Abstract
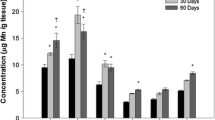
Manganese (Mn), iron (Fe), copper (Cu), and zinc (Zn) are essential nutrients which aid in the proper functioning of cells, but high concentrations of these metals can be toxic to various organs. Little is known about the endogenous concentrations of these metals in the cochlea, the auditory portion of the inner ear which is extremely small and difficult to access. To fill this gap, a trace quantitative digestion and inductively coupled plasma mass spectrometry method was developed to determine the concentrations of these metals in the stria vascularis, organ of Corti, and spiral ganglion, three critically important parts of the cochlea (≤1.5 mg); these values were compared to those in specific brain regions (≤20 mg) of rats. Rats were sacrificed and the cochlea and brain regions were carefully isolated, digested, and analyzed to determine baseline concentrations of Mn, Fe, Cu, and Zn. In the cochlea, Mn, Fe, Cu, and Zn concentrations ranged from 3.2–6, 73–300, non-detect, and 13–200 µg/g respectively. In the brain, Mn, Fe, Cu, and Zn concentrations ranged from 1.3–2.72, 21–120, 5.0–10.6, and 33–47 µg/g respectively. Significant differences (p < 0.05) were observed between the tissue types within the cochlea, and between the cochlea and brain. This validated method provides the first quantitative assessment of these metals in the three key subdivisions of the cochlea compared to the levels in the brain; Mn, Fe, and Zn levels were considerably higher in the cochlea than brain.




Similar content being viewed by others
References
Agirdir B, Bilgen I, Dinc O, Ozçağlar H, Fişenk F, Turhan M, Oner G (2002) Effect of zinc ion on cadmium-induced auditory changes. Biol Trace Elem Res 88:153–163
Alexander GJ, Meltzer HL (1975) Onset of audiogenic seizures in rodents after intake of near-toxic doses of rubidium chloride. J Pharmacol Exp Ther 194:480–487
Apostoli P, Catalani S, Zaghini A, Mariotti A, Poliani PL, Vielmi V, Semeraro F, Duse S, Porzionato A, Macchi V, Padovani A, Rizzetti MC, De Caro R (2013) High doses of cobalt induce optic and auditory neuropathy. Exp Toxicol Pathol 65:719–727
Aschner M (1997) Manganese neurotoxicity and oxidative damage. In: Connor JR (ed) Metals and oxidative damage in neurological disorders. Plenum, New York, pp 77–93
Bartley JC, Reber EF (1961) Toxic effects of stable strontium in young pigs. J Nutr 75:21–28
Bird ED, Grant LG, Ellis WH (1967) Measurement of the effect of phenothiazine on the manganese concentration in the basal ganglia of subhuman primates by activation analysis. In: Nuclear activation techniques in the life sciences, vol 44, Amsterdam, pp 491–499
Chan AW, Minski MJ, Lai JC (1983) An application of neutron activation analysis to small biological samples: simultaneous determination of thirty elements in rat brain regions. J Neurosci Methods 7:317–328
Choi Y-H, Hu H, Mukherjee B, Miller J, Park SK (2012) Environmental cadmium and lead exposures and hearing loss in U.S. adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ Health Perspect 120:1544–1550
Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, Salvi RJ (2011a) Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res 282:196–203
Ding D, Roth J, Salvi R (2011b) Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 32:233–241
Ding D, Salvi R, Roth JA (2014) Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. Biometals 27:731–744
Donaldson J, Pierre TS, Minnich JL, Barbeau A (1973) Determination of Na+, K+, Mg2+, Cu2+, Zn2+, and Mn2+ in rat-brain regions. Can J Biochem 51:87–92
Erikson KA, Shihabi ZK, Aschner JL, Aschner M (2002) Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res 87:143–156
Erikson KM, Syversen T, Steinnes E, Aschner M (2004) Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J Nutr Biochem 15:335–341
Fieve RR, Meltzer HL (1974) Proceedings: rubidium salts–toxic effects in humans and clinical effects as an antidepressant drug. Psychopharmacol Bull 10:38–50
Fjerdingstad E, Danscher G, Fjerdingstad EJ (1974a) Zinc content in hippocampus and whole brain of normal rats. Brain Res 79:338–342
Fjerdingstad EJ, Danscher G, Fjerdingstad E (1974b) Hippocampus: selective concentration of lead in the normal rat brain. Brain Res 80:350–354
Fjerdingstad E, Danscher G, Fjerdingstad EJ (1977) Changes in zinc and lead content of rat hippocampus and whole brain following intravital dithizone treatment as determined by flameless atomic-absorption spectrophotometry. Brain Res 130:369–373
Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM (2006) DMT1: which metals does it transport? Biol Res 39:79–85
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain-I. J Neurochem 13:655–669
Gupta S (2014) Cell therapy to remove excess copper in Wilson’s disease. Ann N Y Acad Sci 1315:70–80
Han S, Li W, Jamil U, Dargan K, Orefice M, Kemp FW, Bogden JD (1999) Effects of weight loss and exercise on the distribution of lead and essential trace elements in rats with prior lead exposure. Environ Health Perspect 107:657–662
Hirata Y (2002) Manganese-induced apoptosis in PC12 cells. Neurotoxicol Teratol 24:639–653
Ikeda T, Takahashi K, Kabata T, Sakagoshi D, Tomita K, Yamada M (2010) Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve 42:140–143
Jacobsen P (1998) Chemically-Induced Hearing Disorders. In: Stellman JM (ed) Encyclopedia of Occupational Health and Safety, vol 1. International Labour Office, Geneva
Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25:643–655
Kaler SG (2014) Translational research investigations on ATP7A: an important human copper ATPase. Ann N Y Acad Sci 1314:64–68
Kuo YM, Zhou B, Cosco D, Gitschier J (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98:6836–6841
Larsen NA, Pakkenberg H, Damsgaard E, Heydorn K (1979) Topographical distribution of arsenic, manganese, and selenium in the normal human brain. J Neurol Sci 42:407–416
Larsen NA, Pakkenberg H, Damsgaard E, Heydorn K, Wold S (1981) Distribution of arsenic, manganese, and selenium in the human-brain in chronic renal-insufficiency, Parkinsons-disease, and amyotrophic lateral sclerosis. J Neurol Sci 51:437–446
Liu H, Ding D, Sun H, Jiang H, Wu X, Roth JA, Salvi R (2014) Cadmium-induced ototoxicity in rat cochlear organotypic cultures. Neurotox Res 26:179–189
McCarthy RC, Kosman DJ (2014) Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One 9:e89003
Mizuno D, Kawahara M (2013) The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia. Int J Mol Sci 14:22067–22081
Murata K, Araki S, Yokoyama K, Uchida E, Fujimura Y (1993) Assessment of central, peripheral, and autonomic nervous system functions in lead workers: neuroelectrophysiological studies. Environ Res 61:323–336
Nose Y, Kim BE, Thiele DJ (2006) Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4:235–244
Oberto A, Marks N, Evans HL, Guidotti A (1996) Lead (Pb+2) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J Pharmacol Exp Ther 279:435–442
Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilincarslan S, Oner G (2001) Effects of cadmium on the hearing system. Acta Otolaryngol 121:393–397
Pelclova D, Sklensky M, Janicek P, Lach K (2012) Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol 50:262–265
Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90:467–477
Reaney SH, Bench G, Smith DR (2006) Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci 93:114–124
Rose JE, Gross NB, Geisler CD, Hind JE (1966) Some neural mechanisms in the inferior colliculus of the cat which may be relevant to localization of a sound source. J Neurophysiol 29:288–314
Roth J, Ponzoni S, Aschner M (2013) Manganese homeostasis and transport. Met Ions Life Sci 12:169–201
Seth PK, Husain R, Mushtaq M, Chandra SV (1977) Effect of manganese on neonatal rat: manganese concentration and enzymatic alterations in brain. Acta Pharmacol Toxicol (Copenh) 40:553–560
Sharp PA (2003) Ctr1 and its role in body copper homeostasis. Int J Biochem Cell Biol 35:288–291
Sokoloff L (1981) Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab 1:7–36
Stamelou M, Tuschl K, Chong WK, Burroughs AK, Mills PB, Bhatia KP, Clayton PT (2012) Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov Disord 27:1317–1322
Takeda A, Ishiwatari S, Okada S (1999) Manganese uptake into rat brain during development and aging. J Neurosci Res 56:93–98
United States Environmental Protection Agency (1996) Method 3050B: acid digestion of sediments, sludges, and soils. Washington D.C.
Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, Uemura K (2013) Inducer of heme oxygenase-1 cobalt protoporphyrin accelerates autophagy and suppresses oxidative damages during lipopolysaccharide treatment in rat liver. Hepatol Res 43:91–96
Wang L, Ding D, Salvi R, Roth JA (2014) Nicotinamide adenine dinucleotide prevents neuroaxonal degeneration induced by manganese in cochlear organotypic cultures. Neurotoxicology 40:65–74
Young ED, Spirou GA, Rice JJ, Voigt HF, Rees A (1992) Neural organization and responses to complex stimuli in the dorsal cochlear nucleus [and discussion]. Philos Trans 336:407–413
Zhang S, Chen X, Yang Y, Zhou X, Liu J, Ding F (2011) Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Mol Cell Biochem 354:161–170
Zhang J, Cao R, Cai T, Aschner M, Zhao F, Yao T, Chen Y, Cao Z, Luo W, Chen J (2013) The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox Res 24:478–490
Zou W, Yan M, Xu W, Huo H, Sun L, Zheng Z, Liu X (2001) Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res 64:646–653
Acknowledgments
Research supported by National Institute for Occupational Safety and Health (NIOSH) award #R010HH010311-01. Stacia Wegst-Uhrich acknowledges her IGERT fellowship support from the National Science Foundation (NSF), grant #DGE-0654305, titled “Ecosystem Restoration through Interdisciplinary Exchange” Traineeship Program. We acknowledge the NSF Major Research Instrumentation Program CHE0959565 for the ICP-MS. We are grateful to the Colón lab for the use of the microbalance.
Conflict of interest
The authors state that they have no conflicts of interest that affect the objectivity of this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wegst-Uhrich, S.R., Mullin, E.J., Ding, D. et al. Endogenous concentrations of biologically relevant metals in rat brain and cochlea determined by inductively coupled plasma mass spectrometry. Biometals 28, 187–196 (2015). https://doi.org/10.1007/s10534-014-9814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9814-8