Abstract
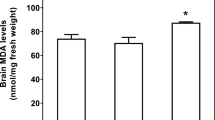
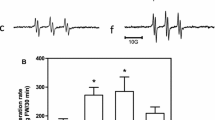
This work was aimed to test the hypothesis that sub-chronic administration of iron-dextran (Fe-dextran) (six doses of 50 mg Fe-dextran/kg) to rats triggers a transient oxidative stress in brain and mechanisms of cellular antioxidant defence. After 2 h of administration of the 6th dose, a significant increase of total Fe, the labile Fe pool (LIP), the lipid radical (LR•)/α-tocopherol (α-T) content ratio were observed, as compared to values in control brain homogenates. The ascorbyl radical (A•)/ascorbate (AH−) content ratio and the oxidation rate of 2′,7′-dichlorodihidrofluorescein (DCFH-DA) were significantly higher in Fe-dextran treated rats, as compared to values in brain from control rats after 4 h treatment. An increase in both catalase (CAT) and superoxide dismutase (SOD) activity was observed at 8 and 1–2 h, respectively. No significant changes were detected in the nuclear factor-κB (NF-κB) levels in nuclear extracts from rat brains after 1–8 h of Fe-dextran administration. After 2 h of Fe administration Fe concentration in cortex, striatum and hippocampus was significantly increased as compared to the same areas from control animals. Both, CAT and SOD activities were significantly increased in cortex after Fe administration over control values, without changes in striatum and hippocampus. Taken as a whole, sub-chronic Fe administration enhances the steady state concentration of Fe in the brain LIP that favors the settlement of an initial oxidative stress condition, both at hydrophilic and lipophilic compartments, resulting in cellular protection evidenced by antioxidant enzyme upregulation.







Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aust SD, Morehouse LA, Thomas CE (1985) Role of metals in oxygen radical reactions. J Free Radic Biol Med 1:3–25
Bayraktar UD, Bayraktar S (2010) Treatment of iron deficiency anemia associated with gastrointestinal tract diseases. World J Gastroenterol 16:2720–2725
Brand A, Schonfeld E, Isharel I, Yavin E (2008) Docosahexaenoic acid–dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. J Neurochem 105:1325–1335
Brumby PE, Massey V (1967) Determination of nonheme iron, total iron and cooper. Methods Enzymol 10:463–474
Buettner GR (1987) Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med 3:259–303
Chevion M, Leibowitz S, Aye NN, Novogrodsky O, Singer A, Avizemer O, Bulvik B, Konijn AM, Berenshtein E (2008) Heart protection by ischemic preconditioning: a novel pathway initiated by iron and mediated by ferritin. J Mol Cell Cardiol 45:839–845
Chtourou Y, Fetoui H, Gdoura R (2014) Protective Effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res 158:376–383
Courderot-Masuyer C, Lahet JJ, Verges B, Brun JM, Rochette L (2000) Ascorbyl free radical release in diabetic patients. Cell Mol Biol 46:1397–1401
Czerniczyniec A, Karadayian AG, Bustamante J, Cutrera RA, Lores-Arnaiz S (2011) Paraquat induces behavioral changes and cortical and striatal mitochondrial dysfunction. Free Radic Biol Med 51:1428–1436
Daba A, Gkouvatssos K, Sebastiani G, Pantopoulos K (2013) Differences in activation of mouse hepcidin by dietary iron and parenterally administered iron dextran: compartmentalization is critical for iron sensing. J Mol Med 91:95–102
Darbari D, Loyevsky M, Gordeuk V, Kark JA, Castro O, Rana S (2003) Fluorescence measurements of the labile iron pool of sickle erythrocytes. Blood 102:357–364
Das M, Das DK (2008) Molecular mechanism of preconditioning. IUBMB Life 60:199–203
Deryckere F, Gannon F (1994) A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques 16:405
Desai I (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–146
Doba T, Burton GW, Ingold KU (1985) Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta 835:298–303
Drögue W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49:835–842
Fretham SJB, Carlson ES, Georgieff MK (2011) The role of iron in learning and memory. Adv Nutr 2:112–121
Galleano M, Aimo L, Puntarulo S (2002) Ascorbyl radical/ascorbate ratio in plasma from iron overloaded rats as oxidative stress indicator. Toxicol Lett 133:193–201
Galleano M, Tapia G, Puntarulo S, Varela P, Videla LA, Fernandez V (2011) Liver preconditioning induced by iron in a rat model of ischemia/reperfusion. Life Sci 89:221–228
Gey KF, Stähelin HB, Puska P, Evans A (1987) Relationship of plasma level of vitamin C to mortality from ischemic heart disease. Ann N Y Acad Sci 498:110–123
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Hidalgo C, Núñez MT (2007) Calcium, iron and neuronal function. IUBMB Life 59:280–285
Jiang H, Song N, Wang J, Ren LY, Xie JX (2007) Peripheral iron dextran induced degeneration of dopaminergic neurons in rat substantia nigra. Neurochem Int 51:32–36
Kakhlon O, Cabantchik ZI (2002) The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med 33:1037–1046
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116
Korantzopoulos P, Vlachou C, Kotsia A, Kalantzi K, Barbouti A, Galaris D, Goudevenos JA (2012) Leukocyte labile iron pool in patients with systolic heart failure. Hellenic J Cardiol 53:95–100
Kotake Y, Tanigawa T, Tanigawa M, Ueno I, Allen DR, Lai CS (1996) Continuous monitoring of cellular nitric oxide generation by spin trapping with an iron dithiocarbamate complex. Biochim Biophys Acta 1289:362–368
Kruszewski M (2003) Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res 531:81–92
Kutnink MA, Hawkes WC, Schaus EE, Omaye ST (1987) An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem 166:424–430
Lai EK, Crossley C, Sridhar R, Misra HP, Janzen EG, McCay PB (1986) In vivo spin trapping of free radicals generated in brain, spleen, and liver during γ radiation of mice. Arch Biochem Biophys 244:156–160
Laurie SH, Tancock NP, McGrath SP, Sanders J (1991) Influence of complexation on the uptake by plants of iron, manganese, copper and zinc: I. Effect of EDTA in a multimetal and computer simulation study. Exp Bot 42:509–513
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malanga G, Calmanovici G, Puntarulo S (1997) Oxidative damage to chloroplasts from Chlorella vulgaris exposed to ultraviolet-B radiation. Physiol Plant 101:455–462
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Metzler B, Jehle J, Theurl I, Ludwiczek S, Obrist P, Pachinger O, Weiss G (2007) Short term protective effects of iron in a murine model if ischemia/reperfusion. Biometals 20:205–215
Minakata K, Suzuki O, Saito S, Harada N (1993) Ascorbate radical levels in human sera and rat plasma intoxicated with paraquat and diquat. Arch Toxicol 67:126–130
Moon MS, McDevitt EI, Zhu J, Stanley B, Krzeminskym J, Amin S, Aliaga C, Miller TG, Isom HC (2012) Elevated hepatic iron activates NF-E2-related factor2-regulated pathway in a dietary iron overload mouse model. Toxicol Sci 129:74–85
Morales P, Vargas R, Videla LA, Fernández V (2014) Nrf2 activation in the liver of rats subjected to a preconditioning sub-chronic iron protocol. Food Funct 5:243–250
Muñoz JP, Chiong M, García L, Troncoso R, Toro B, Pedrozo A, Díaz-Elizondo J, Salas D, Parra V, Núñez MT, Hidalgo C, Lavandero S (2010) Iron induces protection and necrosis in cultured cardiomyocytes: role of reactive oxygen species and nitric oxide. Free Radic Biol Med 48:526–534
Nakagawa K, Kanno H, Miura Y (1997) Detection and analyses of ascorbyl radical in cerebrospinal fluid and serum of acute lymphoblastic leukemia. Anal Biochem 254:31–35
Pietrangelo A (2003) Iron-induced oxidant stress in alcoholic liver fibrogenesis. Alcohol 30:121–129
Pietri S, Culcasi M, Albat B, Albérici G, Menasché P (1994) Direct assessment of the antioxidant effects of a new heart preservation solution, Celsior. A hemodynamic and electron spin resonance study. Transplantation 58:739–742
Piloni NE, Puntarulo S (2010) Iron role in the oxidative metabolism of animal and plant cells. Effect of iron overload. In: Gimenez MS (ed) Metals in biology systems. Research Signpost, Transworld Research Network, Trivandrum, pp 29–50
Piloni NE, Fermandez V, Videla LA, Puntarulo S (2013) Acute iron overload and oxidative stress in brain. Toxicology 314:174–182
Prus E, Fibach E (2008) Flow cytometry measurement of the labile iron pool in human hematopoietic cells. Cytometry 73A:22–27
Puntarulo S (2005) Iron, oxidative stress and human health. Mol Aspects Med 26:299–312
Puntarulo S, Simontacchi M, Galleano M, Caro A, Malanga G, Kozak RG (1995) Effect of oxidative stress on iron reduction rates in biological systems. J Braz Assoc Adv Sci 47:402–406
Robello E, Galatro A, Puntarulo S (2007) Iron role in oxidative metabolism of soybean axes upon growth: effect of iron overload. Plant Sci 172:939–947
Videla LA (2009) Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol 1:72–78
Walcourt A, Kurantsin-Mills J, Kwagyan J, Adenuga BB, Kalinowski DS, Lovejoy DB, Lane DJ, Richardson DR (2013) Anti-pasmodial activity of aroylhydrazone and thiosemicarbazone iron chelators: effect on erythrocyte membrane integrity, parasite development and the intracellular labile iron pool. J Inorg Biochem 129:43–51
Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434:365–381
Wu J, Ding T, Sun J (2013) Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology 34:243–253
Acknowledgments
This study was supported by grants from the University of Buenos Aires, ANPCyT and CONICET (to SP) and FONDECYT 1110006 (to VF). SP is career investigator from CONICET, and NP is a CPA member from CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piloni, N.E., Perazzo, J.C., Fernandez, V. et al. Sub-chronic iron overload triggers oxidative stress development in rat brain: implications for cell protection. Biometals 29, 119–130 (2016). https://doi.org/10.1007/s10534-015-9902-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9902-4