Abstract
While seagrass meadows are perceived to be pertinent blue carbon reservoirs, they also potentially release methane (CH4) into the atmosphere. Seasonal and diurnal variations in CH4 emissions from a subtropical hypersaline lagoon dominated by Halodule wrightii in southern Texas, USA, on the northwest coast of the Gulf of Mexico were investigated. Dissolved CH4 concentrations decreased in the daytime and increased overnight during the diel observation period, which could be explained by photosynthesis and respiration of seagrasses. Photosynthetic oxygen was found to significantly reduce CH4 emissions from seagrass sediment. Diffusive transport contributed slightly to the release of CH4 from the sediment to the water column, while plant mediation might be the primary mechanism. The diffusive CH4 flux at the sea-air interface was 12.3–816.2 µmol/m2 d, over the range of the sea-air fluxes previously reported from other seagrass meadows. This was related to relatively higher dissolved CH4 concentrations (11.6–258.2 nmol/L) in a mostly closed lagoon with restricted water exchange. This study emphasizes seagrass meadows in the subtropical hypersaline lagoon as a source of atmospheric CH4, providing insights into the interactions between seagrass ecosystems and methane dynamics, with potential implications for seagrass meadow management and conservation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass meadows are enormous blue carbon reservoirs in coastal areas and have great capabilities in climate mitigation due to their efficiency in capturing and converting light energy into organic matter and utilizing dissolved inorganic carbon (Purvaja et al. 2020; Ricart et al. 2020; Rosentreter et al. 2021). They are also recognized as a source of atmospheric methane (CH4), which could offset < 0.5% ̵ 2% of blue carbon deposited in sediment (Al-Haj and Fulweiler 2020; Rosentreter et al. 2021; Yau et al. 2023). Compared with other coastal vegetation, mangroves, and salt marshes, seagrasses are a weaker source. However, the restoration of seagrass meadows in South Bay, Virginia, US, the world’s largest successful seagrass restoration project until 2020, observed an eightfold larger CH4 emissions after restoration (Oreska et al. 2020). This revealed the importance of including CH4 emissions in a thorough benefit analysis and evaluation of coastal seagrass restoration.
Although methanogenesis is usually believed to be depressed by sulfate reduction in saline environments, methanogenesis has been observed to appear concurrently with sulfate reduction in sulfate-rich anoxic sediment (Lyimo et al. 2018; Oremland et al. 1982), which could be explained by the existence of non-competitive substrates such as methylamines and methanol that can feed methanogens (Kelley et al. 2015; King 1984; Oremland and Polcin 1982; Reeburgh 2007). In addition to methanogenesis related to sedimentary organic carbon fermentation, Schorn et al. (2022) discovered that CH4 production from seagrass Posidonia oceanica in the Mediterranean Sea was supported by methylated compounds produced by seagrass (Schorn et al. 2022). These findings further suggested a large potential for CH4 emissions from seagrass meadows in saline coastal waters, which should be integrated into local and global CH4 budget assessments.
While studies on CH4 cycling in seagrass ecosystems are sparse compared to the globally wide distribution of seagrass meadows (Fig. 1a), the present studies have shown high spatial and temporal variations in CH4 production from seagrass meadows (Al-Haj and Fulweiler 2020; Rosentreter et al. 2021). For example, in seagrass meadows in the Red Sea, where relatively extensive research was performed, spatial variations in benthic CH4 emissions along the Red Sea coast reached two magnitudes, from 0.81 ± 0.34 to 401.32 ± 95.59 µmol/m2 d (Burkholz et al. 2020; Garcias-Bonet and Duarte 2017), and sea-air CH4 fluxes 16.1 ± 95.59–25.04 ± 1.33 µmol/m2 d (Saderne et al. 2023). Temporally, no diel or seasonal variations were found in the Red Sea (Garcias-Bonet and Duarte 2017). In contrast, seasonal observations of seagrass meadows in Virginia USA and Chilika, India, showed that higher CH4 emissions were related to the presence of seagrass (Banerjee et al. 2018; Oreska et al. 2020). Diel changes in CH4 fluxes in Swan Bay, Australia, and Ria Formosa Lagoon, southern Portugal, were found due to the impact of tidal processes (Bahlmann et al. 2015; Ollivier et al. 2022). Such high spatial and temporal variations in CH4 emissions suggest diverse influences from hydrological, physicochemical and biotic environments in coastal areas.
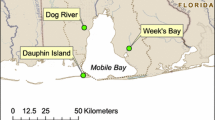
a Distribution of global seagrass meadows (dark green) (UNEP-WCMC and Short 2021) and locations where methane emissions from seagrass meadows have been investigated (orange dots) and location of this study (red star). b An overview of the Gulf of Mexico and the study area. The study area, Upper Laguna Madre, is located northwest of the Gulf of Mexico and southeast of Texas, USA, marked by the white rectangle. c Sampling sites. Yellow: LM1, LM9, LM10, P7, and P9 are located in the seagrass meadow; White: LM6, LM7, LM8, LM11, LM12, P4 and P6 are located in the Gulf Intracoastal Water Way (channel); P8 is at the Packery Channel; LM7, LM11, LM12 are close to LM1, LM9, and LM10, respectively; and P7 and P9 are close to P4. Sites in seagrass meadows are the primary study objects in this study
Having extensive seagrass habitats, Laguna Madre in the northwestern Gulf of Mexico is among the world’s most hypersaline lagoons (Thorhaug et al. 2017). Haloduel wrightii (H. wrightii, shoal grass) is the dominant seagrass species in this region due to its high tolerance to a wide range of environmental conditions, such as salinity, temperature, and light. H. wrightii is also considered a pioneer species because it can recover quickly after disturbance events (Rivera-Guzmán et al. 2016). The survival of H. wrightii in such a hypersaline environment highly relies on photosynthetic oxygen transported to the rhizome to inhibit the growth of sulfide (Eldridge and Morse 2000), which could modify the sedimentary redox gradients (Lee and Dunton 2000). Photosynthetic oxygen might inhibit CH4 production in sediment, as indicated in previous studies (Oremland 1975; Oremland and Taylor 1977). A decrease in dissolved CH4 concentrations at noon observed in seagrass meadows P. oceanica in the Mediterranean Sea also implied the impact of photosynthesis (Yau et al. 2023). Hence, photosynthetic oxygen is expected to reduce CH4 emissions from H. wrightii meadows. Considering that the Upper Laguna Madre is a lagoon with limited water exchange with the Gulf of Mexico and has minor freshwater input, it should be the major source of CH4 to the atmosphere. Hence, the Upper Laguna Madre provides an ideal environment to explore the impact of seagrass ecosystems on CH4 emissions. This investigation focused on long-term and diurnal variations in dissolved CH4 concentrations and sea-air CH4 fluxes in seagrass meadows in the Upper Laguna Madre. We also investigated the transport of CH4 at the sediment–water interface and discussed factors that influence CH4 emissions to the atmosphere. The presented data will expand current seagrass meadow methane studies along the Gulf of Mexico and include a specific species of seagrass that has not been examined. This research provides a more thorough understanding of CH4 cycling in subtropical seagrass meadows.
Materials and methods
Study area
The Upper Laguna Madre is a shallow lagoon (water depth is approximately 1 m) located on the southeast coast of Texas along the northwest Gulf of Mexico (Fig. 1). It is separated from the Gulf of Mexico by the sandy barrier Padre Island. Water exchange with the Gulf of Mexico is limited because it is connected only through the narrow Packery Channel. The Upper Laguna Madre has a semiarid subtropical climate. This system is one of the most hypersaline lagoons in the world, with evaporation consistent in excess of precipitation and no freshwater input except low rainfall (Eldridge and Morse 2000; Tunnell et al. 2002). Its annual salinity ranged from 29.0 ± 8.3 psu to 45.7 ± 9.4 psu between 1976 and 2016 (tpwd.texas.gov). In the middle of the lagoon, there is a 38 m (125 feet) wide and 3.7 m (12 feet) deep channel for boating and marine transportation. It is a part of the Gulf Intracoastal Waterway of Texas (GIWW-T) along the Texas coastline and handles 67% of all GIWW traffic (Kruse et al. 2016).
Upper Laguna Madre is a crucial seagrass habitat, with seagrass meadows covering approximately 66% of the floor (Dunton and Reyna 2019). H. wrightii (56.0 ± 39.1%, 2018) dominated the region, followed by S. filiforme (9.2 ± 23.1%, 2018) and H. engelmannii (0.5 ± 4.7%, 2018) (Dunton and Reyna 2019). H. wrightii (shoal grass) prefers to root at a surface in the oxic zone. Its rhizome internodes are 0.75–3.5 cm long (Phillips and Menez 1988). Due to Upper Laguna Madre hypersalinity, only a few submerged phytoplankton can survive, e.g., the harmful “brown tide” organism Aureoumbra lagunensis (Cira et al. 2021). Seagrass meadows covering the Upper Laguna Madre provide vital nursery habitat for many birds, fish, and invertebrate species. It also serves as a major area for public recreation, e.g., boating and fishing.
Study method and sampling
To acquire long-term variations in dissolved CH4 concentrations and sea-air CH4 fluxes, surface water and ambient air samples were collected at Upper Laguna Madre in June 2018, January, March, May, July, August 2019, and January 2020 and bimonthly from July 2020 to May 2021. Diurnal variations were studied with sampling at site LM1 (27°32′39.16"N, 97° 17′9.5"W, seagrass meadow) of Upper Laguna Madre (Fig. 1) on August 13th and 14th, 2019. During each cruise, water incubation experiments were performed at LM1 to determine CH4 production and consumption in the water column. Floating chambers were applied at LM1 to measure in situ sea-air CH4 fluxes and in situ gas transfer velocities. To determine CH4 transport at the sediment–water interfaces, sediment cores were collected at LM1 and LM9 in May 2019 (spring) and at LM1 in August 2019 (summer) and September 2020 (autumn). Benthic chambers were deployed for diurnal observation in August 2019 to measure the sediment–water CH4 fluxes directly.
Surface water and ambient air sampling
Surface water and ambient air samples were collected to determine the dissolved CH4 concentration, sea-air CH4 flux, and dissolved inorganic carbon (DIC) as described in Yu et al. (2023). In brief, triplicate surface water samples were overflowed three times the volume into serum glass bottles, saturated CuSO4 solution was added to inhibit microbial growth, and then the samples were immediately capped without headspace using airtight butyl rubber septa and crimp sealed (Coffin and Mueller 2020; Rose et al. 2013). Fourteen additional water samples were collected simultaneously with the first samples at LM1 for the water incubation experiment, with CuSO4 solution added to every two samples in 1, 2, 3, 4, 5, 6, and 12 h. Ambient air was sampled at approximately 1 m above the water surface using a syringe and injected into a serum butyl-stoppered glass filled with Milli-Q water that was previously sparged by ultrahigh purity N2 to measure atmospheric CH4 concentrations and calculate the sea-air CH4 flux. Plastic bottles were used to collect chlorophyll-a (Chl-a) samples. All water and air samples were stored in an ice box in the dark during the cruise, transported to the lab on the same day and stored in a refrigerator.
In situ salinity, pH, dissolved oxygen (DO), and temperature were measured using a multiparameter sonde (HI98194, Hanna Instruments). Synchronic weather data (wind speed, wind direction, and temperature), water level, and current data from nearby meteorological and hydrological stations were acquired online (NOAA TIDES & CURRENTS, station Bird Island (#8776139) and Packery Channel (#8775792); Local Climatological Data, station NAS, Fig. 1). These data were applied to calculate the CH4 flux and analyze factors controlling CH4 emissions at the sea-air interface. During diurnal observations, all sampling and in situ measurements were performed every 4 h to determine 24-h variations at LM1.
Floating chamber experiment
During each cruise, three floating chambers (3 L, 154 cm2 in area) were placed at LM1 to measure total in situ CH4 emissions from surface water to the atmosphere and calculate the standard gas transfer velocity (Yu et al. 2023). In the diurnal experiment, floating chambers were placed both in the daytime and during the night. Air samples in floating chambers were collected every 15 min in one hour using a 30 mL syringe from the top of the chamber by inserting a small gauge needle into the small hole (1/16 inch) through the sealing tape. Once the needle was drawn from the chamber, the pierced hole was sealed immediately using tape. Without delay, air samples were injected into vials filled with Milli-Q water that had previously been purged with ultrahigh purity N2.
Sediment core collection and benthic chamber experiment
Sediment cores were collected at LM1 and/or LM9 using 50 cm polycarbonate tubes (6.67 cm in diameter) in May and August 2019 and September 2020. Porewater samples were immediately drawn from cores across 2 cm intervals using Rhizon samplers (Coffin et al. 2013; Seeberg-Elverfeldt et al. 2005; Treude et al. 2014) equipped with 30 ml syringes from holes (1/8 inch in diameter) that were predrilled and sealed. Core samples inserted with Rhizon samplers were stored in an ice box and transported to the lab with the other samples. With a full syringe, porewater samples were transferred to 30 ml vials previously filled with ultrahigh purity N2 gas, and 0.2 mL saturated CuSO4 solution was injected immediately and then stored in the refrigerator until measurement.
Benthic chamber experiments were performed using 50 cm polycarbonate tubes (6.67 cm in diameter) in seagrass meadows (LM1 and LM9) in August 2019. The chambers were inserted into the sediment 15 cm below the surface and submerged under water. When the water became clear, the opening was sealed immediately. After 24 h, the overlying water in each chamber was collected using a 60 mL gastight syringe and transferred to serum glass bottles previously filled with ultrahigh purity N2 gas; the septa were sealed immediately without headspace, and 0.5 mL saturated CuSO4 solution was added.
Analytical methods
All samples were stored in a refrigerator before measurement. Concentrations of dissolved and airborne CH4 were measured via headspace equilibration technique and gas chromatography (GC, Agilent 6890 N) (Magen et al. 2014; Reeburgh 2007). DIC concentrations were determined with a UIC CM5017 Coulometer (Dickson et al. 2007). Chlorophyll a (Chl-a) concentrations were measured using a Turner 10-AU fluorometer. Porewater H2S in porewater was determined by colorimetric analysis of the methylene blue method (Cline 1969). The above works were performed in the Isotope Core Laboratory at Texas A&M University-Corpus Christi. The δ13C-CH4 values of some samples were analyzed at the Stable Isotope Lab of the University of California-Davis.
Calculation of fluxes
Floating chamber methane flux
The sea-air CH4 flux acquired using floating chambers is called the chamber flux in this paper. If the CH4 mixing ratio increased linearly (R2 > 0.95), a diffusion flux was calculated using the increase rate of the chamber CH4 mixing ratio. If there was a dramatic increase, it suggested that potential bubble CH4 was released from water. When the ratio of chamber flux and diffusion flux (Equation (2)) was larger than 2.5, it was treated as ebullition input (Wu et al. 2019). The ebullitive flux was calculated using the difference between the final and initial mixing ratios.
where Ffc is chamber flux (µmol/m2 h); s is growth rate of CH4 mixing ratios (ppm/min) in linear increase or CH4 variation before and after observation (ppm/hr) when ebullitive input is determined; P is gas pressure inside the chamber (Pa); R is universal gas law constant (8.314510 m3 Pa K−1 mol−1); T is in situ air temperature (K); \(\frac{P}{R\times T}\) is to convert volume mixing ratio to molar concentration based on Law of Avogadro’s; \({V}_{chamber}\) is volume of the chamber (m3); \({A}_{chamber}\) is area of the chamber connecting surface water (m2); and t is time coefficient (convert from minutes to hours).
Diffusive methane flux at the sea-air interface
The diffusive CH4 flux at the sea-air interface was calculated using the gas-transfer model (Wanninkhof 1992).
where F is flux of gas to the atmosphere (mmol/m2 d or µmol/m2 h); Cobs represents measured concentration of dissolved CH4 in water (nmol/L); Ceq is concentration of CH4 in equilibrium with the atmosphere at in situ temperature (nmol/L), calculated for each sample from the temperature- and salinity-dependent equilibrium relationship (Wiesenburg and Guinasso 1979); and kv is gas transfer velocity (cm/h), calculated based on the Schmidt number (Sc) normalized to k660, which is called the standard gas transfer velocity.
where \(Sc\) is defined as the kinematic viscosity of water divided by the gas diffusion coefficient, which is dependent on water temperature and salinity (Wanninkhof 1992, 2014). The calculation of \(Sc\) followed Wanninkhof 2014 since water temperatures exceeded 30°C. k660 is calculated using the in situ floating chamber flux (kfc) and empirical equations (kmodel).
Floating chambers:
Empirical equations:
J2008 (Jiang et al. 2008):
W2014 (Wanninkhof 2014):
VD2019 (Van Dam et al. 2019):
R2001 (Raymond and Cole 2001):
where \(\mu\) is hourly mean wind speed at 10 m at nearby weather stations. Monthly average wind speeds at the NAS station (NOAA Local Climatological Data, https://www.ncdc.noaa.gov/cdo-web/datasets/LCD/stations/WBAN:12926/detail) were applied to calculate monthly sea-air CH4 fluxes during the study period. Hourly diel sea-air CH4 fluxes in August 2019 were calculated using hourly wind speeds at the Bird Island station (NOAA Tides& Currents, https://tidesandcurrents.noaa.gov/stationhome.html?id=8776139). kfc, kJ2008, kW2014, kVD2019 and kR2001 are the gas transfer velocities (kv) acquired using an in situ floating chambers and four empirical equations proposed by Jiang et al. 2008, Wanninkhof 2014, Van Dam et al. 2019 and Raymond and Cole 2001, respectively. Correspondingly, the sea-air fluxes were presented as Ffc, FJ2008, FW2014, FVD2019 and FR2001 (Fig. S1). The latter four were grouped as Fmodel. Based on the relationship between Fmodel and Ffc under conditions of temperature and wind speed, we unified CH4 fluxes using empirical equations R2001, W2014 and J2008 (Supporting Information, SI1).
Methane flux at the sediment–water interface
Diffusive CH4 flux at the sediment–water interface was calculated using Fick's first law(Berner 1980).
\({J}_{s}\) is the sediment–water interface diffusive CH4 flux; \(\varnothing\) is sediment porosity measured from dried sediment weight at 80°C (Coffin et al. 2014, 2013; Morin and Morse 1999); \({D}_{0}\) is the diffusion coefficient for CH4 in water (1.5 × 10–5 cm2 s−1) (Broecker and Peng 1974); \(\theta\) is tortuosity, calculated using \({\theta }^{2}=1-ln\left({\varnothing }^{2}\right)\) (Boudreau 1996); and \(\frac{dc}{dz}\) is the CH4 gradient in porewater. The gradient between porewater with the maximum dissolved CH4 concentration and the first layer of porewater was applied to represent sediment–water CH4 fluxes.
The benthic chamber CH4 flux at the sediment–water interface was calculated through the variation in CH4 concentration in the overlying water before and after 24 h in situ observation.
where \({F}_{inc}\) is sediment–water flux (µmol/m2 h or µmol/m2 d); S is variation in CH4 concentration in the overlying sediment chamber water before and after observation (nmol/L); \({V}_{chamber}\) is the volume of overlying water in the chamber (m3); \({A}_{chamber}\) is the area of the chamber (m2); and t is time coefficient (converted from minutes to hours or days).
Statistical analysis
Statistical analyses were performed using R software (version 3.6.1). Dissolved CH4 concentrations and sea-air CH4 flux were logarithmically processed before analysis to acquire normal distributions. Linear models were built to determine factors that influence CH4 concentrations. Akaike information criterion correlation (AICc) was used in model selection. The variance information factor (VIF) was used to check collinearity. The Shapiro–Wilk W test at the p < 0.05 level of significance was used to assess the normality of residuals in the model. Homoscedastic was tested by the Brevsch-Pagan test with p > 0.05. One-way ANOVA was applied for variation comparison of CH4 concentrations and sea-air fluxes across seasons. The t test was performed to check differences in estimated CH4 fluxes across the methods applied.
Results
Dissolved methane and other parameters in the water column
Dissolved methane
Dissolved CH4 concentrations over the seagrass meadow were 62.3 ± 49.9 nmol/L (11.6–258.2 nmol/L, median: 52.5 nmol/L). During bimonthly observations from July 2020 to May 2021, CH4 concentrations were highest in early autumn, decreased dramatically to their lowest values in winter, and then increased in spring (Fig. 2a). Overall, concentrations in winter (42.6 ± 40.2 nmol/L) were lower than those in other seasons, although there was no statistical significance (p > 0.05). Dissolved CH4 concentrations in autumn and summer (70.9 ± 79.2 nmol/L) and 67.9 ± 48.8 nmol/L) were relatively higher, and those in spring (61.1 ± 34.2 nmol/L) were in the middle.
During the August 2019 diurnal observation, the dissolved CH4 concentration decreased and reached its lowest value (28.3 nmol/L) before sunset, while the overnight CH4 concentration increased and reached its highest value (79.1 nmol/L) before sunrise (Fig. 3). The δ13C values of dissolved CH4 from seagrass meadows were −57.8‰ to −57.3‰ during the diel observation in August 2019 and − 58.7‰ in July 2020, indicating a biogenic origin (Kirschke et al. 2013; Schroll et al. 2020; Whiticar et al. 1986).
Water incubation experiments showed that variations in dissolved CH4 concentration in the water were no more than 20% in 24 h (Fig. S6), indicating that bacterial consumption and production in the water column were limited. In most cases, a decrease in dissolved CH4 concentration occurred in the first hour after the start of the incubation experiments, and then the CH4 concentration slightly increased (Fig. S6).
Other parameters and their relationship to dissolved methane
The seasonal water temperature during the study period was 14.5–35.1 °C and was highest in summer (Fig. S4). Salinity ranged from 19.9 to 47.1 psu, with an average of 34.9 ± 7.0 psu. Although annual variances existed, pH (7.6–8.9, 8.2 ± 0.2) was lower in winter and spring and higher in summer, and DIC (0.84–2.88 mmol C/L, 1.65 ± 0.37 mmol C/L) varied oppositely (R2 = 0.67, p = 6.8 × 10–13).
Statistical analyses showed the long-term variation in CH4 concentrations during the study period can partially be explained by either of the two statistical models: (1) the model with pH, DO and temperature (∆[CH4] = 18.4 + 0.009 × DO%—2.1 × pH + 0.077*temperature, adjusted R2: 0.23, p = 0.0022), or (2) the model with DIC and temperature (∆[CH4] = 0.35 + 1.14 × DIC + 0.066 × temperature, adjusted R2: 0.19, p = 0.0037). In both models, temperature was positively related to dissolved CH4 concentration (p < 0.0071). DIC was positively related to the dissolved CH4 concentration (p = 0.0012), while pH had the opposite relationship (p = 0.00028). DIC and pH could not exist in the same model because of the collinearity (R2 = 0.67, p = 6.8 × 10–13).
Dissolved CH4 concentrations in diel observations were inversely related to DO (R2 = 0.39, p = 0.136) and Chl-a concentrations (R2 = 0.13, p = 0.425) and positively correlated with DIC (R2 = 0.54, p = 0.061, Fig. S7). When comparing CH4 concentrations with DO and Chl-a concentrations four hours later, the correlation between CH4 and DO (R2 = 0.75, p = 0.011) and Chl-a concentrations (R2 = 0.91, p = 0.00087) increased. A positive relationship between dissolved CH4 concentration and DIC was similar to the long-term observations. In contrast to the seasonal data, there was an opposite relationship between CH4 concentration and water temperature (R2 = 0.73, p = 0.014), indicating that temperature was not the major factor that led to the increase in CH4 concentrations overnight in summer.
Methane transport at the sea-air interface
Sea-air CH4 flux acquired using floating chambers
Variations in CH4 concentrations in floating chambers reflected in situ CH4 transport from water to the atmosphere. Concentrations in floating chambers increased linearly over one hour, except in July 2020, suggesting that diffusion was a primary pathway of CH4 emissions at the sea-air interface (Fig. S5). The most significant increase occurred in September 2020, indicating the largest transport of diffusive CH4 from water to the atmosphere in early autumn (Fig. 4). The diffusive CH4 fluxes in the chamber were positively related to the dissolved CH4 concentration in the water (R2 = 0.83, p = 0.0007). Gas transfer velocities derived from floating chamber fluxes (kfc) ranged from 5.1 to 28.0 cm/h, revealing considerable temporal discrepancies. When applying kfc to all sampling sites, the derived sea-air fluxes (Ffc) were 217.8 ± 181.9 µmol/m2 d (12.2–788.9 µmol/m2 d) (Fig. S1). During diurnal observations, hourly CH4 flux patterns varied similarly to CH4 concentrations (R2 = 0.94, p = 0.00027), decreasing before sunset and rising overnight (Fig. 3).
In July 2020, there was a dramatic increase in one chamber 30 min after the start of the experiment, which could be attributed to the input of ebullitive CH4 (Figs. 4, S5). This ebullition flux was 16.8 times the diffusive flux in chambers simultaneously and 2.2 times the highest diffusive flux observed and was the only ebullitive event caught during the study period.
Based on the relationship between δ13C-CH4 and 1/CH4 (Fig. 4) obtained by conducting keeling plots (Garcias-Bonet and Duarte 2017), the δ13C of dissolved CH4 entering the chamber in August 2019 and July 2020 was − 57.7‰, consistent with dissolved CH4 in water. The δ13C of ebullitive CH4 was − 65.8‰, indicating the stable carbon isotopic signature of CH4 input from deeper sediment directly.
Diffusive sea-air CH4 flux acquired using empirical equations
In addition to using kfc, sea-air diffusive CH4 fluxes were calculated using four kmodels based on four empirical equations (Fig. S1). Although CH4 fluxes obtained using different gas transfer velocities were somewhat different, they varied following a similar pattern, being lowest in winter and higher in summer and autumn. Continuous bimonth observations from July 2020 to May 2021 showed that fluxes were highest in summer and early autumn, decreased to the lowest values in winter and increased during spring. These results revealed that more CH4 was released from water to the atmosphere in summer and autumn than in winter.
We unified the sea-air diffusive CH4 fluxes over seagrass meadows based on the relationship between Ffc and Fmodel (Yu et al. 2023; Supporting Information SI 1). The average of the unified CH4 fluxes (221.1 ± 190.7 µmol/m2 d) was similar to the average of all Fmodel (207.1 ± 163.6 µmol/m2 d) (t test, p = 0.68). Long-term variations in sea-air diffusive CH4 fluxes discussed in the following sections will use unified CH4 data.
Generally, the sea-air diffusive CH4 flux was significantly lower in winter (72.4 ± 75.6 µmol/m2 d) than in other seasons (p < 0.017). CH4 fluxes were highest in summer (277.0 ± 178.3 µmol/m2 d), but they were not significantly different from those in spring and autumn (203.8 ± 180.5 µmol/m2 d and 227.9 ± 187.8 µmol/m2 d, respectively). From July 2020 to May 2021, CH4 fluxes were highest in summer and early autumn and decreased to their lowest values in winter, followed by increases during spring. This revealed that more CH4 was released from water to the atmosphere in summer and autumn than in winter (Fig. 2).
Methane transport at the sediment–water interface
Porewater CH4, DIC and sulfide concentration profiles in seagrass sediment cores showed significant temporal variations (Fig. S8), indicating a dynamic biogeochemical process in the seagrass surface sediment (Barber and Carlson 1993; Lee and Dunton 2000). Porewater CH4 concentrations in the surface sediment (< 25 cm) were highest in September 2020 (831.4 ± 961.2 nmol/L), lowest in August 2019 (37.1 ± 13.3 nmol/L), and moderate in May 2019 (107.4 ± 105.1 nmol/L), which positively corresponded to sulfide concentrations (557.1 ± 685.8 µmol/L, 35.8 ± 33.6 µmol/L, 55.0 ± 53.5 µmol/L, respectively; R2 = 0.998, p = 0.031). This result indicated a synchronized variation in porewater CH4 and sulfide affected by photosynthetically sourced oxygen in the sediment.
The sediment–water CH4 fluxes estimated using Fick's first law of diffusion at the sediment–water interface were 0.064 µmol/m2 d, 0.008–0.023 µmol/m2 d, and 0.613 µmol/m2 d in May 2019, August 2019 and September 2020, respectively. The values were lower than the fluxes calculated using the variation in CH4 concentrations in the overlying water of sediment benthic chambers before and after the 24-h observation in August 2019 (37.7–47.0 µmol/m2 d). This result suggested that diffusion was not a primary mechanism for CH4 exchange from sediment to water.
Discussion
Impact of seagrass photosynthesis and respiration on diel methane emissions
Photosynthetic oxygen is essential to seagrass not only due to seagrass metabolism but also because oxygen can be delivered to seagrass rhizome sediment to inhibit toxic compounds such as H2S (Borum et al. 2007; Eldridge and Morse 2000). Photosynthetic oxygen can also oxidize sediment CH4 and impede methanogenesis in sediment (Alongi et al. 2008; Oremland 1975; Oremland and Taylor 1977). During diurnal observations (Fig. 3), DO was highest in the afternoon (139.1%) and decreased to its lowest level before sunset (22.4%), which could be explained by photosynthesis in the seagrass ecosystem in the daytime and oxygen consumption by dominant respiration overnight (Borum et al. 2007; Marbà et al. 2006). The lowest DIC and highest pH before sunset and increase in DIC and decrease in pH overnight suggested that they were related to CO2 in the photosynthetic and respiration processes of seagrass ecosystems (CO2 + H2O ↔ HCO3− + H+) (Marbà et al. 2006). Similar diel curves of DIC and DO in seagrass meadows were reported at Laguna Madre in September 1996 when water exchange was weak (Ziegler and Benner 1998). Moreover, depression in porewater sulfide in seagrass sediment in Corpus Christi Bay and Lower Laguna Madre during mid-day was demonstrated to be attributed to photosynthetic oxygen (Lee and Dunton 2000).
Confirming the influence of photosynthetic oxygen on CH4 emissions, the dissolved CH4 concentration was inverse to DO, showing decreases before sunset and increasing overnight to a sunrise peak in concentration. The highest δ13C-CH4 (− 57.3‰) appeared when the dissolved CH4 concentration was lowest, and lower δ13C-CH4 (− 57.7‰) was accompanied by higher CH4 concentrations, implying methanotrophic oxidation (Garcias-Bonet and Duarte 2017). The δ13C of diffusive CH4 entering the floating chambers (− 57.7‰) was similar to δ13C-CH4 in the water but approximately 8‰ larger than ebullitive CH4 (−65.8‰). This 13C enrichment is a further indication of CH4 oxidation in the sediment. In comparison, the δ13C of dissolved CH4 at the site along the channel (− 59.5‰ and − 59.8‰ in August 2019 and July 2020, respectively), adjacent to the seagrass meadow, was approximately 2‰ lower, suggesting more CH4 oxidation over living seagrass meadows than in the channel water.
Although DO concentrations provided the capacity to oxidize all CH4, our water incubation experiments showed that less than 10% of CH4 was decomposed in 24 h. This was much smaller than the difference in diurnal variations (28.3–79.1 nmol/L), which was up to 2.8 times. Therefore, the major change did not occur in the water column but should be associated with sediment. The lowest CH4 concentration appeared four hours after the DO peak, and overall CH4 concentrations were more significantly related to DO concentrations four hours earlier (R2 = 0.75, p = 0.0113). This time lag further implied that CH4 oxidation occurred in sediment, given that photosynthetic oxygen passively diffused over a few hours through seagrass lacunae, reaching rhizome sediment from leaves to roots (Borum et al. 2007). Similar to this study, lag periods between methanogenesis and gaseous composition were found from the diurnal variation in photosynthetic oxygen and CH4 in sediment bubbles and gases in the rhizome of the seagrass T. testudinum in Florida Bay (Oremland and Taylor 1977).
Diel experiments exhibited an overnight increase in CH4 concentrations associated with the respiration of the seagrass ecosystem. This was reflected in a decrease in DO and an increase in DIC (Fig. 3). Photosynthetic oxygen decreased overnight, leading to less oxidation in rhizome sediment. Consequently, there was more potential for CH4 production and emissions. Similarly, 44-h observations in Swan Bay, Victoria, Australia, also showed that CH4 increased immediately after sunset, although it was influenced by tidal processes synchronically (Ollivier et al. 2022).
Dissolved CH4 concentrations increased at an average rate of 5.15 nmol/L h overnight. Assuming the average water depth was 0.65 m (Fig. S3), the increase in CH4 would be 3.3 µmol/m2 h. Considering that CH4 was released at a rate of 7.7 µmol/m2 h to the atmosphere (Fig. 5), the CH4 input to the water should be 11.0 µmol/m2 h at night (Supporting information, SI2). Applying the same method, the CH4 daytime input should be 6.2 µmol/m2 h. Hence, approximately 40% less CH4 entered the water column during the daytime than overnight due to photosynthetic oxygen.
Diel CH4 cycling in the seagrass meadow in August 2019 (summer). Left: daytime (13 h); Right: nighttime (11 h). Δ[CH4] represents average variation rate of dissolved CH4 concentrations in water column (nmol/L hr: hourly variation in daytime and nighttime, and nmol/L d: daily variation); -: decrease; + : increase. Variation in the CH4 concentration was calculated from diurnal observations. Diffusive CH4 fluxes at the water-atmosphere interface were averages of diffusive fluxes in daytime and nighttime. Diffusive CH4 fluxes at the sediment–water interface were calculated using Fick’s first law. Vertical arrows represent diffusive transport from water to atmosphere and from sediment to water, respectively. Total benthic CH4 fluxes at sediment–water interface were calculated based on the diel CH4 variations, including all kinds of transport (plant-mediation, diffusive and ebullitive), indicating by horizontal arrows
Long-term variations showed a significant relationship between DIC and pH, indicating that DIC variations might be related to CO2 metabolism in seagrass meadows. Similar to diurnal variations, DIC was positively related to the dissolved CH4 concentration, while pH had the opposite relationship.
Sediment–water methane transport
The diffusive CH4 fluxes at the sediment–water interface calculated using Fick’s first law ranged from 0.008 to 0.613 µmol/m2 d, much lower than the sediment–water emissions obtained using benthic chambers (37.7–47.0 µmol/m2 d in August 2019) and long-term water–air CH4 fluxes (and 12.3–816.2 µmol/m2 d). This result indicated that diffusion was not a major mechanism for emissions of CH4 from seagrass sediment. Our findings were similar to the observations in South Bay, Virginia that diffusive fluxes were negligible in comparison with CH4 emissions captured in water (20.3–314.4 µmol/m2 d) (Oreska et al. 2020).
Porewater CH4 concentrations in this study (15.8 ± 4.0–831.4 ± 961.2 nmol/L) were at a similar level (nmol/L) as in other coastal seagrass sediments, such as Zostera marina in South Bay, Virginia, USA (5–300 ± 250 nmol/L) (Oreska et al. 2020), Posidonia oceanica in Bay of Fetovaia, Mediterranean Sea (37 ± 7 nmol/L) (Schorn et al. 2022), Thalassia testudinum in Florida Bay, USA (25.8 ± 11.1–3380 ± 530 nmol/L) (Barber and Carlson 1993)and Posidonia oceanica in Portlligat Bay, Mediterranean Sea (400–4600 nmol/L) (Yau et al. 2023). Sediment-air diffusive flux in Portlligat Bay calculated using the Fick’s first law (0.25 ± 0.1, 0.1–0.4 µmol/m2 d) was enough to support the transport of CH4 from water to the atmosphere (0.12 ± 0.10 µmol/m2 d) (Yau et al. 2023). Since porewater CH4 concentrations in other regions were all no larger than in Portlligat Bay, diffusive fluxes based on the porewater CH4 gradients would in the same level or less. These diffusive fluxes were much less than CH4 emissions from sediment and surface water in most of other reported seagrass meadows (Tables S1 and S2). It suggested diffusion was not a primary export mechanism from sediment in many seagrass meadows. This contrasts with freshwater ecosystems and some tropical mangroves, where porewater CH4 concentrations (mmol/L) can be up to six orders of magnitude higher, and diffusive transport plays a significant role in CH4 emissions (Chuang et al. 2016; Huttunen et al. 2006).
In addition to diffusive transport, other transport approaches include the advection of fluid, ebullition of bubbles and plant mediation. Advective transport of porewater CH4 reported in the seagrass meadow at Ria Formosa Lagoon in southern Portugal was prompted by tidal immersion (Bahlmann et al. 2015). Since the Upper Laguna Madre is relatively isolated and has weak water exchange with the Gulf of Mexico, the tidal impact on CH4 emissions is negligible.
Ebullitive emissions of CH4 during July 2020 were approximately 17-fold the synchronic diffusive flux (Fig. 4). The δ13C of ebullitive CH4 (− 65.8‰) was much more negative than δ13C-CH4 in water, indicating that it was a direct sediment source. Bubbles containing CH4 have been observed within interstices of Thalassia testudinum seagrass sediment in the Florida Keys and Bahamas (Oremland and Taylor 1977). While bubbles are a possible CH4 transport process from sediment to the water column, this was observed only once in a chamber experiment; however, ebullitive CH4 transport from seagrass meadows still needs further investigation.
Plant-mediated transport of CH4 has been reported in emergent and submerged macrophytes (Chanton et al. 1992; Fonseca et al. 2017; Zhang et al. 2019). CH4 has been found in the seagrass Thalassia testudinum rhizome from an inward diffusion of CH4 in sediment (Oremland 1975; Oremland and Taylor 1977). Research on seagrass meadows of Posidonia oceanica in the Mediterranean Sea also proposed possible plant-mediated transport of CH4 (Schorn et al. 2022). In this study, considering diffusion to be minor and ebullition observed once, plant mediation appears to be the dominant process for CH4 transport from sediment to water in this study. Sediment–water fluxes calculated based on diel CH4 cycling showed continuous CH4 input from sediment during daytime and overnight (Fig. 5). This observation supported the interpretation of continuous transport via the seagrass internal lacunae system. Although sediment–water fluxes acquired using benthic chambers were lower than the calculated fluxes due to the limitation of benthic chambers (Oreska et al. 2020), such as lowering the CH4 concentration gradient between overlying water and sediment porewater due to lack of hydrodynamic effect, the data also suggested that the release of CH4 from sediment was much larger than the value expected from diffusion. This further implied the potential of plant mediation of CH4 emissions from sediment.
Other CH4 sources, such as riverine input and submarine groundwater discharge, contributed minorly to the upper Laguna Madre (Murgulet et al. 2022).
Sea-air methane transport
The CH4 fluxes at the sea-air interface of seagrass meadows were 12.3 –816.2 µmol/m2 d (221.1 ± 190.7 µmol/m2 d, median: 163.7 µmol/m2 d), over the range reported for other seagrass meadows (0.11 ± 0.14–135.3 ± 133.3 µmol/m2 d) (Table S1). Both dissolved CH4 concentrations and gas transfer velocity can influence CH4 emissions from water to the atmosphere.
Comparing the mean values of sea-air CH4 fluxes and dissolved CH4 concentrations, the current global dataset showed that dissolved CH4 concentrations were significantly positively related to CH4 fluxes (R2 = 0.695, p = 2 × 10–4), indicating an important role of dissolved CH4 concentrations in CH4 transport to the atmosphere, although the fluxes were acquired using different methods. Dissolved CH4 concentrations in this study (11.6–258.2 nmol/L, 62.2 ± 49.9 nmol/L) were larger than those in Chilika Lagoon, India, Red Sea, East Harbor, Massachusetts, USA and Portlligat Bay, Mediterranean Sea but lower than those in Pleasant Bay, Massachusetts (Table S1). The Upper Laguna Madre has quite limited water exchange with the outside open water, minor freshwater input and a relatively long residence time (Bugica et al. 2020). The topography of this system lowers the potential for dissolved CH4 to be exchanged or diluted except when transported to the atmosphere. Note that our study showed an accumulation of CH4 in the water after a diel cycle in summer. Concentrations of CH4 were comparable to those at mangrove creeks (3.1–219.2 nmol/L, 57.8 ± 44.2 nmol/L) located north of Upper Lagua Madre, where water exchange by tidal processes could reduce CH4 emissions (Yu et al. 2023), despite carbon sequences in seagrass being less than the mangrove sediments. This result indicated that restricted water exchange with a longer residence time caused increased dissolved CH4 concentrations. Moreover, different from the findings in the Mediterranean Sea (Yau et al. 2023), the consumption of CH4 in the water column was less than 20%. This was probably another reason for the relatively high level of dissolved CH4.
Gas transfer velocity is another pertinent issue controlling sea-air flux at the sea-air interface. Comparing fluxes acquired using different empirical equations, FW2014 was smallest (130.7 ± 107.4 µmol/m2 d), which was half of the largest estimated flux FR2001 (277.8 ± 228.3 µmol/m2 d). Except for FW2014, there was no significant difference between other equation-derived fluxes. The selection of empirical equations can largely influence the estimation of sea-air fluxes. The mean value of Ffc (217.8 ± 181.9 µmol/m2 d) that was applied to acquire in situ gas transfer velocities was close to that of FVD2019 (211.6 ± 173.9 µmol/m2 d). This is reasonable since the empirical equation for FVD2019 was set up for gas exchange at an estuary (Van Dam et al. 2019). However, even the minimum FW2014 in our study (17.0–557.1 µmol/m2 d, 130.7 ± 107.4 µmol/m2 d, median: 103.2 µmol/m2 d) was also slightly over the previously reported range, indicating a larger gas exchange through our study area. This observation differed from the experiment using the 3He/SF6 tracer in Florida Bay, USA, which proposed a smaller gas exchange coefficient (k600 = 0.143 µ2) than equation W2014 (k660 = 0.251 µ2) due to the wind fetch being limited by seagrasses (Dobashi and Ho 2023). The morphologic difference between the dominant seagrass species was a potential reason since the canopy height of T. testudinum (up to 35 cm), which is dominant in Florida Bay, is larger than that of H. wrightii (20.9 ± 8.4–23.8 ± 7.4 cm) in this study area (Capistrant-Fossa et al. 2023; Dunton and Reyna 2019). The parameterization of water–air gas exchange is challenging in estuaries, and the application of empirical equations should be cautious.
Factors influencing long-term methane emissions
The highest CH4 concentrations appeared in early autumn, with the lowest in winter, an observation concurrent with the seasonal pattern of seagrass biomass. An investigation at Laguna Madre from 1989 to 1992 showed a peak growth of leaves of Halodule wrightii between early May and mid-June and a second elevated growth in later summer, as well as a rapid drop in autumn and winter (Dunton 1994). Applying biomass data of Halodule wrightii in the Upper Laguna Madre from 1989 to 1997 (Burd and Dunton 2001), seasonal variations in CH4 concentrations were positively related to seagrass biomass (Fig. 6). This relationship was not statistically significant (below-ground: R2 = 0.76, p = 0.064), probably because the seagrass biomass data were not synchronous with CH4 concentrations. The impact of seagrass meadow presence on CH4 emissions has also been reported in other seagrass regions (Alongi et al. 2008; Banerjee et al. 2018; Oreska et al. 2020). Considering our observations of seagrass photosynthesis and respiration on CH4 emissions, it is reasonable to recognize seagrass growth as an important component of CH4 cycling.
Seasonal variations in seagrass biomass and dissolved CH4 concentrations (above: aboveground biomass; below: belowground biomass). Raw biomass data of seagrass Halodule Wrightii in Upper Laguna Madre from May 1989 to October 1997 were collected from a paper published in 2001 (Burd and Dunton 2001) by digitalizing the figures in the paper using WebPlotDigitizer. Seasonal average aboveground and belowground biomass data were calculated based on the raw data
Seasonal dynamics in the abundance and cycling of methanogens, controlled by substrate and nutrients, is another explanation for the observed variations. Methanogenic archaeal taxa have been found to be abundant in spring in temperate seagrass in the Bohai Sea, China, because temperature could limit the efficiency of methanogenesis of the genus Methanolobus (Liu et al. 2021). Our study also showed that long-term variations in CH4 concentrations were positively related to water temperature (p = 0.00727). A recent study discovered that CH4 from seagrass P. oceanica in the Mediterranean Sea was generated from methylated compounds produced by seagrass rather than buried organic carbon fermentation (Schorn et al. 2022). Long-term CH4 emissions can also be influenced by plant presence considering that growth in seagrass meadows is seasonally dependent. Since both seagrass growth and microbial activities are influenced by temperature, this study cannot distinguish which factor contributed more to CH4 emissions. Moreover, salinities in the majority of the studied seagrass meadows were larger than 30 psu, and nearly half were over 35 psu (Fig. S9). Higher CH4 emissions have been observed from seagrass meadows with higher salinity (Fig. S9). CH4 emissions from seagrass meadows would be ubiquitous regardless of sulfate in sediment, further indicating the coexistence of sulfate reduction and methanogenesis (Oremland et al. 1982; Schorn et al. 2022).
Conclusion
Both dissolved CH4 concentrations and sea-air CH4 fluxes decreased in the daytime and increased overnight. The relationship between CH4 concentrations and DO, DIC and pH indicated the impact of photosynthesis and respiration of seagrass meadows on CH4 emissions. Photosynthetic oxygen was estimated to reduce approximately 40% of CH4 emissions in the daytime in the diurnal experiments. Dissolved CH4 concentrations and sea-air CH4 fluxes were lowest in winters during the study period. Temperature, DIC or pH could partly explain the seasonal variations, which were probably related to seagrass biomass and methanogenesis. Dissolved CH4 concentrations in this study (11.6–258.2 nmol/L, 62.2 ± 49.9 nmol/L) were higher than those in many other seagrass meadows. It could be explained by less exchange or dilution of dissolved CH4, except for release to the atmosphere, due to this being a closed coastal system with minimum freshwater or groundwater inflow and a longer residence time. The comparison with CH4 emissions from nearby mangrove creeks implied that water exchange with open water, such as tidal processes, could help reduce CH4 release from coastal vegetated areas.
Diffusive CH4 fluxes at the sediment–water interface calculated using Fick’s first law were 0.008–0.613 µmol/m2 d, lower than the water‒air CH4 fluxes of 12.3–816.2 µmol/m2 d and the sediment–water CH4 fluxes acquired using benthic chambers (e.g., 37.7–47.0 µmol/m2 d in August 2019). This result suggested that diffusion was not the primary process for the transport of CH4 in the sediment–water inference. Porewater CH4 concentrations in surface sediment were positively related to sulfide concentrations, further proving the influence of photosynthetic oxygen on CH4 production in sediment. It also suggested that plant mediation was a dominant control of CH4 from sediment to the water column, particularly considering the few chances to catch ebullitive CH4 and minor tidal influence.
Data availability
Data for this research will be freely available upon request, as well as retrieved from the Texas A&M University-Corpus Christi Repository (https://tamucc-ir.tdl.org/items/5520da4b-e593-4202-8478-5ed1dff8629f).
References
Al-Haj AN, Fulweiler RW (2020) A synthesis of methane emissions from shallow vegetated coastal ecosystems. Glob Chang Biol 26(5):2988–3005. https://doi.org/10.1111/gcb.15046
Alongi DM, Trott LA, Undu MC, Tirendi F (2008) Benthic microbial metabolism in seagrass meadows along a carbonate gradient in Sulawesi, Indonesia. Aquat Microb Ecol 51(2):141–152
Bahlmann E, Weinberg I, Lavrič J, Eckhardt T, Michaelis W, Santos R, Seifert R (2015) Tidal controls on trace gas dynamics in a seagrass meadow of the Ria Formosa lagoon (southern Portugal). Biogeosciences 12(6):1683–1696
Banerjee K, Paneerselvam A, Ramachandran P, Ganguly D, Singh G, Ramesh R (2018) Seagrass and macrophyte mediated CO2 and CH4 dynamics in shallow coastal waters. PLoS ONE 13(10):e0203922. https://doi.org/10.1371/journal.pone.0203922
Barber TR, Carlson PR (1993) Effects of seagrass die-off on benthic fluxes and porewater concentrations of ∑CO2, ∑H2S, and CH4 in florida bay sediments. In: Oremland RS (ed) Biogeochemistry of global change. Springer, Boston, pp 530–550
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton University Press, Princeton
Borum J, Sand-Jensen K, Binzer T, Pedersen O, Greve TM (2007) Oxygen movement in seagrasses. Seagrasses: biology, ecologyand conservation. Springer, Berlin, pp 255–270
Boudreau BP (1996) The diffusive tortuosity of fine-grained unlithified sediments. Geochim Cosmochim Acta 60(16):3139–3142. https://doi.org/10.1016/0016-7037(96)00158-5
Broecker WS, Peng T-H (1974) Gas exchange rates between air and sea. Tellus 26(1–2):21–35
Bugica K, Sterba-Boatwright B, Wetz MS (2020) Water quality trends in Texas estuaries. Mar Pollut Bull 152:110903. https://doi.org/10.1016/j.marpolbul.2020.110903
Burd AB, Dunton KH (2001) Field verification of a light-driven model of biomass changes in the seagrass Halodule wrightii. Mar Ecol Prog Ser 209:85–98
Burkholz C, Garcias-Bonet N, Duarte CM (2020) Warming enhances carbon dioxide and methane fluxes from Red Sea seagrass (Halophila stipulacea) sediments. Biogeosciences 17(7):1717–1730. https://doi.org/10.5194/bg-17-1717-2020
Capistrant-Fossa KA, Tapia SA, Dunton KH (2023) A long-term seagrass monitoring program for Corpus Christi Bay, Upper Laguna Madre, and Baffin Bay
Chanton JP, Martens CS, Kelley CA, Crill PM, Showers WJ (1992) Methane transport mechanisms and isotopic fractionation in emergent macrophytes of an Alaskan tundra lake. J Geophys Res Atmos 97(D15):16681–16688. https://doi.org/10.1029/90jd01542
Chuang PC, Young MB, Dale AW, Miller LG, Herrera-Silveira JA, Paytan A (2016) Methane and sulfate dynamics in sediments from mangrove-dominated tropical coastal lagoons, Yucatán, Mexico. Biogeosciences 13(10):2981–3001. https://doi.org/10.5194/bg-13-2981-2016
Cira EK, Palmer TA, Wetz MS (2021) Phytoplankton dynamics in a low-inflow estuary (Baffin Bay, TX) during drought and high-rainfall conditions associated with an El Niño Event. Estuaries Coasts 44(7):1752–1764. https://doi.org/10.1007/s12237-021-00904-7
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14(3):454–458. https://doi.org/10.4319/lo.1969.14.3.0454
Coffin RB, Hamdan L, Smith J, Rose P, Plummer R, Yoza B, Pecher I, Montgomery M (2014) Contribution of vertical methane flux to shallow sediment carbon pools across Porangahau ridge, New Zealand. Energies 7(8):5332–5356. https://doi.org/10.3390/en7085332
Coffin RB, Mueller J (2020) Carbon isotope forensics for methane source identification. Remediat J 30(2):55–62
Coffin RB, Smith JP, Plummer RE, Yoza B, Larsen RK, Millholland LC, Montgomery MT (2013) Spatial variation in shallow sediment methane sources and cycling on the Alaskan Beaufort Sea Shelf/Slope. Mar Pet Geol 45:186–197. https://doi.org/10.1016/j.marpetgeo.2013.05.002
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements, North Pacific Marine Science Organization
Dobashi R, Ho DT (2023) Air–sea gas exchange in a seagrass ecosystem–results from a 3He∕SF6 tracer release experiment. Biogeosciences 20(6):1075–1087. https://doi.org/10.5194/bg-20-1075-2023
Dunton KH (1994) Seasonal growth and biomass of the subtropical seagrass Halodule wrightii in relation to continuous measurements of underwater irradiance. Mar Biol 120(3):479–489. https://doi.org/10.1007/BF00680223
Dunton KH, Reyna NE (2019) A long-term seagrass monitoring program for Corpus Christi Bay and Upper Laguna Madre, Program CBBaE, Coastal Bend Bays and Estuaries Program
Eldridge PM, Morse JW (2000) A diagenetic model for sediment–seagrass interactions. Mar Chem 70(1):89–103. https://doi.org/10.1016/S0304-4203(00)00018-9
Fonseca ALD, Marinho CC, Esteves FD (2017) Floating aquatic macrophytes decrease the methane concentration in thewater column of a tropical coastal lagoon: implications for methane oxidation and emission. Braz Arch Biol Technol 60:16. https://doi.org/10.1590/1678-4324-2017160381
Garcias-Bonet N, Duarte CM (2017) Methane production by seagrass ecosystems in the Red Sea. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00340
Huttunen JT, Väisänen TS, Hellsten SK, Martikainen PJ (2006) Methane fluxes at the sediment-water interface in some boreal lakes and reservoirs. Boreal Environ Res 11(1):27–34
Jiang L-Q, Cai W-J, Wang Y (2008) A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnol Oceanogr 53(6):2603–2615. https://doi.org/10.4319/lo.2008.53.6.2603
Kelley CA, Chanton JP, Bebout BM (2015) Rates and pathways of methanogenesis in hypersaline environments as determined by 13C-labeling. Biogeochemistry 126(3):329–341. https://doi.org/10.1007/s10533-015-0161-9
King GM (1984) Utilization of hydrogen, acetate, and “noncompetitive”; substrates by methanogenic bacteria in marine sediments. Geomicrobiol J 3(4):275–306. https://doi.org/10.1080/01490458409377807
Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L, Cameron-Smith P, Castaldi S, Chevallier F, Feng L, Fraser A, Heimann M, Hodson EL, Houweling S, Josse B, Fraser PJ, Krummel PB, Lamarque J-F, Langenfelds RL, Le Quéré C, Naik V, O’Doherty S, Palmer PI, Pison I, Plummer D, Poulter B, Prinn RG, Rigby M, Ringeval B, Santini M, Schmidt M, Shindell DT, Simpson IJ, Spahni R, Steele LP, Strode SA, Sudo K, Szopa S, van der Werf GR, Voulgarakis A, van Weele M, Weiss RF, Williams JE, Zeng G (2013) Three decades of global methane sources and sinks. Nat Geosci 6(10):813–823. https://doi.org/10.1038/ngeo1955
Kruse CJ, Ellis D, Protopapas A, Norboge N, Glover B (2016) Texas Gulf intracoastal waterway master plan: technical report, College Station, Texas
Lee K-S, Dunton KH (2000) Diurnal changes in pore water sulfide concentrations in the seagrass Thalassia testudinum beds: the effects of seagrasses on sulfide dynamics. J Exp Mar Biol Ecol 255(2):201–214. https://doi.org/10.1016/S0022-0981(00)00300-2
Liu PY, Zhang HK, Song ZL, Huang YY, Hu XK (2021) Seasonal dynamics of bathyarchaeota-dominated benthic archaeal communities associated with seagrass (Zostera japonica) meadows. J Mar Sci Eng 9(11):15. https://doi.org/10.3390/jmse9111304
Lyimo LD, Gullström M, Lyimo TJ, Deyanova D, Dahl M, Hamisi MI, Björk M (2018) Shading and simulated grazing increase the sulphide pool and methane emission in a tropical seagrass meadow. Mar Pollut Bull 134:89–93. https://doi.org/10.1016/j.marpolbul.2017.09.005
Magen C, Lapham LL, Pohlman JW, Marshall K, Bosman S, Casso M, Chanton JP (2014) A simple headspace equilibration method for measuring dissolved methane. Limnol Oceanogr Methods 12:637–650. https://doi.org/10.4319/lom.2014.12.637
Marbà N, Holmer M, Gacia E, Barron C (2006) Seagrass beds and coastal biogeochemistry. Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 135–157
Morin J, Morse JW (1999) Ammonium release from resuspended sediments in the Laguna Madre estuary. Mar Chem 65(1):97–110. https://doi.org/10.1016/S0304-4203(99)00013-4
Murgulet D, Lopez CV, Douglas AR (2022) Radioactive and stable isotopes reveal variations in nearshore submarine groundwater discharge composition and magnitude across low inflow northwestern Gulf of Mexico estuaries. Sci Total Environ 823:153814. https://doi.org/10.1016/j.scitotenv.2022.153814
Ollivier QR, Maher DT, Pitfield C, Macreadie PI (2022) Net drawdown of greenhouse gases (CO2, CH4 and N2O) by a temperate Australian seagrass meadow. Estuaries Coasts 45(7):2026–2039. https://doi.org/10.1007/s12237-022-01068-8
Oremland RS (1975) Methane Production in shallow-water, tropical marine sediments. Appl Microbiol 30(4):602–608
Oremland RS, Marsh LM, Polcin S (1982) Methane production and simultaneous sulphate reduction in anoxic, salt marsh sediments. Nature 296(5853):143–145. https://doi.org/10.1038/296143a0
Oremland RS, Polcin S (1982) Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol 44(6):1270–1276. https://doi.org/10.1128/aem.44.6.1270-1276.1982
Oremland RS, Taylor BF (1977) Diurnal fluctuations of O2, N2, and CH4 in the rhizosphere of Thalassia testudinum. Limnol Oceanogr 22(3):566–570. https://doi.org/10.4319/lo.1977.22.3.0566
Oreska MPJ, McGlathery KJ, Aoki LR, Berger AC, Berg P, Mullins L (2020) The greenhouse gas offset potential from seagrass restoration. Sci Rep 10(1):7325. https://doi.org/10.1038/s41598-020-64094-1
Phillips RC, Menez EG (1988) Seagrasses. Smithsonian Institution Press, Washington, DC
Purvaja R, Ganguly D, Hariharan G, Arumugam K, Ramesh R (2020) In situ photosynthetic activities and associated biogeochemical changes in three tropical seagrass species. Front Earth Sci. https://doi.org/10.3389/feart.2020.467548
Raymond PA, Cole JJ (2001) Gas exchange in rivers and estuaries: choosing a gas transfer velocity. Estuaries 24(2):312–317. https://doi.org/10.2307/1352954
Reeburgh WS (2007) Oceanic methane biogeochemistry. Chem Rev 107(2):486–513. https://doi.org/10.1021/cr050362v
Ricart AM, York PH, Bryant CV, Rasheed MA, Ierodiaconou D, Macreadie PI (2020) High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci Rep 10(1):5865. https://doi.org/10.1038/s41598-020-62639-y
Rivera-Guzmán NE, Moreno-Casasola P, Espinosa EC, Ruiz AEL, Vega CM, Peralta-Peláez LA, Higueredo LES, Medina KPAR, Aguayo KVS (2016) The Biological Flora of Coastal Dunes and Wetlands: Halodule wrightii Ascherson. J Coastal Res 33(4):938–948. https://doi.org/10.2112/jcoastres-d-14-00162.1
Rose PS, Millholland LC, Plummer RE, Reed A, Montgomery MT, Boyd TJ, Coffin RB (2013) Natural gas at seep site on the UK Continental Shelf, North Sea, Washinton, DC
Rosentreter JA, Al-Haj AN, Fulweiler RW, Williamson P (2021) Methane and nitrous oxide emissions complicate coastal blue carbon assessments. Glob Biogeochem Cycles 35(2):006858. https://doi.org/10.1029/2020GB006858
Saderne V, Dunne AF, Rich WA, Cadiz R, Carvalho S, Cúrdia J, Kattan A (2023) Seasonality of methane and carbon dioxide emissions in tropical seagrass and unvegetated ecosystems. Commun Earth Environ 4(1):99. https://doi.org/10.1038/s43247-023-00759-9
Schorn S, Ahmerkamp S, Bullock E, Weber M, Lott C, Liebeke M, Lavik G, Kuypers MMM, Graf JS, Milucka J (2022) Diverse methylotrophic methanogenic archaea cause high methane emissions from seagrass meadows. Proc Natl Acad Sci 119(9):e2106628119. https://doi.org/10.1073/pnas.2106628119
Schroll M, Keppler F, Greule M, Eckhardt C, Zorn H, Lenhart K (2020) The stable carbon isotope signature of methane produced by saprotrophic fungi. Biogeosciences 17(14):3891–3901. https://doi.org/10.5194/bg-17-3891-2020
Seeberg-Elverfeldt J, Schlüter M, Feseker T, Kölling M (2005) Rhizon sampling of porewaters near the sediment-water interface of aquatic systems. Limnol Oceanogr Methods 3(8):361–371. https://doi.org/10.4319/lom.2005.3.361
Thorhaug A, Poulos HM, López-Portillo J, Ku TCW, Berlyn GP (2017) Seagrass blue carbon dynamics in the Gulf of Mexico: Stocks, losses from anthropogenic disturbance, and gains through seagrass restoration. Sci Total Environ 605–606:626–636. https://doi.org/10.1016/j.scitotenv.2017.06.189
Treude T, Krause S, Maltby J, Dale AW, Coffin RB, Hamdan LJ (2014) Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: implications for deep sulfur cycling. Geochim Cosmochim Acta 144:217–237. https://doi.org/10.1016/j.gca.2014.08.018
Tunnell J, Hilburn N, Withers K (2002) Comprehensive bibliography of the Laguna Madre of Texas and Tamaulipas. Center for Coastal Studies, Texas A&M University-‐Corpus Christi for The Nature Conservancy of Texas
UNEP-WCMC, Short FT (2021) Global distribution of seagrasses (version 7.1). Seventh update to the data layer used in Green and Short (2003). UN Environment Programme World Conservation Monitoring Center, Cambridge, UK. https://doi.org/10.34892/x6r3-d211
Van Dam BR, Edson JB, Tobias C (2019) Parameterizing air-water gas exchange in the shallow, microtidal new river estuary. J Geophys Res Biogeosci 124(7):2351–2363. https://doi.org/10.1029/2018JG004908
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. J Geophys Res 97(C5):7373–7382. https://doi.org/10.1029/92JC00188
Wanninkhof R (2014) Relationship between wind speed and gas exchange over the ocean revisited. Limnol Oceanogr Methods 12(6):351–362. https://doi.org/10.4319/lom.2014.12.351
Whiticar MJ, Faber E, Schoell M (1986) Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim Cosmochim Acta 50(5):693–709. https://doi.org/10.1016/0016-7037(86)90346-7
Wiesenburg DA, Guinasso NL (1979) Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J Chem Eng Data 24(4):356–360
Wu S, Li S, Zou Z, Hu T, Hu Z, Liu S, Zou J (2019) High methane emissions largely attributed to ebullitive fluxes from a subtropical river draining a rice paddy watershed in China. Environ Sci Technol 53(7):3499–3507. https://doi.org/10.1021/acs.est.8b05286
Yau YYY, Reithmaier G, Majtényi-Hill C, Serrano O, Piñeiro-Juncal N, Dahl M, Mateo MA, Bonaglia S, Santos IR (2023) Methane emissions in seagrass meadows as a small offset to carbon sequestration. J Geophys Res 128(6):007295. https://doi.org/10.1029/2022JG007295
Yu H, Organ H, Xu D, Coffin R (2023) Tidal control and mangrove dieback impact on methane emissions from a subtropical mangrove estuary. Limnol Oceanogr 68(4):753–766. https://doi.org/10.1002/lno.12307
Zhang M, Xiao Q, Zhang Z, Gao Y, Zhao J, Pu Y, Wang W, Xiao W, Liu S, Lee X (2019) Methane flux dynamics in a submerged aquatic vegetation zone in a subtropical lake. Sci Total Environ 672:400–409. https://doi.org/10.1016/j.scitotenv.2019.03.466
Ziegler S, Benner R (1998) Ecosystem metabolism in a subtropical, seagrass-dominated lagoon. Mar Ecol Prog Ser 173:1–12
Acknowledgements
This work was financially supported by an Institutional Grant (Grants-in-Aid of Graduate Research Program) to the Texas Sea Grant College Program from the NOAA, Texas A&M University-Corpus Christi Texas Comprehensive Research Funds, and Geological Society of America Graduate Student Grants. We are very grateful to Dr. Paula Rose for her help in laboratory work and to Mr. Chriss Shope and Mr. Derry Xu for their help during sampling. We also sincerely appreciate suggestions from Dr. James Gibeaut and Dr. Joseph Felix at Texas A&M University-Corpus Christi and Mr. Kyle Capistrant-Fossa at the University of Texas at Austin Marine Science Institute.
Funding
This work was supported by Grants-in-Aid of Graduate Research Program of the Texas Sea Grant College Program from the NOAA, Texas A&M University-Corpus Christi Texas Comprehensive Research Funds, and Geological Society of America Graduate Student Grants.
Author information
Authors and Affiliations
Contributions
Hao Yu and Richard Coffin contributed to the study conception and design. Sample collection and measurement, and data analyses were performed by Hao Yu and Hannah Organ. The first draft of the manuscript was written by Hao Yu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Additional information
Responsible Editor: Feng Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, H., Coffin, R. & Organ, H. Dynamics of methane emissions from northwestern Gulf of Mexico subtropical seagrass meadows. Biogeochemistry 167, 723–741 (2024). https://doi.org/10.1007/s10533-024-01138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-024-01138-y