Abstract
Phyllostachys heterocycla is well-known for its high diversity of bioactive metabolites, which are the reason for its various potential medical uses for which anticancer activity has been proven. Herein, Phyllostachys heterocycla extract was prepared in two different metallic nanoparticle formulas such as iron oxide nanoparticle-boron, and iron oxide nanoparticle-humic acid (Fe2O3 NP-B and Fe2O3 NP-HA) with average particle sizes of 12.25 nm and 15.80 nm, respectively. Phyllostachys heterocycla extract and the two nano-formulas were investigated to obtain their cytotoxic activity. The crude extract exhibited potent cytotoxic activity against the ovarian (OVCAR-3) cancer cell line, with IC50 values of 16.3 µg/mL. In comparison, the two nano-loaded forms displayed a much more promising cytotoxic activity with IC50 values of 0.9 µg/mL for Fe2O3 NP-HA, and 6.4 µg/mL for Fe2O3 NP-B. Additionally, NP-HA and NP-B showed potent cytotoxic activities against prostate (PC-3) and pancreatic (Panc1) cancer cell lines with IC50 values of 2.31, 6.3 µg/mL for Fe2O3 NP-HA, and 14.9, 16.8 µg/mL for Fe2O3 NP-B. For apoptosis investigation, Fe2O3 NP-HA induced total ovarian apoptotic cell death by a 87.34-fold change, and necrosis by 1.29-fold change. Regarding cell cycle analysis, Fe2O3 NP-HA-PHE arrested the cell proliferation of OVCAR-3 cells in S-phase, with an increased cell population at S-phase of 42.6%. Additionally, it confirmed the apoptosis mechanism by inhibiting the antiapoptotic gene and activating the proapoptotic gene markers. Moreover, upon continuation of our phytochemical investigation of the plant, additional chemical components of the crude extract of Phyllostachys heterocycla were isolated using various chromatographic techniques. As a result, six compounds were isolated. By using different spectroscopic data, the chemical structures of the pure isolated compounds were assigned as stigmasterol (1), glyceryl monobehenate (2), vanillic acid (3), ferulic acid (4), catechin (5), and thymidine (6). These isolated compounds were previously reported for their potent cytotoxic activities against panel of cancer cell lines including pancreatic cancer and prostate cancer cell lines (Ferulic acid), beside the anti-tumor potential against ovarian cell lines (Stigmasterol). In addition to the cytotoxic activity against human larynx carcinoma HepG-2 cell lines (Catechin), human breast cancer MCF7 (Thymidine), and human colon cancer cell line HT-29 (Vanillic acid). Which may explain the significant cytotoxic and anticancer properities of the crude extract of Phyllostachys heterocycla.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is regarded among the leading causes of mortality around the globe [1], and it is considered as a serious obstacle hindering life spans in most countries worldwide [2]. Incidence of cancer cases and cancer deaths were estimated as 19.3 and 10 million, respectively [3,4,5]. Ovarian cancer is one of the most commonly seen types of gynecologic cancers [6], with the worst prognosis and the highest death rate [7], and it is threefold more lethal than breast cancer [8]. When it comes to males, prostate cancer is attributed as the fifth most common cause of mortality and the second most prevalent kind of cancer among men around the world [9]. Pancreatic cancer is also among the deadliest cancers worldwide [10], coming as the seventh top reason behind cancer-related mortality in both genders [11], and as the eleventh most common type of cancer [12].
About 25% of the recently approved anti-cancer drugs were derived from natural sources [13], this is mainly because of the fact that natural products present a remarkable chemical diversity, which makes them a goldmine for bioactive metabolites [14, 15]. That diversity of natural products is the primary source of inspiration for the discovery of novel anticancer drugs [16,17,18].
The medicinal properties and pharmacological importance of bamboo made it one of several possible natural sources of anticancer medications that gained global attention [19]. Bamboo has a lot of medicinal potential, and its shoots and leaves may be used in a way that is both safe and kind to the environment [20]. A large number of bioactive metabolites, including flavonoids, phenolic acids, phenolic glycosides, and glycosides, have been identified by phytochemical analysis of plants belonging to the Phyllostachys genus [21], fatty acids [22], and sterols [23]. The therapeutic use of the leaves of bamboo included managing hypertension, arteriosclerosis, cardiovascular diseases, and most importantly, cancer [24]. Bamboo shoots also showed antioxidant activity [25], and a protective role against benign prostatic hyperplasia [26], beside their anticancer effects against different cell lines [27].
In the field of biomedicine, nanoparticles serve as both delivery vehicles and therapeutic agents for natural extracts [28]. They offered unique and enhanced physicochemical characteristics compared to their equivalent bulk materials [29,30,31]. Because of their small size (between 1 and 100 nm), Nanoparticles exhibit a high ratio of surface area to volume. which provides them with special features and improves their catalytic, magnetic, mechanical, and optical properties, hence broadening their potential for biomedical application [32, 33]. Because of this large surface, nanoparticles can easily absorb large amounts of various substrates, which include drugs/extracts, and subsequently distribute these absorbed compounds across the target tissue [34,35,36]. By adjusting the shape and size of nanoparticles, monodispersed nanoparticles can be generated which facilitates their internalization through the cells [37]. Nanoparticles employed in the realm of biomedicine are categorized according to the chemical components they contain. Inorganic nanoparticles such as metals and metal oxides are used in biomedicine [38].
To our best knowledge, no prior investigations focused on the cytotoxic effect of the nano-formulated crude extract of Phyllostachys heterocycle as a biomass-assisted green NPs synthesis. In this context, and for better results, we aimed to investigate the cytotoxic effects of the nano-delivered crude extract of the bamboo plant (Phyllostachys heterocycla) against ovarian (OVCAR-3), prostate (PC-3), and pancreatic (Panc1) cancer cell lines. Additionally, this study was aimed to look into the mechanism for apoptosis-induction and gene expression analysis. Moreover, an investigation of the chemical profile of the extract proceeded to assign the potential activity to specific compounds isolated from the crude extract.
Results and Discussion
Characterization of iron Oxide Nanocomposites (Fe2O3 NP-B-PHE and Fe2O3 NP-HA-PHE)
The production of iron oxide nanocomposites was carried out via gamma-ray synthesis. The synthetically produced iron oxide nanocomposites had a dark brown hue and did not clump with time. The electrostatic attraction was used to stabilize the coated B-PHE and HA-PHE. Using HRTEM, it was possible to see mono-dispersed iron oxide nanocomposites with rounded shapes and average particle sizes of 20.5 nm for Fe2O3 NPs-B-PHE and 30.5 nm for Fe2O3 NPs-HA-PHE (Fig. 1a and b, respectively). High-rate mono-dispersed nanoparticles with consistent particle size were shown in HRTEM images covering thin layers (B-PHE or HA-PHE). The mean Fe2O3 NPs-B-PHE particle size allotment was determined to be 23.0 nm by 91.2%, as illustrated in (Fig. 1c), and 33.1 nm by 86.6% for Fe2O3 NPs-HA-PHE as shown in (Fig. 1d). DLS was also employed to estimate particle size distribution.
Our findings showed that the mean particle size determined from the HRTEM image analysis was smaller than the particle size dispersion obtained from the DLS investigation. The hydrodynamic radius around Fe2O3 NPs-HA-PHE or Fe2O3 NPs-B-PHE and surrounded by water molecules was measured using the DLS method. As the explanations said, this contributes to increase the sizes of the synthesized Fe2O3 NPs [39].
Moreover, (Fig. 2) illustrates the investigation of the created iron oxide nanocomposites’ external morphology, purity, and elemental composition. Based on the SEM analysis of the produced Fe2O3 NPs-B-PHE (Fig. 2a), the synthesized Fe2O3 NPs remained stable with the boron layer, and the extracted PHE, the Fe2O3 NPs encompassed a semi-spherical building and uniformly distributed as a brilliant particle at the outermost layer of boron and the extracted PHE. Similar events occurred in an instance of Fe2O3 NPs-HA-PHE, in which SEM imaging revealed the arrangement of Fe2O3 NPs with HA and PHE layers, and bright particles were found on the outside (Fig. 2b).
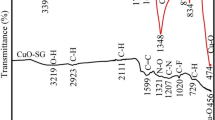
The Fe, B, and O atoms were present in the produced Fe2O3 NPs-B-PHE, and the lack of additional atoms that would have been detected as impurities in the EDX analysis proved the anticipated nanocomposites’ increased purity. As shown in (Figure S1-a), there were traces of sulfur owing to ferrous sulphate precursors, the sodium atom in the present resulted from pH fixing, the carbon atom (C) matched the container utilized for the SEM imaging procedure and the carbon of the extracted PHE. It was projected that Fe and O atoms from the manufactured Fe2O3 NPs and S, C, and O atoms from the HA layers would each make up a percentage of the Fe2O3 NPs-HA-PHE, which presented the same issue. The carbon atom (C) also resembled the SEM imaging technique’s container and the carbon of the extracted PHE. Finally, the existence of the Si atom was brought on by certain impurities in the imaging process (Figure S1-a).
Assessment of in Vitro Cytotoxic Activity of P. Heterocycla NP
MTT Assay
Samples of crude extract and its nano forms of NP-B and NP-HA were investigated for their cytotoxicity against ovarian (OVCAR-3), prostate (PC-3), and pancreatic (Panc1) cancer cell lines using MTT assay. Cytotoxicity results as shown in (Table 1) and (Fig. 3), crude extract exhibited moderate cytotoxic activity against OVCAR-3 cells with IC50 values of 16.3 µg/mL, while nano-loaded form exhibited potent cytotoxicity with promising IC50 values. NP-HA showed potent cytotoxic activities against OVCAR-3, PC-3, and Panc1 with IC50 values of 0.9, 2.31, 6.3 µg/mL. While NP-B exhibited promising cytotoxic activities against OVCAR-3, PC-3, and Panc1 with IC50 values of 6.4, 14.9, and 16.8 µg/mL. Interestingly, both nano forms exhibited safe cytotoxicity results with much higher IC50 values against WISH normal cells. These results highlighted the potent cytotoxicity of the NP-HA against OVCAR-3 cells; hence, it was worthy to be further tested for mechanism of action.
As well reported in previous literature [40], humic acid, as a phenolic polymer, exhibited pro-oxidant and cytotoxic effects against cancer cells, so incorporating humic acid with nanoparticles exhibited potent cytotoxicities with much lower IC50 values compared to other Boron-containing nanoparticles and extract and Doxorubicin. Compared to previous study utilized α-Fe2O3 nanoparticles that showed moderate cytotocicity against A549 acncer cells with IC50 value of 51.2 µg/mL, in our study, cytotoxixity values against the tested cell lines were greatly improved to be potent.
Apoptosis-induction Activity
Annexin V/PI Staining
Annexin V/PI labeling was used to screen for the apoptosis-induction mechanism of NP-HA cytotoxic activity in OVCAR-3 cells. As seen in (Fig. 4), NP-HA induced total ovarian apoptotic cell death by 33.19% compared to 0.38% in the untreated control cells. So, it induced apoptosis in OVCAR-3 cells by 87.34-fold change. Moreover, it induced cell death through necrosis by a 1.29-fold change.
Cell Cycle Analysis
Through cell cycle analysis, which exposed the cell population at each phase in both untreated and treated cells, OVCAR-3 cells were examined to determine at which points in the cell cycle divisions were halted. As seen in Fig. 3, NP-HA has significantly increased cell population at S-phase by 42.6%, in comparison to 31.7% for the control, so it arrested cell division at S-phase. Upon treatment, cell population in the G1 phase was non-significantly increased, while the cell population in the G2/M phase was decreased. Accordingly, NP-HA arrested the cell proliferation of OVCAR-3 cells in the S-phase.
Apoptosis-induction assessment using flow cytometry of untreated and treated OVCAR-3 cancer cells (IC50 = 0.9 µM, 48 h). A: Annexin V/PI staining of untreated and treated OVCAR-3 cancer cells. Quadrant charts show Q1 (necrotic cells), Q2 (late apoptotic cells), Q3 (normal cells), Q4 (early apoptotic cells). B: Cell cycle analysis of untreated and treated OVCAR-3 cancer in G1, S, and G2/M phases. *(P ≤ 0.05) and **(P ≤ 0.001) were significantly different between untreated and treated cells using unpaired t-test using GraphPad prism software
Gene Expression Analysis Using RT-PCR
Analysis of gene expression for apoptosis-related genes in both untreated and treated cells was performed to investigate the apoptosis-inducing effects of NP-HA in OVCAR-3 cells. As seen in (Fig. 5), NP-HA upregulated P53 gene by 5.8-fold, Bax gene by 4.8-fold, and caspases 3,8,9 by 4.7, 7.9, and 8.7-fold, respectively. While it downregulated the Bcl-2 gene by 0.58-fold, These findings validated the mechanism by which treatment induced cell death in OVCAR-3 cells.
Gene expression analysis of untreated and treated OVCAR-3 cells with NP-HA (IC50 = 0.9 µM, 48 h). β-actin was used as a housekeeping gene. The fold of change is calculated by 2^ − ΔΔCT, where ΔΔCT is the difference between mean values of genes CT values in the treated and control groups. Blue dashed line represents the fold change of untreated cells = 1. **(P ≤ 0.001) significantly different between untreated and treated cells using unpaired t-test using GraphPad prism software
Spectroscopic data of the Isolated Compounds
Stigmasterol (1)
White crystals; Molecular formula: C29H48O, m/z: 413.2660 [M + H] +, 1H NMR (500 MHz, HDO/DMSO): δH 5.41 (1H, d, J = 5 Hz, H-6), 5.20 (1H, m, H-20), 5.08 (1H, m, H-20), 3.85 (1H, d, J = 10 Hz, H-3), 1.05 (s, 3 H, H-29), 1.01 d, 3 H, J = 5.0 Hz, H-19, 0.86 (brs, H-24, 26, 27), and 0.67 (s, 3 H, H-28). 13C-NMR (125 MHz, DMSO): δC 37.6 (C-1), 32.4 (C-2), 71.1 (C-3), 43.3 (C-4), 141.7 (C-5), 121.0 (C-6), 32.0 (C-7), 31.9 (C-8), 51.2 (C-9), 36.7 (C-10), 21.2 (C-11), 39.8 (C-12), 42.9 (C-13), 56.7 (C-14), 24.3 (C-15), 28.4 (C-16), 56.0 (C-17), 40.6 (C-18), 21.3 (C-19), 138.7 (C-20), 129.2 (C-21), 45.8 (C-22), 25.5 (C-23), 11.8 (C-24), 29.2 (C-25), 19.8 (C-26), 19.4 (C-27), 18.9 (C-28), and 12.0 (C-29). The spectral data are consistent with the reported literature [41] of stigmasterol.
Glyceryl 1- monobehenate (2)
White powder; Molecular formula: C25H50O4, 414.7665 [M + H]+, 1H NMR (500 MHz, HDO/DMSO): δH 0.86 (3 H, t, J = 7.5 Hz, terminal CH3), 1.27 (brs, nCH2), 1.62 (2 H, m, CH2- CH2- COO), 2.37 (2 H, t, J = 7.5 Hz, CH2-CO2), 4.05 (2 H, m, H-3), 4.37 (1H, m, H-2), 4.56 (1H, dd, J = 10, 5 Hz. H-1b), 4.65 (1H, dd, J = 11.5, 4.9 Hz. H-1a). 13C-NMR (125 MHz, DMSO): δC 14.1 (Terminal CH3), 22.7 (CH2- CH3), 25.0 (CH2- CH2- COO), 29.7 (nCH2), 31.9 (CH2- CH2- CH3), 34.2 (CH2- COO), 63.8 (C-3), 66.5 (C-1), 70.5 (C-2), and 173.8 (CO2). These data confirmed the presence of long chain fatty acid ester as a 1-monoglyceride, in agreement with the literature [42]. The length of the alkyl chain was determined using MS mass spectroscopy, which was carried for the whole compound, peak was exhibited at m/z = 414.7665 [M + H]+ suggesting molecular formula as C25H50O4. Thus, compound 2 was identified as 1- glyceride monobehenate. (Glyceryl docosanoate).
Vanillic acid (3)
Yellowish white powder; Molecular formula: C8H8O4, m/z: 169.0175 [M + H] +, 1H NMR (500 MHz, HDO/DMSO): δH 3.80 (3 H, s, OCH3), 6.85 (1H, d, J = 8.0 Hz, H-5), 7.43 (1H, d, J = 2.0 Hz, H-2), 7.44–7.46 (1H, dd, J = 8.0 and 2.0 Hz, H-6). 13C-NMR (125 MHz, DMSO): δC 55.9 (0-CH3), 113.0 (C-5), 115.5 (C-2), 122.0 (C-1), 124.0 (C-6), 147.6 (C-3), 151.5 (C-4), 167.8 (COOH). By comparison with previously reported results [43], compound 3 was assigned as vanillic acid.
Ferulic acid (4)
White powder; Molecular formula: C10H10O4, m/z: 195.0311 [M + H] +, 1H NMR (500 MHz, HDO/DMSO): δH 3.80 (3H, s, -OCH3), 6.36 (1H, d, J = 15 Hz, H-2’), 6.79 (1H, d, J = 10 Hz, H-6), 7.08 (1H, dd, J = 8 and 2 Hz, H-5), 7.26 (1H, d, J = 2.5 Hz, H-3), 7.49 (1H, d, J = 15 Hz, H-1’), 9.68 (1H, s, -COOH). 13C-NMR (125 MHz, DMSO): δC 13CNMR (500 MHz, DMSO) δ: 56.08 (-OCH3), 123.27 (C-5), 115.95 (C-2), 115.99 (C-2’), 111.46 (C-3), 126.18 (C-4), 145.05 (C-1’), 148.33 (C-6), 149.46 (C-1), 168.55 (C-3’). These findings along with previously published article [44], come in consistency with the suggested structure of ferulic acid (4-hydroxy-3-methoxycinnamic acid).
Catechin (5)
White powder; Molecular formula: C15H14O6, m/z: 291.0855 [M + H] +, 1H NMR (500 MHz, HDO/DMSO): δH 2.49 (1H, dd, J = 5, 15 Hz, H-4b), 2.70 (1H, dd, J = 5, 15 Hz, H-4a), 4.02 (1H, m, H-3), 4.74 (1H, d, J = 5 Hz, H-2), 5.75 (1H, d, J = 2.3 Hz, H-6), 5.91 (1H, d, J = 2.3 Hz, H-8), 6.67 (1H, d, J = 8.5 Hz, H-6’), 6.70 (1H, d, J = 8.5 Hz, H-5’), 6.91 (1H, s, H-2’), 3.77 (3-OH), 8.90 (2 H, s, 5-OH, 7-OH), 9.09 (1H, s, 4’-OH), and 9.29 (1H, s, 3’-OH). 13C-NMR (125 MHz, DMSO): δC 78.5 (C-2), 65.3 (C-3), 28.7 (C-4), 156.6 (C-5), 95.5 (C-6), 157.0 (C-7), 94.6 (C-8), 156.2 (C-9), 99.0 (C-10), 131.1 (C-1’), 115.2 (C-2’), 144.8 (C-3’), 144.9 (C-4’), 115.3 (C-5’) and 118.5 (C-6’). All of these findings along with comparison of previously reported article [45], come in agreement with the suggested structure of catechin.
Thymidine (6)
White powder; Molecular formula: C10H14N2O5, m/z: 241.0817 [M + H] +, 1H NMR (500 MHz, HDO/DMSO): δH 11.30 (1H, s, NH), 7.70 (1H, s, H-6), 6.17 (1H, t, J = 6.8, H-1′), 5.27 (1H, brs, 3′-OH), 5.07 (1H, brs, 5′-OH), 4.23 (1H, m, H-3′), 3.76 (1H, q, J = 3.5, H-4′), 3.56 (2 H, m, H-5′), 2.07 (2 H, m, H-2′), and 1.77 (3 H, s, 5-CH3). 13C-NMR (125 MHz, DMSO): δC 164.2 (C-4); 150.9 (C-2), 136.6 (C-6), 109.8 (C-5), 87.7 (C-4′), 84.2 (C-1′), 70.9 (C-3′), 61.8 (C-5′), 39.8 (C-2′), and 12.7 (CH3). These NMR data are in good agreement with the literature [46]. Thus, based on these data, Compound 6 was assigned as thymidine. The chemical structures of these six isolated compounds are shown in (Fig. 6).
The six freshly isolated compounds together with the previously reported ones from the plant [22, 23, 47] were previously assigned as crucial agents to combat cancer. For example, Ferulic Acid exhibited a significant cytotoxic activity against human pancreatic cancer cell line [48], and human prostate cancer cell lines by affecting apoptotic, cell cycle, colony formation behavior, and invasion of human pancreatic cancer cells [49]. Catechin also showed cytotoxic activity against human larynx carcinoma HepG-2 cell lines, and human colon adenocarcinoma HCT 15, 116. This anticancer activity is due to cytotoxicity, nuclear fragmentation, and DNA fragmentation associated with apoptosis [50]. Additionally, thymidine exhibited selective inhibitory activity on cell proliferation of human breast cancer cell line MCF7 [51]. Moreover, vanillic acid was reported for its significant inhibition activity on the proliferation of human colon cancer cell line HT-29 [52]. Finally, stigmasterol was reported for its significant anticancer potential and cytotoxicity against MCF-7, NIH/3T3 and HeLA cells [53]. stigmasterol was also reported for its anti-tumor potential against lung cancer [54], liver cancer [55], gastric cancer [56], and ovarian cancer [57] through inhibiting growth and promoting apoptosis in the tumor cells [58]. All of these findings suggest the explanation for the significant cytotoxic and anticancer potentials of the crude extract of the Phyllostachys heterocycla.
In relation to our observed cytotoxicity, ferulic acid might have contributed against the used human pancreatic cancer cell line (Panc1), and human prostate cancer cell lines (PC-3) by the same reported mechanism of action against similar cancer cells by affecting apoptotic, cell cycle, colony formation behavior, and invasion of cancer cells [49], these suggestions were inforced by our cell cycle analysis of the extract. Additionally, the crude extract activity against ovarian cancer cell line (OVCAR-3) might be attributed to the presence of isolated compound stigmasterol because of its reported anti-tumor activity against ovarian cancer through inhibiting growth and promoting apoptosis in the tumor cells [58]. This appoptotic effect was confirmed through our apoptosis investigation which validates the suggested action.
In addition to that, the other isolated compounds were previously reported against panel of cancer cell lines through different mechanisms such as nuclear fragmentation and DNA fragmentation associated with apoptosis [50]. These reports suggested its strong possible correlation of the isolated compounds to the cytotoxic activity of the crude extract. Thus, we strongly suggest that all the identified compounds of the extract could be synergetically responsiple for the obseved cytotoxic activities. Moreover, the isolated compounds bioavailability studies suggested their promising potential as a lead compounds in treating cancer as ferulic acid is reported for its rapid absorption and rapid clearance from the body after oral administration in rats [59].
Catechin bioavailability studies also showed its wide range of distribution in different tissues, such as the lung, eye, brain, gastrointestinal tract, bladder, and kidney [60]. However, for the poorly bioavailabile compounds such as vanillic acid [61], and stigmasterol [62], their bioavailibility is suggested to be enhanced through our nanoformulation.
The isolated compounds were also reported for their huge range of bioavailability which support their potential role as anticancer molecules in the phyllostachyes heterocycla extract. For example, Vanillic acid was reported for its various bioactivities against cancer, cardiovascular, diabetes, obesity, and hepatic diseases [61]. Ferulic acid also has a broad spectrum of biological activity, including anti-inflammatory, antibacterial, anti-cancer (skin, lung, breast, and colon cancer), anti-arrhythmic, and antithrombotic activity [63].
Catechin also showed positive bioactivity as an anti-cancer, anti-inflammatory, anti-allergenic, anti-microbial and anti-viral effect [64]. Stigmasterol showed range of bioactivities as it was found to be active as anticancer, anti-inflammatory, anti-diabetic, immunomodulatory, neuroprotective, anti-osteoarthritis, antioxidant, antiparasitic, antifungal, and antibacterial properties [65]. In addition to the strong antibacterial bioactivity of behenatic acid [66].
The lethal effects of NP-HA and NP-B formulations on various cancer cell lines varies significantly, which can be attributed to changes in their chemical composition, biological targeting, cellular absorption, synergistic interactions, and cellular response mechanisms. To best utilize these formulations for certain cancer types or patient demographics, more research into these parameters may help clarify the fundamental causes of the observed variances.
Materials and methods
Plant Material and the Extraction Process
Moso bamboo (Phyllostachys heterocycla) shoots were collected from Joyama garden in Isahaya town, Nagasaki prefecture, Japan (October 2011), and preserved at -24 °C until further processing. The plant was identified by Koji Yamada, Garden for Medicinal Plants, School of Pharmacy, Nagasaki University, Japan. A specimen was deposited in the herbarium section in department of pharmacognosy, Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt with the registration number KY-11. The extraction of Moso bamboo shoot skin was performed as previously described [20] to give a residue of the crude extract of Phyllostachys heterocycla.
Metallic Nanoparticle Formula
Chemicals and Reagents
Analytical-grade chemicals as ferrous sulphate heptahydrate, boric acid, and humic acid (all accessible from Sigma Aldrich in the UK) were used to make various nanocomposites.
Gamma Radiation
Gamma radiation was employed at the NCRRT in Cairo, Egypt, as a green method [67,68,69]. To liquefy the starting materials, radiation from the [60] Co-Gamma chamber 4000-A-India was used to irradiate the tested solutions at a dose rate of 1.041 kGy/hour.
Preparation of iron Oxide Nanocomposites (Fe2O3 NP-B and Fe2O3 NP-HA) by P. Heterocycla Extract (PHE)
The Fe2O3 NP-Boron (B)-PHE and Fe2O3 NP-humic acid (HA)-PHE were produced in the presence of gamma rays, which function as a distinguished reducing agent, in accordance with the following publications [70, 71] with a few minor alterations. Free reducing electrons (e− aq) were responsible for the gamma rays’ ability to eliminate metal ions. Fe2O3 NP-B was synthesized by dissolving 22 g of ferrous sulphate heptahydrate in 250 mL of distilled water and stirring the mixture for about 30 min. Then, to make the boric acid solution, 3 g of boric acid was placed in 250 mL of distilled water. A final volume of 500 mL was achieved after combining the solutions of ferrous sulphate and boric acid. Just before the gamma radiation, the pH value of the created sample was measured and corrected with sodium hydroxide to neutrality (pH = 7). After that, the extracted PHE was added to the solution, and it was subjected to a set dose of 35.0 kGy gamma radiation. Fe2O3 NP-H was produced by dissolving 4.2 g of ferrous sulphate heptahydrate in 100 mL of distilled water and stirring the mixture for around 30 min; then, the humic acid solution was made by dissolving 0.5 g of humic acid in 50 mL of distilled water. A final volume of 150 mL was achieved by combining the solutions of ferrous sulphate and humic acid. Prior to gamma radiation, the prepared sample was checked for pH; after pH correction using sodium hydroxide, the sample was adjusted to be neutral (pH = 7). After that, the extracted PHE was added to the solution, and it was subjected to a set dose of 35.0 kGy gamma radiation.
Characterization of iron Oxide Nanocomposites (Fe2O3 NP-B-PHE and Fe2O3 NP-HA-PHE)
The surface structure and external appearance of iron oxide nanocomposites were investigated by the Japanese firm JEOL JSM-5600 LV using a SEM. The elemental accounting of the generated iron was carried out using an EDAX detector (JEOL JSM-5600 LV, Japan). To ascertain the intermediate particle size diffusion, the created iron oxide nanocomposites were put through a dynamic light scattering test (DLS-PSS-NICOMP 380-ZLS particles sized system, St. Barbara, California, USA). A high-resolution transmission electron microscope (HR-TEM, JEM2100, Jeol, Japan) was used to study the exact shape and intermediate particle size of the produced iron oxide nanocomposites.
Assessment of in Vitro Cytotoxic Activity of P. Heterocycla
Cytotoxicity Investigation Using the MTT Assay
The DMEM/F12 medium L-Glutamine (Lonza Verviers SPRL, Belgium, cat#12–604 F) was used to cultivate the ovary (OVAR-3), pancreas cancer (Panca1), prostate cancer (PC-3) and normal cell (WISH) lines purchased from the National Research Institute, Egypt. Fetal bovine serum (Sigma-Aldrich, MO, USA) and penicillin-streptomycin at a concentration of 10% and 1%, respectively, have been added to the cell lines (Lonza, Belgium). All cell cultures were maintained in a 37 °C, 5% CO2 incubator (NuAire). A total of 96 wells were used, and each well contained 5000 cells in triplicate. Samples have been added to the cells on day two at doses of 0.01, 0.1, 1, 10, and 100 µg/mL. Cell viability was assessed 48 h later using MTT solution (Promega, USA) [72]. The plate was incubated at 37 degrees for three hours after MTT dye (20 µL) was added to each well. Then, an ELISA microplate reader was used to quantify the absorbance (at 570 nm) (BIO-RAD, model iMark, Japan) [73].
Annexin V/PI Staining and cell Cycle Analysis
OVCAR-3 cells (3–5 105 cells/well) were cultured onto 6-well culture plates and incubated overnight. Treatment of cells with compound NP-HA at IC50 concentrations for 48 hours was then performed. The cells and medium supernatants were then washed with ice-cold PBS. After that, 100 L of annexin binding buffer solution was added to the cell suspension “25 mM CaCl2, 1.4 M NaCl, and 0.1 M Hepes/NaOH, pH 7.4” and incubation with “Annexin V-FITC solution (1:100) and propidium iodide (PI)” at a concentration of 10 µg/mL in the dark for about 30 min. The Cytoflex FACS system was then used to obtain the stained cells. cytExpert was used to analyze the data [74,75,76].
Real time-polymerase Chain Reaction for the Selected Genes
The expression levels of proapoptotic genes (P53, Bax, Caspases-3, 8, 9) and antiapoptotic genes (Bcl-2 were analyzed to gain insight into the apoptotic mechanism. The IC50 values for OVCAR-3 cells were then used to treat the cells with compound NP-HA for 48 h. After treatment, a standard RT-PCR test was performed. Then, the Ct values were calculated to obtain the expression levels of each gene in comparison to that of the β-actin housekeeping gene in all of the samples [74, 77].
Isolation and Purification of Compounds 1–6
The crude extract of Phyllostachys heterocycla was suspended in about 4 L of distilled water and then extracted against n-hexane, Ethyl acetate, n-Butanol successively. The n-hexane extract (37 g) was then subjected to a Silica gel column. The elution was proceeded using n-hexane: Ethyl acetate gradient and observed using TLC to assemble the resulting similar fractions. Two fractions were chosen for further investigation which proceeded as the following: the first fraction was chromatographed on a silica gel column packed with n-hexane and eluted gradually using n-hexane: Ethyl acetate. The eluted fractions were monitored to combine similar fractions together to obtain a semi-pure sub-fraction which was purified by crystallization on cold using (1:1) n-hexane: Ethyl acetate and finally washed with Ethyl acetate to yield pure compound 1 (20.5 mg). The second fraction was applied onto a silica gel chromatographic column, and step gradient elution using Chloroform: Methanol was performed. Similar Fractions were combined upon investigation using TLC. Final purification was attained by crystallization on cold using (1:1) n-hexane: Ethyl acetate to yield pure compound 2 (12.4 mg). The Ethyl acetate extract (14.8 g) was applied on a silica gel column and eluted gradually using Chloroform: Methanol. The eluted fractions were investigated by TLC to compile similar ones together. One main fraction was chromatographed against a Silica gel column, eluted through step gradient of n-hexane: Ethyl acetate and observed by TLC to compile the resulting similar fractions. Three subfractions were obtained and further examined as follows; the first subfraction was subjected to a Sephadex LH-20 column. Elution was proceeded using (Chloroform: Methanol = 1:1) to yield the pure compound 3 (9.9 mg). The second subfraction was applied on a silica gel column and gradually eluted using Chloroform: Methanol to yield the pure compound 4 (7.9 mg). The third (final) sub-fraction was also purified over a silica gel column, elution was done using Chloroform: Methanol (9:1) to yield pure compound 5 (6.1 mg). The n-Butanol extract (37 g) was applied onto a Silica gel column, then eluted using gradient concentrations of Chloroform: Methanol. Monitoring by TLC was done to compile resulting similar subfractions. The main subfraction was finally purified using a Sephadex LH-20 column and elution system of (Chloroform: Methanol = 1:1) to obtain the pure compound 6 (2.2 gm).
Conclusions
In conclusion, the current study addressed the cytotoxicity of Phyllostachys heterocycla extract on its nano-form of iron oxide nanocomposites (Fe2O3 NP-B-PHE and Fe2O3 NP-HA-PHE) against ovarian cancer cell line (OVCAR-3) with IC50 values of 6.41 µg/mL, and 0.9 µg/mL, respectively, prostate cancer cell line (PC-3) with IC50 of 14.9 µg/mL, and 2.31 µg/mL, respectively, and pancreatic cancer cell line (Panc1) with IC50 value of 6.3 µg/mL, and 8.3 µg/mL, respectively. For apoptosis investigation, NP-HA-PHE induced total ovarian apoptotic cell death by 33.19% compared to 0.38% in the untreated control cells. So, it induced apoptosis in OVCAR-3 cells by 87.34-fold and cell death through necrosis by 1.29-fold change. Regarding cell cycle analysis, NP-HA-PHE arrested the cell proliferation of OVCAR-3 cells in the S-phase with an increased cell population at S-phase of 42.6%. It also upregulated the proapoptotic genes P53, Bax, and caspases 3,8,9 by 5.8, 4.8, 4.7, 7.9, and 8.7-fold, respectively, while downregulating the antiapoptotic gene Bcl-2 by 0.58-fold. Moreover, chemical profile investigation of Phyllostachys heterocycla extract resulted in the isolation of six compounds Stigmasterol (1), Glyceryl 1- monobehenate (2), Vanillic acid (3), Ferulic acid (4), Catechin (5), and Thymidine (6). In our future work, we want to investigate the in vivo application of the produced NPs to precisely define their mode of action, as well as their antioxidant and antibacterial potentials to build a comprehensive profile for their biomedical employment. Among the discovered phytoconstituents, we also need to identify the exact compounds that are on the NP’s surface and function as reducing, capping, and stabilizing agents. It is critical to carry out more phytochemical research on Phyllostachys heterocycla in order to identify novel bioactive substances that may have therapeutic use. These molecules have the potential to impact future investigations and clinical uses by deepening our knowledge of the chemistry of natural products, directing efforts towards drug discovery, and providing encouraging leads for the creation of novel cancer treatments.
Data Availability
No datasets were generated or analysed during the current study.
References
Momenimovahed, Z.; Ghoncheh, M.; Pakzad, R.; Hasanpour, H.; Salehiniya, H. Incidence and mortality of uterine cancer and relationship with Human Development Index in the world. Cukurova Med J. 2017, 42, 233–40.
Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R. L.; Torre, L. A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018, 68, 394–424.
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021, 71, 209–249.
Merriel, S. W. D.; Ingle, S. M.; May, M. T.; Martin, R. M. Retrospective cohort study evaluating clinical, biochemical and pharmacological prognostic factors for prostate cancer progression using primary care data. BMJ open. 2021, 11, e044420.
Ganesh, K.; Massague, J. Targeting metastatic cancer. Nature medicine. 2021, 27, 34–44.
Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: epidemiology and risk factors. International Journal of Women’s Health. 2019, 11, 287–299.
Coburn, S. B.; Bray, F.; Sherman, M. E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. International journal of cancer. 2017, 140, 2451–2460.
Yoneda, A.; Lendorf, M. E.; Couchman, J. R.; Multhaupt, H. A. Breast and ovarian cancers: a survey and possible roles for the cell surface heparan sulfate proteoglycans. Journal of Histochemistry & Cytochemistry. 2012, 60, 9–21.
Rawla, P. Epidemiology of prostate cancer. World journal of oncology. 2019, 10, 63.
Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD. 2016.
Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: IARC CancerBase. Globocan. 2012, 2013, 11.
Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015, 136, E359-E386.
Newman, D. J.; Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of natural products. 2020, 83(3), 770–803.
Huang, M.; Lu, J. J.; Ding, J. Natural products in cancer therapy: Past, present and future. Natural products and bioprospecting. 2021, 11, 5–13.
Mohammed, H.A.; Emwas, A.H.; Khan, R.A. Salt-tolerant plants, halophytes, as renewable natural resources for cancer prevention and treatment: roles of phenolics and flavonoids in immunomodulation and suppression of oxidative stress towards cancer management. International Journal of Molecular Sciences. 2023, 24, 5171.
Demain, A. L.; Vaishnav, P. Natural products for cancer chemotherapy. Microbial biotechnology. 2011, 4, 687–699.
Palanisamy, C.P.; Cui, B.; Zhang, H.; Panagal, M.; Paramasivam, S.; Chinnaiyan, U.; Jeyaraman, S.; Murugesan, K.; Rostagno, M.; Sekar, V.; Natarajan, S.P. Anti-ovarian cancer potential of phytocompound and extract from South African medicinal plants and their role in the development of chemotherapeutic agents. American journal of cancer research. 2021, 11, 1828.
Sowmya, S.; Perumal, P.C.; Ravi, S.; Anusooriya, P.; Shanmughavel, P.; Murugesh, E.; Chaithanya, K.K.; Gopalakrishnan, V.K. 1-Pentacosanol Isolated from Stem Ethanolic Extract of Cayratia trifolia (L.) is A Potential Target for Prostate Cancer-In SILICO Approach. Jordan Journal of Biological Sciences. 2021, 14.
Nirmala, C.; Bisht, M. S.; Bajwa, H. K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends in Food Science & Technology. 2018, 77, 91–99.
Nirmala, C.; Bisht, M. S. 10 WBC Reports: Bamboo: A prospective ingredient for functional food and nutraceuticals. Bamboo Journal. 2017, 30, 82–99.
Xu, X. B.; Jiang, H.; Sun, J.; Tang, F.; Guo, X. F.; Wang, J. Chemical constituents and antioxidant properties of Phyllostachys prominens Gramineae (WY Xiong) leaf extracts. Tropical Journal of Pharmaceutical Research. 2016, 15, 569–575.
Abdelhameed, R. F.; Habib, E. S.; Ibrahim, A. K.; Yamada, K.; Abdel-Kader, M. S.; Ibrahim, A. K.; Ahmed, S.A.; Badr, J.M.; Nafie, M. S. Chemical profiling, cytotoxic activities through apoptosis induction in MCF-7 cells and molecular docking of Phyllostachys heterocycla bark nonpolar extract. Journal of Biomolecular Structure and Dynamics. 2022, 40, 9636–9647.
Abdelhameed, R. F.; Nafie, M. S.; Ibrahim, A. K.; Yamada, K.; Abdel-Kader, M. S.; Ibrahim, A. K.; Ahmed, S.A.; Badr, J.M.; Habib, E. S. Cytotoxic, apoptosis-inducing activities, and molecular docking of a new sterol from bamboo shoot skin phyllostachys heterocycla var. pubescens. Molecules. 2020, 25, 5650.
Park, H. S.; Lim, J. H.; Kim, H. J.; Choi, H. J.; Lee, I. S. Antioxidant flavone glycosides from the leaves of Sasa borealis. Archives of Pharmacal Research. 2007, 30, 161–166.
Park, E. J.; Jhon, D. Y. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT-Food Science and Technology. 2010, 43, 655–659.
Song, K. H.; Seo, C. S.; Yang, W. K.; Gu, H. O.; Kim, K. J.; Kim, S. H. Extracts of Phyllostachys pubescens leaves represses human steroid 5-alpha reductase Type 2 promoter activity in BHP-1 cells and ameliorates testosterone-induced benign prostatic hyperplasia in rat model. Nutrients. 2021, 13, 884.
Sharma, V.; Nirmala, C. Therapeutic potential of bamboo shoots against cancer: an overview. In: Proceedings of 11th World Bamboo Congress, August 14–18, 2018, Xalapa, Mexico. 2018.
Aouf, D.; Khane, Y.; Fenniche, F.; Albukhaty, S.; Sulaiman, G.M.; Khane, S.; Henni, A.; Zoukel, A.; Dizge, N.; Mohammed, H.A.; Abomughaid, M.M. Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application. Nanotechnology Reviews. 2024, 13, 20240002.
Heera, P.; Shanmugam, S. Nanoparticle characterization and application: an overview. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 379–386.
Mellatyar, H.; Talaei, S.; Nejati-Koshki, K. Targeting HSP90 gene expression with 17-DMAG nanoparticles in breast cancer cells. Asian Pacific Journal of Cancer Prevention. 2016, 17, 2453–2457.
Palanisamy, C.P.; Poompradub, S.; Sansanaphongpricha, K.; Jayaraman, S.; Subramani, K.; Sonsudin, F.Biosynthesis of silver nanoparticles (AgNPs) using ethanolic extract of Nigella sativa (L.) seeds promotes wound healing via PDGF and VEGF signalling pathways activation. Biocatalysis and Agricultural Biotechnology. 2023, 54, 102970.
Borm, P. J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; Krutmann, J., Oberdorster, E. The potential risks of nanomaterials: a review carried out for ECETOC. Particle and fibre toxicology. 2006, 3, 1–35.
Manimaran, D.; Elangovan, N.; Mani, P.; Subramanian, K.; Ali, D.; Alarifi, S.; Palanisamy, C.P.; Zhang, H.; Rangasamy, K.; Palanisamy, V.; Mani, R. Isolongifolene-loaded chitosan nanoparticles synthesis and characterization for cancer treatment. Scientific Reports. 2022, 12, 19250.
Stapleton, P. A.; Nurkiewicz, T. R. Vascular distribution of nanomaterials. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2014, 6, 338–348.
Pei, J.; Yan, Y.; Jayaraman, S.; Rajagopal, P.; Natarajan, P.M.; Umapathy, V.R.; Gopathy, S.; Roy, J.R.; Sadagopan, J.C.; Thalamati, D. A review on advancements in the application of starch-based nanomaterials in biomedicine: Precision drug delivery and cancer therapy. International Journal of Biological Macromolecules. 2024, 130746.
Abdellatif, A.A.; Mohammed, H.A.; Khan, R.A.; Singh, V.; Bouazzaoui, A.; Yusuf, M.; Akhtar, N.; Khan, M., Al-Subaiyel, A.; Mohammed, S.A.; Al-Omar, M.S. Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity. Nanotechnology Reviews. 2021, 10, 1493–1559.
Chithrani, B. D.; Chan, W. C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano letters. 2007, 7, 1542–1550.
Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. Journal of Drug Delivery Science and Technology. 2021, 61, 102316.
Baraka, A.; Dickson, S.; Gobara, M.; El-Sayyad, G. S.; Zorainy, M.; Awaad, M. I.; Hatem, H.; Kotb, M.M.; Tawfic, A. F. Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chemical Papers. 2017, 71, 2271–2281.
Yang, H.-L.; Hseu, Y.-C.; Hseu, Y.-T.; Lu, F.-J.; Lin, E.; Lai, J.-S. Humic Acid Induces Apoptosis in Human Premyelocytic Leukemia HL-60 Cells. Life Sci. 2004, 75, 1817–1831, doi:https://doi.org/10.1016/j.lfs.2004.02.033.
Chaturvedula, V. S. P.; Prakash, I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242.
Hernández-Galicia, E.; Calzada, F.; Roman-Ramos, R.; Alarcón-Aguilar, F. J. Monoglycerides and fatty acids from Ibervillea sonorae root: isolation and hypoglycemic activity. Planta medica. 2007, 73, 236–240.
Ghareib, H. R. A.; Abdelhamed, M. S.; Ibrahim, O. H. Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium murale on tomato plants. Weed biology and management. 2010, 10, 64–72.
Sajjadi, S. E.; Shokoohinia, Y.; Moayedi, N. S. Isolation and Identification of Ferulic Acid From Aerial Parts of Kelussia odoratissima Mozaff. Jundishapur Journal of Natural Pharmaceutical Products. 2012, 7, 159.
Usman, A.; Thoss, V.; Itodo, A. U. Isolation of Catechin from Trichilla Emetica Whole Seeds. International Journal Of Science for Global Sustainability. 2016, 2, 6–6.
Xu, X. B.; Tang, F.; Guo, X. F.; Wang, J.; Yao, X.; Sun, J.; Xun, H. Isolation, identification and determination of six nucleosides and two amino acids from bamboo shoots of Gramineae Phyllostachys prominens (WY Xiong). Tropical Journal of Pharmaceutical Research. 2015, 14, 2239–2246.
Abdelhameed, R. F.; Habib, E. S.; Ibrahim, A. K.; Yamada, K.; Abdel-Kader, M. S.; Ahmed, S. A.; Ibrahim, A.K.; Badr, J.M.; Nafie, M. S. Chemical constituent profiling of Phyllostachys heterocycla var. Pubescens with selective cytotoxic polar fraction through EGFR inhibition in HepG2 cells. Molecules. 2021, 26, 940.
Fahrioğlu, U.; Dodurga, Y.; Elmas, L.; Seçme, M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene, 2016, 576, 476–482.
Eroğlu, C.; Seçme, M.; Bağcı, G.; Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumor Biology. 2015, 36, 9437–9446.
Manikandan, R.; Beulaja, M.; Arulvasu, C.; Sellamuthu, S.; Dinesh, D.; Prabhu, D.; Babu, G.; Vaseeharan, B.; Prabhu, N. M. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microscopy research and technique. 2012, 75, 112–116.
Ooi, S. O.; Sim, K. Y.; Chung, M. C. M.; Kon, O. L. Selective antiproliferative effects of thymidine. Experientia. 1993, 49, 576–581.
Ramadoss, D. P.; Sivalingam, N. Vanillin extracted from Proso and Barnyard millets induce apoptotic cell death in HT-29 human colon cancer cell line. Nutrition and Cancer. 2020, 72, 1422–1437.
Ayaz, M.; Sadiq, A.; Wadood, A.; Junaid, M.; Ullah, F.; Khan, N. Z. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids. 2019, 141, 30–35.
Song, N.; Wang, J.; Lai, Z.; Liang, S.; Zou, W.; Wang, J.; Zheng, D.; Li, Y.; He, Y.; Cheng, J. Arisaema heterophyllum blume monomer stigmasterol targets PPARγ and inhibits the viability and tumorigenicity of lung adenocarcinoma cells NCI-H1975. Evidence-Based Complementary and Alternative Medicine. 2022, 2022.
Kim, Y. S.; Li, X. F.; Kang, K. H.; Ryu, B.; Kim, S. K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB reports. 2014, 47, 433.
Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON. 2018, 23, 1420–1425.
Bae, H.; Song, G.; Lim, W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics. 2020, 12, 488.
Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Frontiers in Oncology. 2020, 12, 1101289.
Modi, M.A.; Kale, N.V.; Patel, J.H.; Varia, R.D.; Modi, F.D.; Vihol, P.D. Pharmacokinetics of ferulic acid following oral administration ethyl ferulate alone and in combination with piperine in rats. Ann. Phytomed. 2019, 8, 194–198.
Chu, K.O.; Pang, C.C. Pharmacokinetics and disposition of green tea catechins. Pharmacokinetics and adverse effects of drugs: mechanisms and risks factors. 2018, 17, 18–36.
Kaur, J.; Gulati, M., Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; Chaitanya, M.V.N.L. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends in Food Science & Technology. 2022, 122, 187–200.
Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules. 2022, 27, 523.
Raj, N.D.; Singh, D. A critical appraisal on ferulic acid: Biological profile, biopharmaceutical challenges and nano formulations. Health Sciences Review. 2022, 5, 100063.
Bae, J.; Kim, N.; Shin, Y.; Kim, S.Y.; Kim, Y.J. Activity of catechins and their applications. Biomedical Dermatology. 2020, 4, 1–10.
Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants. 2022, 11, 1912.
Xie, Y.; Peng, Q.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Xiao, Y.; Zhao, J.; Zhang, Q. Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Frontiers in Microbiology. 2021, 12, 645484.
Sivaselvam, S.; Selvakumar, R.; Viswanathan, C.; Ponpandian, N. Rapid one-pot synthesis of PAM-GO-Ag nanocomposite hydrogel by gamma-ray irradiation for remediation of environment pollutants and pathogen inactivation. Chemosphere. 2021, 275, 130061.
El-Batal, A. I.; Nada, H. G.; El-Behery, R. R.; Gobara, M.; El-Sayyad, G. S. Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC advances. 2020, 10, 9274–9289.
Fathy, R. M.; Mahfouz, A. Y. Eco-friendly graphene oxide-based magnesium oxide nanocomposite synthesis using fungal fermented by-products and gamma rays for outstanding antimicrobial, antioxidant, and anticancer activities. Journal of Nanostructure in Chemistry. 2021, 11, 301–321.
Seino, S.; Kinoshita, T.; Otome, Y.; Nakagawa, T.; Okitsu, K.; Mizukoshi, Y.; Nakayama, T.; Sekino, T.; Niihara, K.; Yamamoto, T. A. Gamma-ray synthesis of magnetic nanocarrier composed of gold and magnetic iron oxide. Journal of magnetism and magnetic materials. 2005, 293, 144–150.
He, C.; Qu, J.; Yu, Z.; Chen, D.; Su, T.; He, L.; Zhao, Z.; Zhou, C.; Hong, P.; Li, Y.; Sun, S.; Li, C. Preparation of micro-nano material composed of oyster shell/Fe3O4 nanoparticles/humic acid and its application in selective removal of Hg (II). Nanomaterials. 2019, 9, 953.
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983, 65, 55–63.
Tantawy, E. S.; Amer, A. M.; Mohamed, E. K.; Abd Alla, M. M.; Nafie, M. S. Synthesis, characterization of some pyrazine derivatives as anti-cancer agents: In vitro and in Silico approaches. Journal of Molecular Structure. 2020, 1210, 128013.
Nafie, M. S.; Arafa, K.; Sedky, N. K.; Alakhdar, A. A.; Arafa, R. K. Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chemico-biological interactions. 2020, 324, 109087.
Nafie, M. S.; Amer, A. M.; Mohamed, A. K.; Tantawy, E. S. Discovery of novel pyrazolo [3, 4-b] pyridine scaffold-based derivatives as potential PIM-1 kinase inhibitors in breast cancer MCF-7 cells. Bioorganic & Medicinal Chemistry. 2020, 28, 115828.
Gad, E. M.; Nafie, M. S.; Eltamany, E. H.; Hammad, M. S.; Barakat, A.; Boraei, A. T. Discovery of new apoptosis-inducing agents for breast cancer based on ethyl 2-amino-4, 5, 6, 7-tetra hydrobenzo [b] thiophene-3-carboxylate: Synthesis, in vitro, and in vivo activity evaluation. Molecules. 2020, 25, 2523.
Nafie, M. S.; Mahgoub, S.; Amer, A. M. Antimicrobial and antiproliferative activities of novel synthesized 6-(quinolin‐2‐ylthio) pyridine derivatives with molecular docking study as multi‐targeted JAK2/STAT3 inhibitors. Chemical Biology & Drug Design. 2021, 97, 553–564.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R.F.A.A: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. M.S.N: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. A.K.I: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. A.K.I: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. M.S.A: suggested the research topic, wrote the original draft, and participated in data representation and article revising and editing. S.A.A: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. G.S.E: suggested the nanotechnology topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. A.I.E: suggested the nanotechnology topic, wrote the original draft, and participated in data representation and article revising and editing. K.Y: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. J.M.B: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. E.S.H: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Research Involving Human Participation and/or Animals
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhameed, R.F.A., Nafie, M.S., Ibrahim, A.K. et al. Two Metallic Nanoparticles Formulas of Phyllostachys heterocycla Extract Exhibited Potent Cytotoxicity against Ovarian Cancer Cells through Apoptosis Induction. J Clust Sci (2024). https://doi.org/10.1007/s10876-024-02645-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10876-024-02645-6