresi et al. 2015, 2021) some of us developed a class of potent and selective TSPO ligands, namely the N,N-dialkyl-2-arylindol-3-ylglyoxylamides (PIGAs, Fig. 1), endowed with nanomolar/subnanomolar TSPO affinity and safe profile (Santoro et al. 2016). Specifically, many PIGA compounds have been shown to elicit marked increase in pregnenolone concentration both in vitro and in murine models (Costa et al. 2016; Santoro et al. 2016).
The strategy behind this work relies on the exploitation of TSPO overexpression that occurs in OC. Indeed, owing to the high affinity for TSPO of compounds belonging to the PIGA family, the use of these latter as ligands may allow the selective mitochondrial receptor-mediated delivery of the [Au(PEt)3]+ anticancer pharmacophore to the cancer site. In turn, the activation of such complexes is mediated by the release of [AuPEt3]+, able to inhibit the TrxR and to exert its antiproliferative activity.
For the rational design of the new complex 1 (Scheme 1), we have taken into account the previously described pharmacophore/topological model for the binding of PIGAs to TSPO (Fig. 1), made up of three lipophilic pockets L1, L3, and L4, occupied by the 2-phenyl group, and the aryl/alkyl substituents on the amide nitrogen, respectively, and an H-bond donor group interacting with the amide carbonyl group (Primofiore et al. 2004; Da Settimo et al. 2008; Barresi et al. 2015). Accordingly, the indole nitrogen is suitable to be exploited for the coordination with the [Au(PEt3)]+ anticancer cationic fragment, the “true pharmacophore”, as the 1-NH is supposed not to be involved in a direct interaction with the mitochondrial target protein TSPO.
To this aim, the N-unsubstituted PIGA 4 (Scheme 1) (Primofiore et al. 2004) was selected as representative of the whole class, to be complexed with the [Au(PEt3)]+ fragment.
The novel complex 1 (Scheme 1) was obtained and fully characterized by means of NMR and elemental determination measurements. The cytotoxic activity of complex 1 was tested on A2780 and SKOV-3 human OC cell lines and on HEK293 human embryonic kidney cell line (Table 1). In addition, TrxR activity of the complex was tested on A2780 cell line (Fig. 2) and on isolated enzyme (Table 2).
Materials and methods
All the reagents were provided by Merck-Sigma Aldrich and used without further purification. Et3PAuCl was purchased from Sigma-Aldrich (code:288225). Solvents were also used without further purification. N,N-di-n-hexyl-2-(2-phenyl-1H-indol-3-yl)glyoxylamide 4 was synthesized as previously described (Primofiore et al. 2004). The obtained product was stored at − 20 °C. NMR spectra were recorded at 293 K on a Bruker Avance II 400 MHz; chemical shifts (expressed in parts per million, ppm) were referenced to solvent residual peaks. Elemental analysis was performed using a vario MICRO V4.0.10 Elementar Analysensysteme GmbH.
RPMI 1640 cell culture medium, fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were obtained from Euroclone (Milan, Italy). Thiazolyl Blue Tetrazolium Bromide (MTT) was obtained from Merck-Sigma Aldrich.
Preparation of [N,N-di-n-hexyl-2-(2-phenyl-1-indol-kN-3-yl)glyoxylamido]-AuPEt3 1.
In a 25 mL flask were added 39 mg (0.090 mmol) of N,N-di-n-hexyl-2-(2-phenyl-1H-indol-3-yl)glyoxylamide, 3.00 mL of EtOH and 16 mg (0.12 mmol) of K2CO3. The reaction mixture was stirred at room temperature for 10 min and then transferred to a schlenk tube, containing 32 mg (0.090 mmol) of Et3PAuCl, in which 3 vacuum-nitrogen cycles had previously been performed. After 4 h under magnetic stirring, the suspension was filtered on celite, and the filtrate removed under reduced pressure. A light brown powder was obtained which was washed with Et2O. The product was finally dried under reduced pressure, obtaining 45 mg of [N,N-di-n-hexyl-2-(2-phenyl-1-indol-kN-3-yl)glyoxylamido]-AuPEt2 (0.0603 mmol; yield 67%).
1H-NMR (DMSO-d6, mixture of conformational isomers): 8.02 (s, 1H); 7.62–7.57 (m, 3H); 7.40–7.38 (m, 3H); 7.16–7.09 (m, 2H); 3.00 (bs, 2H); 2.86 (bs, 2H); 1.89–1.81 (m, 6H); 1.41–0.96 (m, 25H); 0.87 (t, 3H, J = 6.9 Hz); 0.76 (t, 3H, J = 7.0 Hz).
31P{1H}-NMR (DMSO-d6): 32.32.
Elemental Analysis (CHN): C34H50AuN2O2P·0.5KCl; required: C = 52.09; H = 6.43; N = 3.57. Found: C = 51.56; H = 6.34; N = 3.48.
Stability study
The stability of [N,N-di-n-hexyl-2-(2-phenyl-1-indol-kN-3-yl)glyoxylamido]-AuPEt2 was assessed by 31P{1H}-NMR experiments recorded UV–Vis spectra at increasing time intervals. A small quantity of the compound was solubilized in DMSO-d6 and NMR spectra were recorded after 2 h and 24 h. Uv–Vis spectra were recorded on a PerkinElmer Lambda 25 Uv–Vis spectrometer.
Cell culture
A2780 human ovarian cancer cell line was purchased from the European Collection of Authenticated Cell Cultures (ECACC, a part of Public Health England) (Lot Nº 13J012, Sigma Aldrich), SKOV-3 human ovarian cancer cell line was gifted from Prof. Enrico Mini, University of Florence, Italy, and HEK (human embryonic kidney) cells were gifted from Prof. Letizia Taddei, University of Florence, Italy. Cells were grown in RPMI1640 medium supplemented with 10% FBS, 1% glutamine and 1% antibiotics at 37ºC and sub-cultured twice weekly. Split 1: 5 (3–6 × 104 cells per mL).
Cell viability assay
Cell viability was assessed using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Briefly, 1 × 104 exponentially growing A2780 cells were seeded in 96 well-microplates for 24 h, then treated with tested compounds 1, 4 and 5 concentrations ranging from 0.003 to 100 µM and incubated for 72 h at 37 °C in a humidified incubator. On the day of the test, cells were treated with 0.5 mg/ml MTT for 1 h at 37ºC. Following precipitation, blue formazan was dissolved in DMSO, and optical density was read at 595 nm in a microplate reader interfaced with Microplate Manager/PV version 4.0 software (BioRad Laboratories). From Absorbance measurements, half-maximal inhibitory concentration (IC50) values of each compound were calculated by using GraphPad Prism Software 6.0.
The effects of these calculated 72-h exposure IC50 doses on A2780 cell viability were also evaluated using an MTT time course assay at 24, 48, and 72 h of drug exposure. The experimental protocol described above was applied. All MTT experiments were performed in triplicate (biological replicates) and in each assay all gold compound concentrations were tested in triplicate (technical replicates).
Thioredoxin reductase activity assay
The total thioredoxin reductase (TrxR) activity in cells was measured by using a commercial colorimetric assay kit (Sigma Aldrich CS0170) based on the reduction of 5,5’-dithiobis (2-nitrobenzoic) acid (DTNB) with NADPH to 5-thio-2 nitrobenzoic acid (TNB) at 412 nm. Since several enzymes present in biological sample can reduce DTNB, the kit also contains an inhibitor solution of mammalian thioredoxin reductase. This inhibitor allows determining the reduction of DTNB due only to TrxR activity. A2780 cells were treated for 24 h with concentrations corresponding to their 72 h-exposure IC50-doses and then lysed with RIPA buffer (50 mM Tris–HCl pH 7.0, 1% (v/v) NP-40, 150 mM NaCl, 2 mM ethylene glycol bis(2-aminoethylether)tetra-acetic acid, 100 mM NaF) supplemented with a cocktail of protease inhibitors. The protein concentrations in the cell lysates were determined by Bradford protein assay kit (Bio-Rad Laboratories) according to the manufacturer’s instructions and then 30 µg of proteins were used for the assay. Results were normalized to the cellular protein content. The experiments were performed in triplicate (three independent biological replicates). The statistical analysis was carried out using one-way ANOVA test followed by Tukey’s multiple comparisons test using Graphpad Prism software v 6.0. P-value p < 0.05 was considered statistically significant.
Thioredoxin reductase inhibition “in vitro”
The half-maximal effective concentration (EC50) values for TrxR inhibition by tested compounds (1, 4 and 5) were determined measuring the ability of the enzyme to directly reduce DTNB in the presence of NADPH. Rat liver TrxR (SigmaAldrich T9698) was diluted in 0.1 M potassium phosphate buffer pH 7.0 to a concentration of 60 nM (2U/mL). Aliquots of 25 µl of this enzyme solution were preincubated, for 5 min at 25 °C, with 25 µl of a solution containing 0.1 M potassium phosphate buffer pH 7.0, 5 mM EDTA, 0.25 mM NADPH and different concentrations of the gold complexes (from 1 mM to 1 nM). Afterwards, the reaction was started with 1 mM DTNB and monitored spectrophotometrically at 412 nm, for about 10 min. The non-interference of the gold compounds with assay components was confirmed by negative control experiments with enzyme free solution. The EC50 values, calculated by using GraphPad Prism Software 6.0, were reported as means ± SD of three independent experiments, each carried out in triplicate.
Results and discussion
The synthetic procedure used for the preparation of N,N-di-n-hexyl-2-(2-phenyl-1-indol-kN-3-yl)glyoxylamide-AuP(Et)2 1 is outlined in Scheme 1 and involved the acylation of the 2-phenylindole 2, commercially available, with oxalyl chloride, in anhydrous ethyl ether, at room temperature, to obtain the corresponding 2-phenylindolylglyoxylyl chloride 3, which was allowed to react at room temperature with di-n-hexylamine in the presence of triethylamine in dry toluene solution, yielding derivative 4 (Primofiore et al. 2004). To a solution of 4 in EtOH, K2CO3 was added and the resulting mixture was reacted at room temperature for 10 min. Subsequently, Et3PAuCl 5 was added, and the resulting mixture was left under stirring at room temperature for further 4 h. Then, the solid was filtered off and the filtrate was evaporated under reduced pressure, obtaining a light brown powder.
The NMR results confirmed the obtainment of the desired complex 1 (Figs. S1–S4). In particular, an highfield shift of the signal in the 31P{1H}-NMR spectrum of 1 (32.2 ppm) with respect to that of precursor 5 (33.87 ppm) (Figs. S1–S2) can be observed. Such results are in line with that reported in literature, where an highfield shift occurs when the chloride ligand of 5 is replaced by a nitrogen donor ligand (Weinstock et al. 1974). Further confirmation of the complex formation is provided by comparison of the 1H-NMR spectra recorded on compound 1 and the uncomplexed TSPO ligand 4. The signals accounting for the aromatic protons in 1 are shifted in comparison to those of the free ligand 4 (Figs. S3–S4). In addition, in the 1 1H-NMR spectrum, the multiplets ranging from 1.89 to 1.81 ppm and 1.10–0.9 ppm, accounting for the CH2 and CH3 of the triethylphosphine ligand coordinated to gold (I) respectively can be observed (Marzo et al. 2017).
Interestingly, 31P NMR spectra recorded at increasing time intervals in presence of organic solvent (DMSO), reveal 1 as highly stable in these conditions, similarly to AF and Et3PAuCl (Marzo et al. 2017). Indeed, no significant changes in the spectra occur (Fig. S5). The substantial stability, even in presence of cell culture medium, was independently confirmed through UV–Vis experiments (Fig. S6). After 24 h of incubation, only minor changes in the profiles were detected. To assess whether these small changes were attributable to partial ligand release, we also recorded the UV–Vis spectrum of the uncomplexed ligand 4 (Fig. S7). From the comparison, it was noticed a ligand release -despite quite limited- upon incubation in water media in physiological-like conditions.
The antiproliferative properties of the new complex 1 was investigated in vitro toward A2780 human ovarian cancer cells and compared with those of uncomplexed-ligand 4 and Et3PAuCl 5. As displayed in Table 1, compounds 1 and 5 produce potent growth inhibition effects with IC50 in the low micromolar range, being 1 the most active one (1.47 µM), with a two-fold gain in potency with respect to the parent compound 5. The result obtained for Et3PAuCl 5 (2.48 µM) is consistent with that previously obtained in the same cell line and recently published (Landini et al. 2020). On the other hand, 4 exhibited a moderate effect with a cytotoxic activity about tenfold less than 1 (Table 1).
The antiproliferative activity was also assessed on another human OC cell line (i.e., SKOV-3), confirming the growth inhibition properties of compounds, that is the higher effectiveness of compound 1 (1.90 µM) with respect to the parent compound 5 (2.73 µM), and the moderate activity of 4 (Table 1). Remarkably, measuring the antiproliferative effects on a non-malignant cell line, HEK293 human embryonic kidney (Table 1), the IC50 values were found to be higher (about sixfold for both compounds 1 and 5), suggesting a selectivity towards cancer cells.
Currently, the thioredoxin reductase (TrxR) enzyme is recognized as the main target of gold-based compounds (Bindoli et al. 2009; Zhang et al. 2019). Therefore, we evaluated the ability of 1, 4 and 5 to inhibit TrxR (Fig. 2). A2780 cancer cells were treated for 24 h with compounds’ concentration corresponding to their 72-h-exposure IC50 dose to avoid the cell death. Indeed, as demonstrated by MTT time course experiments, after 24 h of exposure, the viability of treated cells was comparable to controls (Fig. S8). After 24 h of treatment, A2780 cells exposed to 1 and 5 displayed a residual TrxR activity of only 24% and 32%, respectively, compared to control cells, whereas 4 did not induce a statistically significant decrease of enzyme activity (Fig. 2).
Next, to confirm that the observed decrease of TrxR activity in treated cancer cells could be due to a direct inhibition of the enzyme, we comparatively quantified the inhibitory potency of compound 1 and 5 on the isolated TrxR enzyme. Also compound 4 was tested, showing no detectable activity. The resulting half-maximal effective concentration (EC50) values for enzyme inhibition are reported in Table 2. Overall, both compound 1 and 5 proved to be effective TrxR inhibitors with EC50 values falling in the low nanomolar range (Table 2).
These results suggest a correlation between the antiproliferative properties of 1 and 5 and their inhibitory ability towards TrxR, highlighting 1 as the most active and confirming our working hypotheses that conjugation of the anticancer-active molecular fragment [Au(PEt3)]+ cation with a TSPO ligand targeting mitochondria could retain/improve anticancer effects, thus paving the way to a reliable approach for targeted therapy (Marzo et al. 2017).
Conclusions
In this work, a novel Au(I) complex 1 that is an analogue of AF featuring the replacement of the thiosugar with a TSPO-ligand belonging to the class of PIGAs was prepared and characterized. Similarly to AF and Et3PAuCl, it shows a high stability in DMSO. Notably, the novel complex 1 manifested a fully improved anticancer activity towards two representative models of human OCs (i.e. A2780 line and SKOV-3) and a far lower cytotoxicity toward a normal cell line (i.e. HEK293), offering -to the best of our knowledge- for the first time the proof-of-concept that the conjugation of the active gold fragment [Au(PEt3)]+ with PIGAs may represent a reliable strategy to develop potent anticancer gold-based complexes combined with the possibility to improve mitochondrial delivery.
References
Balfourier A, Kolosnjaj-Tabi J, Luciani N et al (2020) Gold-based therapy: from past to present. Proc Natl Acad Sci USA 117:22639–22648. https://doi.org/10.1073/PNAS.2007285117/SUPPL_FILE/PNAS.2007285117.SD01.TXT
Barresi E, Bruno A, Taliani S et al (2015) Deepening the topology of the translocator protein binding site by novel N, N-dialkyl-2-arylindol-3-ylglyoxylamides. J Med Chem 58:6081–6092. https://doi.org/10.1021/ACS.JMEDCHEM.5B00689/SUPPL_FILE/JM5B00689_SI_002.CSV
Barresi E, Robello M, Costa B et al (2021) An update into the medicinal chemistry of translocator protein (TSPO) ligands. Eur J Med Chem 209:112924. https://doi.org/10.1016/J.EJMECH.2020.112924
Barry NPE, Sadler PJ (2013) Exploration of the medical periodic table: towards new targets. Chem Commun 49:5106–5131. https://doi.org/10.1039/c3cc41143e
Bertrand B, Casini A (2014) A golden future in medicinal inorganic chemistry: the promise of anticancer gold organometallic compounds. Dalton Trans 43:4209–4219. https://doi.org/10.1039/C3DT52524D
Bian M, Fan R, Zhao S, Liu W (2019) Targeting the thioredoxin system as a strategy for cancer therapy. J Med Chem 62:7309–7321. https://doi.org/10.1021/ACS.JMEDCHEM.8B01595/ASSET/IMAGES/LARGE/JM-2018-01595Y_0006.JPEG
Bindoli A, Rigobello MP, Scutari G et al (2009) Thioredoxin reductase: a target for gold compounds acting as potential anticancer drugs. Coord Chem Rev 253:1692–1707. https://doi.org/10.1016/J.CCR.2009.02.026
Chiaverini L, Pratesi A, Cirri D et al (2022) Anti-staphylococcal activity of the auranofin analogue bearing acetylcysteine in place of the thiosugar: an experimental and theoretical investigation. Molecules 27:2578. https://doi.org/10.3390/MOLECULES27082578/S1
Cirri D, Chiaverini L, Pratesi A, Marzo T (2022) Is the next cisplatin already in our laboratory? Comments Inorg Chem. 101080/0260359420222152016
Costa B, Da Pozzo E, Giacomelli C et al (2016) TSPO ligand residence time: a new parameter to predict compound neurosteroidogenic efficacy. Sci Rep. https://doi.org/10.1038/SREP18164
Da Settimo F, Simorini F, Taliani S et al (2008) Anxiolytic-like effects of N, N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J Med Chem 51:5798–5806. https://doi.org/10.1021/JM8003224/SUPPL_FILE/JM8003224_SI_001.PDF
Elie BT, Hubbard K, Layek B et al (2020) Auranofin-based analogues are effective against clear cell renal carcinoma in vivo and display no significant systemic toxicity. ACS Pharmacol Transl Sci 3:644–654. https://doi.org/10.1021/ACSPTSCI.9B00107/ASSET/IMAGES/LARGE/PT9B00107_0002.JPEG
Guarra F, Marzo T, Ferraroni M et al (2018) Interaction of a gold(I) dicarbene anticancer drug with human telomeric DNA G-quadruplex: solution and computationally aided X-ray diffraction analysis. Dalt Trans 47:16132–16138. https://doi.org/10.1039/C8DT03607A
Landini I, Massai L, Cirri D et al (2020) Structure-activity relationships in a series of auranofin analogues showing remarkable antiproliferative properties. J Inorg Biochem 208:111079. https://doi.org/10.1016/J.JINORGBIO.2020.111079
Liu Y, Lu Y, Xu Z et al (2022) Repurposing of the gold drug auranofin and a review of its derivatives as antibacterial therapeutics. Drug Discov Today 27:1961–1973. https://doi.org/10.1016/J.DRUDIS.2022.02.010
Lu Y, Ma X, Chang X et al (2022) Recent development of gold(I) and gold(III) complexes as therapeutic agents for cancer diseases. Chem Soc Rev 51:5518–5556. https://doi.org/10.1039/D1CS00933H
Marzo T, Messori L (2020) A role for metal-based drugs in fighting COVID-19 infection? The case of Auranofin. ACS Med Chem Lett 11:1067–1068. https://doi.org/10.1021/ACSMEDCHEMLETT.0C00190/ASSET/IMAGES/LARGE/ML0C00190_0001.JPEG
Marzo T, Cirri D, Gabbiani C et al (2017) Auranofin, Et3PAuCl, and Et3PAuI are highly cytotoxic on colorectal cancer cells: a chemical and biological study. ACS Med Chem Lett 8:997–1001. https://doi.org/10.1021/acsmedchemlett.7b00162
Marzo T, Massai L, Pratesi A et al (2019) Replacement of the thiosugar of auranofin with iodide enhances the anticancer potency in a mouse model of ovarian cancer. ACS Med Chem Lett 10:656–660. https://doi.org/10.1021/acsmedchemlett.9b00007
Massai L, Messori L, Micale N et al (2017) Gold compounds as cysteine protease inhibitors: perspectives for pharmaceutical application as antiparasitic agents. Biometals 30:313–320. https://doi.org/10.1007/S10534-017-0007-0/TABLES/3
Massai L, Grguric-Sipka S, Liu W et al (2021) Editorial: the golden future in medicinal chemistry: perspectives and resources from old and new gold-based drug candidates. Front Chem 9:109. https://doi.org/10.3389/FCHEM.2021.665244/BIBTEX
Maydaniuk D, Wu B, Truong D et al (2021) New auranofin analogs with antibacterial properties against Burkholderia clinical isolates. Antibiotics 10:1443. https://doi.org/10.3390/ANTIBIOTICS10121443/S1
Nardon C, Boscutti G, Fregona D (2014) Beyond platinums: gold complexes as anticancer agents. Anticancer Res 34:487–492
Nobili S, Mini E, Landini I et al (2010) Gold compounds as anticancer agents: chemistry, cellular pharmacology, and preclinical studies. Med Res Rev 30:550–580. https://doi.org/10.1002/MED.20168
Nutma E, Ceyzériat K, Amor S et al (2021) Cellular sources of TSPO expression in healthy and diseased brain. Eur J Nucl Med Mol Imaging 491(49):146–163. https://doi.org/10.1007/S00259-020-05166-2
Ott I (2009) On the medicinal chemistry of gold complexes as anticancer drugs. Coord Chem Rev 253:1670–1681. https://doi.org/10.1016/j.ccr.2009.02.019
Papadopoulos V, Baraldi M, Guilarte TR et al (2006) Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409. https://doi.org/10.1016/J.TIPS.2006.06.005
Primofiore G, Da Settimo F, Taliani S et al (2004) N, N-dialkyl-2-phenylindol-3-ylglyoxylamides. A new class of potent and selective ligands at the peripheral renzodiazepine receptor. J Med Chem 47:1852–1855. https://doi.org/10.1021/JM030973K/SUPPL_FILE/JM030973K_S.PDF
Santoro A, Mattace Raso G, Taliani S et al (2016) TSPO-ligands prevent oxidative damage and inflammatory response in C6 glioma cells by neurosteroid synthesis. Eur J Pharm Sci 88:124–131. https://doi.org/10.1016/j.ejps.2016.04.006
Sutton BM, Mcgusty E, Walz DT, Dimartino MJ (1972) Oral gold. Antiarthritic properties of alkylphosphinegold coordination complexes. J Med Chem 15:1095–1098. https://doi.org/10.1021/JM00281A001
Trapani A, Palazzo C, De Candia M et al (2013) Targeting of the translocator protein 18 kDa (TSPO): a valuable approach for nuclear and optical imaging of activated microglia. Bioconjug Chem 24:1415–1428. https://doi.org/10.1021/BC300666F/ASSET/IMAGES/LARGE/BC-2012-00666F_0003.JPEG
Wang HH, Su CH, Wu YJ et al (2012) Application of gold in biomedicine: past, present and future. Int J Gerontol 6:1–4. https://doi.org/10.1016/J.IJGE.2011.09.015
Weinstock J, Sutton BM, Kuo GY et al (1974) Oral gold. Synthesis and antiarthritic properties of some large-ring gold chelates. J Med Chem 17:139–140. https://doi.org/10.1021/JM00247A029/ASSET/JM00247A029.FP.PNG_V03
Yeo CI, Ooi KK, Tiekink ERT (2018) Gold-based medicine: a paradigm shift in anti-cancer therapy? Molecules 23:1410. https://doi.org/10.3390/MOLECULES23061410
Zhang X, Selvaraju K, Saei AA et al (2019) Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 162:46–54. https://doi.org/10.1016/j.biochi.2019.03.015
Zoppi C, Messori L, Pratesi A (2020) ESI MS studies highlight the selective interaction of Auranofin with protein free thiols. Dalt Trans 49:5906–5913. https://doi.org/10.1039/d0dt00283f
Acknowledgements
The Authors from the University of Pisa acknowledge the financial support of Rating Ateneo 2022. This work is also supported by the University of Pisa under the ‘‘PRA—Progetti di Ricerca di Ateneo’’ Institutional Research Grants—Project no. PRA_2020_58 ‘‘Agenti innovative e nanosistemi per target molecolari nell’ambito dell’oncologia di precisione’’.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
TG and MM conducted the biological experiments and analyzed the results. LC, EB, and VP synthesized compounds and were involved with data analysis. EB, TM, TG, FDS, DLM, and ST were involved with the conception of the study and interpretation of data. EB, TG, and TM provided general overall supervision of the study. The manuscript was written through the contributions of all authors. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiaverini, L., Baglini, E., Mannelli, M. et al. A complex bearing TSPO PIGA ligand coordinated to the [Au(PEt3)]+ pharmacophore is highly cytotoxic against ovarian cancer cells. Biometals 36, 961–968 (2023). https://doi.org/10.1007/s10534-023-00496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00496-8