Abstract
Seagrasses have some of the highest rates of carbon burial on the planet and have therefore been highlighted as ecosystems for nature-based climate change mitigation. However, information is still needed on the net radiative forcing benefit of seagrasses inclusive of their associated greenhouse gas (GHG) emissions. Here, we report simultaneous estimates of seagrass-associated carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) air–water emissions. Applying in situ sampling within a south-east Australian seagrass ecosystem, this study finds atmospheric GHG emissions from waters above seagrasses to range from − 480 ± 15.96 to − 16.2 ± 8.32 mg CO2-equivalents m2 d−1 (net uptake), with large temporal and spatial variability. Using a combination of gas specific mass balance equations, dissolved stable carbon isotope values (δ13C) and in situ time-series data, CO2-e flux is estimated at − 21.74 mg m2 d−1. We find that the net release of CH4 (0.44 µmol m2 h−1) and net uptake of N2O (− 0.06 µmol m2 h−1) effectively negated each other at 16.12 and − 16.13 mg CO2-e m2 d−1, respectively. The results of this study indicate that temperate Australian seagrasses may function as net sinks of atmospheric CO2-e. These results contribute towards filling key emission accounting gaps both in the Australian region, and through the simultaneous measurement of the three key greenhouse gas species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atmospheric concentrations of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O), have increased substantially since pre-industrial periods and are the three dominant greenhouse gases (GHGs) driving human-induced atmospheric warming (Solomon et al. 2007). Quantifying the global emissions of these gases is therefore of high priority as we move towards strategies for climate change mitigation. In the natural world, photosynthetic assimilation and storage of atmospheric CO2 (i.e. biosequestration), and microbial breakdown of organic material with the subsequent release of GHGs through respiration (i.e. decay), are two of the most fundamental processes influencing the carbon cycle. Coastal vegetated ecosystems such as tidal marshes, mangroves and seagrasses have potential for inclusion into future carbon abatement programmes due to biosequestration rates up to 35 times higher than tropical rainforests (Mcleod et al. 2011), and limited carbon decay due to saline-aquatic redox conditions (Macreadie et al. 2019).
Despite covering only 0.1–0.2% of the ocean’s surface, seagrasses are responsible for an estimated 10–18% of total oceanic carbon burial (Duarte et al. 2005; Mcleod et al. 2011). Seagrasses are amongst the most productive coastal ecosystems with average net primary production equating to ~ 817 g carbon (C) m2 yr−1 (Duarte and Cebrián 1996; Mateo et al. 2006; Fourqurean et al. 2012), with sequestered carbon remaining in their anoxic sediments for millennia (Macreadie et al. 2015). However, a variety of human activities that induced degradation of seagrasses, such as the conversion of coastal areas for aquaculture, boat moorings and eutrophication, can increase organic matter respiration through the perturbation (and oxidation) of sediments and increased nutrient supply, leading to decreased sedimentary carbon and increased GHG release (Short and Wyllie-Echeverria 1996; Waycott et al. 2009; Pendleton et al. 2012; Macreadie et al. 2015).
In order to establish seagrasses’ net radiative forcing benefit and therefore their potential use in natural climate change mitigation, it is crucial to accurately incorporate their baseline atmospheric GHG emissions. Common application of benthic chamber sampling methodologies has facilitated estimates of seagrass CO2 and CH4 flux rates between sediments and the water column (Maher and Eyre 2010; Barrón et al. 2014; Macreadie et al. 2014). However, the proportion of these seagrass sediment produced GHGs that pass through the water column to be emitted to the atmosphere is often unclear. For instance, aerobic microbially respired CO2 can be recycled within photosynthetic cycles of the seagrass meadows, and anaerobically respired CH4 is subject to methanotrophic oxidation in the water column. In addition, air–water GHG flux is a product of specific gas solubility in seawater (subject to temperature and salinity), air–water concentration gradients and gas exchange coefficients (Raymond and Cole 2001; Middelburg et al. 2002; Borges et al. 2004). As such, estimates of seagrass atmospheric GHG emissions, based solely on benthic chamber methodologies, are subject to high variability and are unlikely to accurately capture seagrass-associated atmospheric emissions. Additionally, previous literature is largely constrained by region and seagrass species, whilst predominantly focusing on CO2 flux, with fewer still incorporating CH4 flux (Tokoro et al. 2014; Garcias-Bonet and Duarte 2017; Banerjee et al. 2018) and N2O flux (Murray et al. 2015).
The application of in situ automated cavity ring-down spectroscopy has been shown as an effective method for the quantification of air–water gas flux in estuarine and mangrove systems (Maher, et al. 2013a, b; Maher et al. 2015; Reading et al. 2017). Seagrass meadows often span large areas and experience regionalised variation in environmental conditions that affect GHG dynamics, such as salinity (Bouillon, et al. 2007a, b; Touchette 2007), nutrient inputs (Smith et al. 1999; Duarte 2002), water turbulence (Raymond and Cole 2001) and anthropogenic disturbance (Macreadie et al. 2015). In addition, fluctuations in both light availability (and therefore photosynthesis) (Maher, et al. 2013a, b; Saderne et al. 2013) and tidal pumping of ground waters enriched in GHG solutes, dissolved organic matter and nutrients can influence seagrasses’ net autotrophic/heterotrophic balance over diel and tidal cycles (Santos et al. 2008; Bauer and Bianchi 2011; Gleeson et al. 2013). Automated fixed time-series measurements with high sampling resolution (i.e. minutes) are an effective way to incorporate temporal variation in emissions; however, they do not account for spatial changes in environmental conditions (Gazeau et al. 2005; Call et al. 2015; Maher et al. 2015). Alternatively, and commonly, used are continuous underway or discreet sampling spatial surveys that both inherently incorporate spatial GHG variance due to changes in sediment characteristics and seagrass cover etc., though do not integrate day-night fluctuations. Individually, these methods may not adequately constrain GHG variability, resulting in imprecise emission estimates (Gazeau et al. 2005; Call et al. 2015; Maher et al. 2015).
Here, we measure dissolved GHG concentrations from temperate seagrasses and model emission rates, with the aim to quantify the net GHG balance. To achieve this aim, we quantified CO2, CH4 and N2O dissolved concentrations and diffusive fluxes in a temperate seagrass-dominated estuarine embayment in south-east Australia. We used a combination of fixed time-series measurements over a 44-h period and a continuous underway spatial survey throughout the embayment to assess temporal and spatial variability in GHG fluxes and concentrations (Fig. 1). Furthermore, we measured salinity, temperature, wind speed, tidal depth, dissolved oxygen and isotopic (δ13C) signatures of CO2-C to help determine the major environmental conditions influencing seagrass emissions. This study presents the first use of in situ automated cavity ring-down spectroscopy within an Australian seagrass to determine simultaneous CO2, CH4 and N2O atmospheric flux, including estimates of the cumulative function of temperate seagrasses as a source or sink of GHGs over the study period, providing data that can contribute to regional seagrass emission assessments.
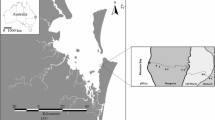
Distribution of seagrass (predominantly Zostera spp.) within temperate Australian Swan Bay. Each yellow circle indicates an individual sample across the spatial survey. The blue circle with point indicates the location of a 44-h time-series sampling setup, which was visually confirmed to be the site of a fringing seagrass meadow. The white square in panel (a) indicates the extent of panel (b). Figure created with ArcMap (V10.2.2), data from Department of Environment, Land, Water & Planning 2017; Department of Economic Development, Jobs, Transport and Resources 2018; Department of Environment, Land, Water & Planning 2019
Materials and Methods
To quantify the spatial and short-term temporal variability in GHG fluxes, we employed a combination of survey and time-series sampling techniques. A time series was conducted for ~ 44 h within a seagrass meadow in Swan Bay, Victoria, Australia (− 38.2701 N, 144.6349 E), and an underway survey was undertaken within Swan Bay between 11:00 and 17:00 (Fig. 1). The experiments were undertaken over a 4-day period from the 4th to 7th of December during the Austral summer. Swan Bay is located within Port Phillip Bay, has an area of ~ 26 km2 and has extensive seagrass beds composed of Zostera muelleri and Heterozostera tasmanica species, which cover ~ 80% of the bay (Fig. 1). Swan Bay experiences a mean annual rainfall of 457 mm, with a mean maximum and mean minimum temperature of 20.4 and 9.4 °C, respectively.
During the time-series measurements, water temperature, depth, DO, pH and salinity were measured using a calibrated water quality sonde (Eureka Manta II) deployed within the seagrass meadow. Using a bilge pump, water was pumped from the location of the water quality sonde to an air–water equilibrator on the shore. The pump was fixed at a depth of 15 cm above the sediment surface. The headspace from the equilibrator was pumped in a closed loop to two cavity ring-down spectrometers (Picarro G2201-i and Picarro G2308) after passing through a desiccant (Drierite) to measure the partial pressure of CO2 (± 200 ppb), CH4 (± 80 ppb), N2O (± 10 ppb) and δ13C-CO2 as described by Maher et al. (2013a, b). A 5-min rolling average was applied to the data, and to account for equilibration times of the various gases data was shifted by 10 min for CO2 and N2O, and 30 min for CH4 (Webb et al. 2016). The same instrument setup was deployed for the underway surveys. The instruments were installed on a small (4 m) shallow draft aluminium boat. The pump and water quality sonde were fixed at a depth of ~ 30 cm, and a GPS (Garmin Map72) was used to record the track. Due to the time lag in gas equilibration times, the process results in a deconvolution of spatial positioning. However, due to the slow transit speed (~ 3 km/h) the effect is small.
The dry molar fractions of each gas were converted to partial pressures using the procedures outlined in Pierrot et al. (2009). Briefly, water vapour pressure and the virial coefficients of N2O were calculated according to Weiss and Price (1980), and the virial coefficients for CO2 were calculated as described by Weiss (1974). Partial pressures were converted to concentrations and percentage saturations using the temperature and salinity-dependent solubility coefficients for each gas (CH4, Wiesenburg and Guinasso (1979); CO2, Weiss (1974); N2O, Weiss and Price (1980)), and atmospheric concentrations of 1.8 ppm, 405 ppm and 328 ppb for CH4, CO2 and N2O respectively.
Air-sea fluxes of each gas were calculated according to
where k is the gas transfer velocity, α is the solubility coefficient and Δpgas is the difference in partial pressure of the gas between the water and atmosphere. As we do not have data on current speeds (although qualitative observations suggest this was low), we used the wind-based gas transfer parametrization of Wanninkhof (2014):
where u is the windspeed measured at a height of 10 m (m s−1) measured on site using a sonic anemometer (Airmar PB200 weather station), and Sc is the Schmidt number of the gas of interest. The Schmidt number was calculated as a function of temperature and salinity according to Wanninkhof (2014), assuming a linear dependence upon salinity.
A mass balance of each individual gas was constructed for a 24-h period of the time series, which accounted for sources and sinks, to determine the production or consumption of each seagrass associated gas:
where Fseagrass is the water column flux from samples within the meadow (mmol m−2 d−1), Fatm is the air water flux (mmol m2 h−1), Δvol is the change in volume (m3 h−1) calculated per m2 by the change in depth and conc is the average concentration of the gas over the time interval of interest. Lateral exchange is accounted for by the \(\Delta vol.conc\) term; however, due to a lack of detailed carbonate chemistry data, there is uncertainty in our approach associated with DIC speciation.
We used the Keeling method to determine the time series δ13C signature of dissolved CO2 source material from within the meadow (Keeling 1958). Keeling methodology is based on the conservation of mass mixing model between the background δ13C (i.e. oceanic DIC which we assume is constant over the short timeframe of the experiment) and source δ13C values, where the source δ13C signature (+ 95% CI) can be determined as the y-intercept of a type II ordinary least squares regression (“lmodel2” function within “lmodel2” package, permutations = 1000) between 1/concentration of measured CO2 and the measured δ13C-CO2 (Pataki et al. 2003; Maher et al. 2017). We assumed that the background concentration and δ13C-CO2 of atmospheric CO2 did not change during the 44-h period. All statistics were performed using R-statistics (V.3.5.3).
Data Analyses
To analyse conditions related to GHG dynamics within seagrass beds of a temperate Australian bay, carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) percent saturation (%sat) levels were separately run through a series of linear models fit with generalized least squares ( “gls()” function within the “nlme” package) (Pinheiro et al. 2017). During spatial surveys, data was removed in small sections where the water sampling unit was exposed to the air (e.g. due to shallow depth). To achieve data normality and meet the assumption of homogeneity of residual variance, CH4 was natural log transformed, and N2O was square root transformed. To test the assumption of model non-collinearity, all pair-wise combinations of environmental variables were assessed using linear models (“lm()” function within “nlme” package): pH, dissolved oxygen (DO), salinity (PSS), tidal depth (m). Both tidal depth and pH were removed from the model due to a relationship with DO of 0.8 and 0.61 adjusted R-squared, respectively. Weak collinearity between salinity and DO was also detected, though a variance inflation factor of 1 indicated that the interaction between these independent variables was negligible to the model (Miles 2014) (“vif()” function within the “car” package). To constrain both salinity and DO covariate residual non-normality, a power variance function was introduced to the model (“varCom()”and “varPower()” function from “nlme” package). Akaike’s information criterion (AIC) model selection was used to confirm the best model fit based on weighted structures, resulting in weights being applied to both DO and salinity. Final model structure was as follows: (Sqrt.GSi ~ Salinity + DO, weighted.variance = Salinity + DO), where GSi represents each Gas Species, CO2, CH4 and N2O. Model validation was conducted using a plot of residual distribution, residuals over predicted values and a quantile–quantile plot (Q-Q plot, “qqnorm()” function in the “stats()” package). Lastly, to obtain an F-statistic, an analysis-of-variance (“anova()” function, type II “marginal”, in “stats” package) was performed on the resulting model objects.
Results
Time Series
Cumulative mass balance of GHGs over a 44 h period, incorporating changes in dissolved gas concentrations, atmospheric flux and water volume, showed a distinct diel pattern in CO2 with net uptake of atmospheric CO2 during the day and net release of CO2 to the atmosphere during the night, with a net negative 24 h flux of − 20.57 μmol m2 h−1 (Fig. 2). Net CO2 forcing was qualitatively associated with high windspeeds during the day and low windspeeds at night (Fig. 3). CH4 maintained a constant net release to the atmosphere, with a 24 h release rate of 0.44 µmol m2 h−1, whilst seagrasses were a net sink of N2O over 24 h, at − 0.06 µmol m2 h−1 (Fig. 2). The estimated δ13C signature of the predominant CO2-C source material, established through Keeling plots, was − 13.71 (− 13.75 to 13.67, 95% confidence interval).
Wind speeds from local weather station used in water-atmosphere gas calculations. The mean ± SE and range of peaks in the day and lows in the night can be viewed in Table 1
Time-series sampling established distinct diel fluctuations in GHGs above seagrass beds, with mean CO2 and CH4 percent saturation (%sat) being 32 and 28% higher during the night, whilst N2O was 11% higher during the day (Fig. 4, Table 1). Day time periods were 83% higher in tidal depth, 80% higher in DO and maintained 66% higher wind speeds (Table 1). Collinearity between these environmental variables, as assessed through linear models, showed that DO%sat was weakly positively correlated with salinity concentrations (P < 0.001, Coef = 37.2, adjusted R-squared = 0.11), whilst pH levels (P < 0.001, Coef = 210.82, adjusted R-squared = 0.61) and tidal depth (m) (P < 0.001, Coef = 0.39, adjusted R-squared = 0.8) showed stronger autocorrelation (Table 1). Tidal depth was only mildly correlated with salinity (P < 0.001, Coef = 41.55, adjusted R-squared = 0.03).
Spatial Survey
Across the survey area, N2O percent saturation (%sat) showed the least variation with a range of 119.36–85.6%, whilst CO2 ranged from 96.4 to 20.1%, and CH4 had the largest variation with a range of 428.5–87.35%. CO2%sat levels were highest at the opening of Swan Bay (Fig. 5a), whilst CH4%sat was highest in areas only sparsely covered by seagrass towards the south-west Swan Bay (Figs. 5b and 1). Across the bay, salinity averaged 33.56 ± 0.07 with a range of 36.04–29.8. Akaike’s information criterion (AIC) determined that spatial survey CO2, CH4 and N2O%sat were best modelled with both salinity and dissolved oxygen (DO). The variance inflation factor for all survey models was low, ranging from 1.004 to 1.006, indicating that the variance around the model was only minutely affected by collinearity between salinity and DO. Water column DO%sat was inversely related to CO2%sat (f(1, 298) = 264.81, P < 0.001, Coef =—0.25) (Fig. 6a), positively related to N2O%sat (f(1, 298) = 17.47, P < 0.001, Coef = 0.001) (Fig. 6e) and did not correlate with CH4 (Fig. 6c). Salinity was not significantly related to CO2 or CH4%sat; however, it was negatively correlated with N2O%sat (f(1,298) = 32.96, P < 0.001, Coef =—0.06) (Fig. 6f). To note, the linear relationships between N2O%sat and both DO%sat and salinity (PSS) retained moderate variance and were heavily influenced by data groupings (Fig. 6e–f), as such inference from these relationships should be treated carefully.
Generalized linear models of greenhouse gas relationships with dissolved oxygen and salinity across Swan Bay. Data was sampled through spatial surveys (one measurement per minute). %sat represents percent saturation, whilst PSS represents the practical salinity scale and * represents a significant correlation (P < 0.05). Data points are representative of CO2; non-transformed, CH4; natural log and N2O; square root. Solid lines indicate the predicted model fit, and shaded areas represent 95% confidence interval
Methodical Comparison
Across both time-series and spatial sampling, CO2 percent saturation (%sat) ranged from 20 to 266, CH4 ranged from 87 to 712% and N2O from 56 to 119%. The mean percent saturation of GHGs were markedly different between survey and time-series methodology, with CO2 and CH4 being 192% and 112% higher in time-series measurements, respectively, whilst N2O was 22% higher in spatial surveys (Fig. 7a). The mean time-series CO2 flux was − 0.46 ± 0.18 mmol m2 d−1, 96% higher than that of spatial sampling, at − 11.48 ± 0.28 mmol m2 d−1 (Fig. 7b). Time-series CH4 flux estimates were also 125% higher than spatial surveys, at 10.29 ± 0.17 μmol m2 d−1 and 4.56 ± 0.19 μmol m2 d−1, respectively (Fig. 7b), whilst N2O mean flux rates were 772% lower in time-series measurements compared to those of surveys, at − 1.07 ± 0.02 and − 0.12 ± 0.02 μmol m2 d−1, respectively (Fig. 7b). Converting these GHG fluxes to CO2-equivalents (CO2-e) using the 20-year sustained global warming potentials of Neubauer and Megonigal (2015) equates to an average time series and spatial survey CO2-e flux of − 16.2 ± 8.32 mg m2 d−1 and − 480.94 ± 15.96 mg m2 d−1, respectively.
Discussion
Through the capture of particulate organic matter and photosynthetic fixation of dissolved carbon dioxide (CO2), seagrass ecosystems play a major role in oceanic carbon storage (Macreadie et al. 2019; Serrano et al. 2019). Recent literature has highlighted seagrasses’ potential in natural carbon-offset strategies; however, their net carbon balance inclusive of atmospheric greenhouse gas (GHG) emissions is yet to be fully established (Macreadie et al. 2019; Serrano et al. 2019). Using a combination of spatial survey and time-series sampling methodologies across a temperate Australian seagrass meadow, we present evidence that during our study, the seagrasses represented net sinks of CO2-equivalent gases inclusive of CO2, methane (CH4) and nitrous oxide (N2O). In addition, we highlight the need for the incorporation of both spatial and temporal focused sampling designs when measuring seagrass emissions and discuss GHG environmental drivers. It is important to note, however, that due to the experiments being undertaken over a short period during the Austral summer period, our results may not be indicative of annual fluxes.
Through mass balance of in situ time-series sampling, seagrasses were shown to be a net sink of GHGs. The seagrass-associated fluxes of GHGs were estimated at − 493.68 μmol CO2 m2 d−1, 10.47 μmol CH4 m2 d−1 and − 1.46 μmol N2O m2 d−1, which at the 20-year sustained global warming potential of CO2 equates to a net flux of − 21.74 mg CO2-e m2 d−1. In addition, we found that the fluxes of CH4 and N2O negated each other at 16.12 and − 16.13 mg CO2-e m2 d−1, respectively. Previous studies on CO2 dynamics have shown that through photosynthetic production and the burial of organic carbon into their sediments, seagrasses act as net autotrophic coastal ecosystems (Gattuso et al. 1998; Gazeau et al. 2005). However, quantification of seagrass sediment gas flux has indicated that a portion of this sequestration may be offset by the release of radiatively potent CH4 (Al‐Haj and Fulweiler 2020; Alongi et al. 2008; Bahlmann et al. 2015; Barber and Carlson 1993; Deborde et al. 2010; Garcias-Bonet and Duarte 2017; Oremland 1975). Our data suggest that although temperate seagrasses are indeed a net source of CH4, these emissions may be counterbalanced by the uptake of N2O; however, as this result is from a single meadow in temperate Australia the finding should be used as a reference for further investigations.
The seagrass-associated CO2 metabolism of − 0.49 mmol CO2 m2 d−1 (uptake) within this study is two orders of magnitude lower than previous global estimates of seagrass net metabolism by Duarte et al. (2010), based on 155 sites at − 99.45 ± 22 mmol CO2 m2 d−1, and more recent estimates of an Australian Zostera meadow at − 99.9 mmol CO2 m2 d−1 by Maher et al. (2011). Whilst the net ecosystem metabolism (NEM = gross primary productivity − ecosystem respiration) of seagrasses varies as a result of a range of biotic and abiotic conditions including, faunal assemblage (Spivak et al. 2009; Kristensen et al. 2012), temperature (Staehr and Borum 2011), seagrass species (Duarte et al. 2010) and sediment quality (Udy and Dennison 1997; Terrados et al. 1999), such a large difference between previous literature and this study is notable. We suggest three reasons for this relationship:
-
1.
Previous estimates of seagrass metabolism are predominantly based on the use of benthic chamber methodology (Duarte et al. 2010), where changes in the concentration of O2 or CO2 are directly linked to the sediment–water interface, whereas this study used an in situ open water sampling technique that inherently homogenizes with other dissolved gas sources, such as tidal pumping and microbial processes, that may affect the net balance of CO2 flux estimates. Microbial breakdown and respiration of dissolved organic carbon associated with seagrass meadows have been shown as a significant term in net seagrass carbon budgets (Barrón et al. 2014; Maher and Eyre 2010) and may have offset the reductions in water column CO2 from photosynthesis (Linto et al. 2014; Call et al. 2015).
-
2.
The average solar irradiance during this sampling period was relatively low due to dense cloud cover at 372.98 ± 9.97 J m2 s−1, whilst the comparable monthly average for this region is ~ 14.4% higher at 426.62 J m2 s−1 (Australian Bureau of Meteorology). Variation in light conditions directly affects photosynthetic production in seagrass beds, with lower irradiance often resulting in lower rates of dissolved CO2 uptake (Gacia et al. 2005; Touchette 2007).
-
3.
A qualitatively large amount of seagrass wrack could be seen along the banks of the seagrass meadow (roughly 10 m from submerged sampling station), suggesting large concentrations of dead biomass in the water column. Microbial decay of leaf biomass is a major contributor to net ecosystem respiration within seagrass systems (Harrison and Mann 1975; Blum and Mills 1991; Mateo and Romero 1997; Liu et al. 2019), indicating a potential for large alternative CO2 inputs and therefore potential offsets of mass-based estimates of net photosynthetic uptake.
Bay wide surveys showed large variations in CO2 and CH4 saturation, whilst N2O saturation remained relatively homogenous (Fig. 5a–c and Table 1). Across medium to large spatial scales, variation in environmental conditions such as, topography, slope/depth, species composition and freshwater inputs (both from riverine and groundwater origins) can directly affect the production and chemical fate of GHGs in coastal systems (Gazeau et al. 2005; Allen et al. 2007; Maher et al. 2015). In accordance with our hypothesis, we found that both dissolved CO2 and CH4 were elevated in localised “hot spots” throughout the bay, whilst CO2 and N2O showed strongly inverse, and weak positive, correlations with dissolved oxygen, respectively (Fig. 6a, c). The photosynthetic productivity of seagrass meadows is reliant on a range of factors that include light availability, nutrient concentrations and substrate suitability, amongst others. In areas less suitable to seagrass establishment, a lack of photosynthetic productivity will lead to lower uptake of dissolved CO2 and less oxygenated water. We found here that CO2 concentrations were highest in the east of the bay, which is more influenced by mixing with oceanic waters (Fig. 5a).
High concentrations of CH4 in the south-eastern area of the bay (Fig. 5b) were less easily explained, whereby no significant relationship with either oxygen or salinity was established, and both wind speed and pH remained relatively homogenous. Previous evaluation of coastal system sediment CH4 flux has correlated higher CH4 emissions with greater seagrass biomass (Bahlmann et al. 2015; Barber and Carlson 1993); however, in contrast to the literature we found the highest CH4 concentrations above sediments with low seagrass colonization (Figs. 5b and 1). The observed pattern in CH4 may instead be due to a combination of organic matter supply from tidal inundation of the large adjacent tidal marsh (Fig. 1) and relatively anoxic sediments in the absence of seagrass O2 production, facilitating a shift towards methanogenic microbial processes (Sansone and Martens 1981; Ollivier et al. 1994).
The concentrations of seagrass-associated dissolved GHGs were unsurprisingly shown to vary markedly over diel cycles. Both CO2 and CH4 percent saturation (%sat) in the water column were found to be 32 and 28% higher at night, whilst N2O was 11% higher during the day (Fig. 4). The greater concentrations of dissolved CO2 during the night are consistent with previous literature and our hypotheses that water column CO2 is largely controlled by photosynthesis/respiration dynamics driven by light (Maher, et al. 2013a, b; Saderne et al. 2013). In addition, high oxygen concentrations facilitate nitrification in aquatic systems, where N2O is produced during the metabolism of ammonium (NH4+) into nitrate (NO3−) (Elkins et al. 1978; Nishio et al. 1983). Higher N2O concentrations during the day, in combination with the positive relationship between N2O and dissolved oxygen saturation (DO%) from spatial surveys, indicate that oxygen-dependent aerobic nitrification is the predominant N2O production pathway in temperate seagrasses (Xia et al. 2013; Johansson et al. 2011), although important to note is that throughout the entire diel cycle, N2O was rarely above 100% atmospheric saturation (Fig. 4c). This suggests that similar to mangroves (Maher et al. 2016), seagrass systems may act as a sink of N2O due to low nutrient concentrations and high rates of complete denitrification (Welsh et al. 2000; Eyre and Ferguson 2002; Eyre et al. 2016). Diel oscillations in autotrophic and heterotrophic respiration cycles within seagrass sediments may also lead to fluctuations in anaerobic methanogenesis and aerobic oxidation of dissolved methane (King et al. 1990; Maher, et al. 2013a, b). However, we did not find a significant relationship between DO and CH4 as would be expected from respiration cycle-driven CH4 production. Alternatively, CH4 concentrations may be more tightly linked to fluctuations in tidal height that alter the exchange of sediment pore water rich in organic matter, with the water column, a process known as tidal pumping (Borges et al. 2003; Bouillon, et al. 2007a, b; Atkins et al. 2013; Maher, et al. 2013a, b; Macklin et al. 2014). Qualitative analyses of diel trends from this study show an inverse relationship between tidal height and CH4%sat (Fig. 4b), a relationship that matches those of previous studies of tidal pumping in mangrove systems (Linto et al. 2014; Call et al. 2015); We therefore suggest that tidal processes were likely the predominant driver of CH4 concentrations.
Large differences in mean atmospheric GHG flux were observed between spatial and temporal sampling methodologies, though both methods estimated flux to be of net uptake into the water column. In coastal ecosystems, variation of environmental conditions, such as DO, salinity, temperature and turbulence, often occurs across both spatial and temporal scales (Smith et al. 1999; Raymond and Cole 2001; Duarte 2002; Bouillon, et al. 2007a, b; Touchette 2007). As photosynthetic production, microbial respiration and gas transfer dynamics are highly linked to these environmental conditions, accounting for both spatial and temporal variation in gas concentrations is important for accurate GHG emission estimates (Gazeau et al. 2005; Call et al. 2015; Maher et al. 2015). Here, net atmospheric GHG uptake rates from bay-wide surveys were ~ 30 times greater than those from the time-series experiment, at − 480 ± 15.96 and − 16.2 ± 8.32 mg CO2-e m2 d−1 respectively, a disparity primarily attributed to much lower CO2 uptake rates during the time-series sampling (Table 1). Variation in GHG flux between spatial and temporal sampling methodologies is in accordance with previous literature from mangrove and estuarine systems (Maher, et al. 2013a, b; Ho et al. 2014; Maher et al. 2015) and further highlights the need for the inclusion of both of these processes when estimating seagrass atmospheric emissions. Important to note, is that whilst the survey methodology from this study maintained a high sampling frequency (i.e. one sample per minute), it was both limited to the outer regions of the bay (Fig. 1) and did not incorporate diel or seasonal variations. The survey was carried out during daylight hours, amplifying the role of primary production on our estimates. Similarly, time-series methodology is constrained to a single point in the bay, whilst diel variation in GHG emissions is likely to vary across the bay. Therefore, the observed variations in flux between the methodologies can only be assessed qualitatively due to the lack of direct overlap, and a robust correlative equation for day-night GHG variations could not be established, though it is of high importance for more accurate future estimates of seagrass emissions based on spatial surveys alone.
The carbon isotopic signature (δ13C) of water column dissolved CO2 indicated that allochthonous terrestrial carbon was not a major contributor to microbial community respiration within Swan Bay. Seagrasses are effective ecosystems for the biosequestration of atmospheric carbon; however, they are also able to trap and bury allochthonous carbon from terrestrial run-off and other coastal ecosystems higher in elevation such as mangroves and tidal marsh (Agawin and Duarte 2002; Duarte et al. 2005; Kennedy et al. 2010; Deegan et al. 2012). In addition, a range of periphytic organisms establish themselves on seagrass blades and may contribute to sedimentary carbon concentrations (Walker and Woelkerling 1988; Gacia et al. 2002). Understanding the carbon source of microbial respiration within seagrasses allows for more targeted management of emissions (Geraldi et al. 2019), for example, terrestrial carbon run-off may be reduced through better up-stream effluent management or increased fencing along water ways. Here, we find that the predominant CO2-C source as established through Keeling plots (Keeling 1958; Pataki et al. 2003; Maher et al. 2017) was − 13.75 ‰, an isotopic value that closely matches that of Zostera spp. and their associated periphyton (− 12.2 – − 13 δ13C) (Thayer et al. 1978). These results suggest that terrestrial C3 carbon inputs do not play a significant role in aquatic metabolism in the bay, and the GHG fluxes measured are likely indicative of in situ processes.
In conclusion, this study provides the first simultaneous in situ assessment of CO2, CH4 and N2O atmospheric emissions from temperate Australian seagrasses. We find that the seagrasses studied were a net CO2-e sink, due to CO2 and N2O uptake outweighing CH4 release. We also note that seagrass atmospheric emissions were heavily linked to diel fluctuations in light availability and tidal pumping, and that omission of these variables when estimating seagrass emissions is likely to lead to inadequate estimates. Finally, we demonstrate large variation in CO2 and CH4 emissions at the habitat scale, highlighting the need for the inclusion of spatial parameters when upscaling seagrass emission estimates. As seagrasses maintain some of the highest rates of carbon sequestration and storage on the planet, they represent potential ecosystems for nature-based carbon offsetting. Further research into seagrasses overall carbon sink potential incorporating their associated GHG emissions is extremely important for future offset investments.
References
Agawin, N.S.R., and G.M. Duarte. 2002. Evidence of direct particle trapping by a tropical seagrass meadow. Estuaries 25: 1205–1209.
Al-Haj, A.N., and R.W. Fulweiler. 2020. A synthesis of methane emissions from shallow vegetated coastal ecosystems. Global Change Biology 26 (5): 2988–3005.
Allen, D.E., R.C. Dalal, H. Rennenberg, R.L. Meyer, S. Reeves, and S. Schmidt. 2007. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biology & Biochemistry 39: 622–631.
Alongi, D., L. Trott, M. Undu, and F. Tirendi. 2008. Benthic microbial metabolism in seagrass meadows along a carbonate gradient in Sulawesi Indonesia. Aquatic Microbial Ecology 51: 141–152.
Atkins, M.L., I.R. Santos, S. Ruiz-Halpern, and D.T. Maher. 2013. Carbon dioxide dynamics driven by groundwater discharge in a coastal floodplain creek. Journal of Hydrology 493: 30–42.
Bahlmann, E., I. Weinberg, J. Lavrič, et al. 2015. Tidal controls on trace gas dynamics in a seagrass meadow of the Ria Formosa lagoon (southern Portugal). Biogeosciences 12: 1683–1696.
Banerjee K., A. Paneerselvam, P. Ramachandran, D. Ganguly, G. Singh, and R. Ramesh. 2018. Seagrass and macrophyte mediated CO2 and CH4 dynamics in shallow coastal waters. PloS One 13: e0203922.
Barber T.R., and P.R. Carlson. 1993. Effects of seagrass die-off on benthic fluxes and porewater concentrations of ∑ CO 2,∑ H 2 S, and CH 4 in Florida Bay sediments. In: Biogeochemistry of Global Change pp. 530–550 Springer.
Barrón, C., E.T. Apostolaki, and C.M. Duarte. 2014. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Frontiers in Marine Science 1: 42.
Bauer, J., and T. Bianchi. 2011. 5.02—Dissolved organic carbon cycling and transformation. Treatise on Estuarine and Coastal Science 5: 7–67.
Blum, L., and A. Mills. 1991. Microbial growth and activity during the initial stages of seagrass decomposition. Marine Ecology Progress Series. Oldendorf 70: 73–82.
Borges A., S. Djenidi, G. Lacroix, J. Théate, B. Delille, and M Frankignoulle. 2003. Atmospheric CO2 flux from mangrove surrounding waters. Geophysical Research Letters 30.
Borges, A.V., J.-P. Vanderborght, L.-S. Schiettecatte, et al. 2004. Variability of the gas transfer velocity of CO 2 in a macrotidal estuary (the Scheldt). Estuaries 27: 593–603.
Bouillon, S., F. Dehairs, B. Velimirov, G. Abril, and A.V. Borges. 2007a. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi Bay, Kenya). J. Geophys. Res. Biogeosciences. 112: G2.
Bouillon, S., J.J. Middelburg, F. Dehairs, et al. 2007b. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences Discuss. 4: 317–348.
Call, M., D.T. Maher, I.R. Santos, et al. 2015. Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring–neap–spring timescales in a mangrove creek. Geochimica Et Cosmochimica Acta 150: 211–225.
Deborde, J., P. Anschutz, F. Guérin, et al. 2010. Methane sources, sinks and fluxes in a temperate tidal Lagoon: The Arcachon lagoon (SW France). Estuarine, Coastal and Shelf Science 89: 256–266.
Deegan, L.A., D.S. Johnson, R.S. Warren, et al. 2012. Coastal eutrophication as a driver of salt marsh loss. Nature 490: 388–392.
Department of Economic Development, Jobs, Transport and Resources. 2018. Port Phillip Bay seagrass mapping at nine aerial assessment regions. [online]. Available from: http://services.land.vic.gov.au/catalogue/metadata?anzlicId=ANZVI0803004651&publicId=guest&extractionProviderId=1.
Department of Environment, Land, Water & Planning. 2017. Victorian Wetland Inventory_Current. [online]. Available from: http://services.land.vic.gov.au/catalogue/metadata?anzlicId=ANZVI0803004912&publicId=guest&extractionProviderId=1.
Department of Environment, Land, Water & Planning. 2019. Watercourse Network 1 : 25,000 - Vicmap Hydro. [online]. Available from: http://services.land.vic.gov.au/SpatialDatamart/dataSearchViewMetadata.html?anzlicId=ANZVI0803002490&extractionProviderId=1.
Duarte, C.M. 2002. The future of seagrass meadows. Environmental Conservation 29: 192–206.
Duarte, C.M., and J. Cebrián. 1996. The fate of marine autotrophic production. Limnology and Oceanography 41: 1758–1766.
Duarte C.M., N. Marbà, E. Gacia, et al. 2010. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles 24: GB4032.
Duarte, C.M., J.J. Middelburg, N.F. Caraco, et al. 2005. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2: 1–8.
Elkins, J.W., S.C. Wofsy, M.B. McElroy, C.E. Kolb, and W.A. Kaplan. 1978. Aquatic sources and sinks for nitrous oxide. Nature 275: 602.
Eyre, B.D., and A.J. Ferguson. 2002. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae-and macroalgae-dominated warm-temperate Australian lagoons. Marine Ecology Progress Series 229: 43–59.
Eyre, B.D., D.T. Maher, and C. Sanders. 2016. The contribution of denitrification and burial to the nitrogen budgets of three geomorphically distinct Australian estuaries: Importance of seagrass habitats. Limnology and Oceanography 61: 1144–1156.
Fourqurean, J.W., C.M. Duarte, H. Kennedy, et al. 2012. Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience 5: 505–509.
Gacia, E., C.M. Duarte, and J.J. Middelburg. 2002. Carbon and nutrient deposition in a Mediterranean seagrass (Posidonia oceanica) meadow. Limnology and Oceanography 47: 23–32.
Gacia, E., H. Kennedy, C.M. Duarte, et al. 2005. Light-dependence of the metabolic balance of a highly productive Philippine seagrass community. Journal of Experimental Marine Biology and Ecology 316: 55–67.
Garcias-Bonet, N., and C.M. Duarte. 2017. Methane production by seagrass ecosystems in the Red Sea. Frontiers in Marine Science 4: 340.
Gattuso, J.-P., M. Frankignoulle, and R. Wollast. 1998. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annual Review of Ecology and Systematics 29: 405–434.
Gazeau, F., C.M. Duarte, J.-P. Gattuso, et al. 2005. Whole-system metabolism and CO 2 fluxes in a Mediterranean Bay dominated by seagrass beds (Palma Bay, NW Mediterranean). Biogeosciences 2: 43–60.
Geraldi, N.R., A. Ortega, O. Serrano, et al. 2019. Fingerprinting Blue Carbon: Rationale and tools to determine the source of organic carbon in marine depositional environments. Frontiers in Marine Science 6: 263.
Gleeson, J., I.R. Santos, D.T. Maher, and L. Golsby-Smith. 2013. Groundwater–surface water exchange in a mangrove tidal creek: Evidence from natural geochemical tracers and implications for nutrient budgets. Marine Chemistry 156: 27–37.
Harrison, P., and K. Mann. 1975. Detritus formation from eelgrass (Zostera marina L.): The relative effects of fragmentation, leaching, and decay. Limnology and Oceanography 20: 924–934.
Ho, D.T., S. Ferrón, V.C. Engel, L.G. Larsen, and J.G. Barr. 2014. Air-water gas exchange and CO2 flux in a mangrove-dominated estuary. Geophysical Research Letters 41: 108–113.
Johansson, A., Å.K. Klemedtsson, L. Klemedtsson, and B. Svensson. 2011. Nitrous oxide exchanges with the atmosphere of a constructed wetland treating wastewater: Parameters and implications for emission factors. Tellus Series B: Chemical and Physical Meteorology 55: 737–750.
Keeling, C.D. 1958. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochimica Et Cosmochimica Acta 13: 322–334.
Kennedy H., J. Beggins, C.M. Duarte, et al. 2010. Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochemical Cycles 24: GB4026.
King, G.M., P. Roslev, and H. Skovgaard. 1990. Distribution and rate of methane oxidation in sediments of the Florida Everglades. Applied and Environmental Microbiology 56: 2902–2911.
Kristensen E., G. Penha-Lopes, M. Delefosse, T. Valdemarsen, C. Quintana, and G. Banta. 2012. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Marine Ecology Progress Series. https://doi.org/10.3354/meps09506.
Linto, N., J. Barnes, R. Ramachandran, J. Divia, P. Ramachandran, and R. Upstill-Goddard. 2014. Carbon dioxide and methane emissions from mangrove-associated waters of the Andaman Islands Bay of Bengal. Estuaries and Coasts 37: 381–398.
Liu, S., S.M. Trevathan-Tackett, C.J.E. Lewis, et al. 2019. Beach-cast seagrass wrack contributes substantially to global greenhouse gas emissions. Journal of Environmental Management 231: 329–335.
Macklin, P.A., D.T. Maher, and I.R. Santos. 2014. Estuarine canal estate waters: Hotspots of CO2 outgassing driven by enhanced groundwater discharge? Marine Chemistry 167: 82–92.
Macreadie, P.I., A. Anton, J.A. Raven, et al. 2019. The future of Blue Carbon science. Nature Communications 10: 1–13.
Macreadie, P.I., M.E. Baird, S.M. Trevathan-Tackett, A.W.D. Larkum, and P.J. Ralph. 2014. Quantifying and modelling the carbon sequestration capacity of seagrass meadows—A critical assessment. Marine Pollution Bulletin 83: 430–439.
Macreadie, P.I., S.M. Trevathan-Tackett, C.G. Skilbeck, et al. 2015. Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Proceedings of the Royal Society b: Biological Sciences 282: 20151537.
Maher D., and B.D. Eyre. 2011. Benthic carbon metabolism in southeast Australian estuaries: Habitat importance, driving forces, and application of artificial neural network models. Marine Ecology Progress Series.
Maher, D.T., K. Cowley, I.R. Santos, P. Macklin, and B.D. Eyre. 2015. Methane and carbon dioxide dynamics in a subtropical estuary over a diel cycle: Insights from automated in situ radioactive and stable isotope measurements. Marine Chemistry 439: 97–115.
Maher D.T., and B.D. Eyre. 2010. Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: Implications for global estimates of benthic DOC fluxes. Journal of Geophysical Research: Biogeosciences 115 (G4).
Maher, D.T., I.R. Santos, L. Golsby-Smith, J. Gleeson, and B.D. Eyre. 2013a. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnology and Oceanography 58: 475–488.
Maher, D.T., I.R. Santos, J.R. Leuven, et al. 2013b. Novel use of cavity ring-down spectroscopy to investigate aquatic carbon cycling from microbial to ecosystem scales. Environmental Science and Technology 47: 12938–12945.
Maher, D.T., I.R. Santos, K.G. Schulz, M. Call, G.E. Jacobsen, and C.J. Sanders. 2017. Blue carbon oxidation revealed by radiogenic and stable isotopes in a mangrove system. Geophysical Research Letters 44: 4889–4896.
Maher, D.T., J.Z. Sippo, D.R. Tait, C. Holloway, and I.R. Santos. 2016. Pristine mangrove creek waters are a sink of nitrous oxide. Science and Reports 6 (1): 1–8.
Mateo M., J. Cebrián, K. Dunton, and T. Mutchler. 2006. Carbon flux in seagrass ecosystems. Seagrasses: Biology, Ecology And Conservation 159–192.
Mateo, M., and J. Romero. 1997. Detritus dynamics in the seagrass Posidonia oceanica: Elements for an ecosystem carbon and nutrient budget. Marine Ecology Progress Series 151: 43–53.
Mcleod, E., G.L. Chmura, S. Bouillon, et al. 2011. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9: 552–560.
Middelburg, J.J., J. Nieuwenhuize, N. Iversen, et al. 2002. Methane distribution in European tidal estuaries. Biogeochemistry 59: 95–119.
Miles J. 2014. Tolerance and variance inflation factor. Wiley StatsRef: Statistics Reference Online.
Murray, R.H., D.V. Erler, and B.D. Eyre. 2015. Nitrous oxide fluxes in estuarine environments: Response to global change. Global Change Biology 21: 3219–3245.
Neubauer, S.C., and J.P. Megonigal. 2015. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 18: 1000–1013.
Nishio, T., I. Koike, and A. Hattori. 1983. Estimates of denitrification and nitrification in coastal and estuarine sediments. Applied and Environmental Microbiology 45: 444–450.
Ollivier, B., P. Caumette, J.-L. Garcia, and R. Mah. 1994. Anaerobic bacteria from hypersaline environments. Microbiological Reviews 58: 27–38.
Oremland, R.S. 1975. Methane production in shallow-water, tropical marine sediments. Applied Microbiology 30: 602–608.
Pataki D., J. Ehleringer, L. Flanagan., et al. 2003. The application and interpretation of Keeling plots in terrestrial carbon cycle research Global Biogeochemical Cycle 17 (1).
Pendleton L., D.C. Donato, B.C. Murray, et al. 2012. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PloS One 7: e43542.
Pierrot, D., C. Neill, K. Sullivan, et al. 2009. Recommendations for autonomous underway pCO2 measuring systems and data-reduction routines. Deep Sea Res. Part II Top. Stud. Oceanogr. 56: 512–522.
Pinheiro J., D. Bates, S. DebRoy, et al. 2017. Package ‘nlme’. Linear Nonlinear Mix. Eff. Models Version.
Raymond, P.A., and J.J. Cole. 2001. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries and Coasts 24: 312–317.
Reading, M.J., I.R. Santos, D.T. Maher, L.C. Jeffrey, and D.R. Tait. 2017. Shifting nitrous oxide source/sink behaviour in a subtropical estuary revealed by automated time series observations. Estuarine, Coastal and Shelf Science 194: 66–76.
Saderne V., P. Fietzek, and P.M.J. Herman. 2013. Extreme variations of pCO2 and pH in a macrophyte meadow of the Baltic Sea in summer: Evidence of the effect of photosynthesis and local upwelling. PloS One 8: e62689.
Sansone, F.J., and C.S. Martens. 1981. Methane production from acetate and associated methane fluxes from anoxic coastal sediments. Science 211: 707–709.
Santos, I.R.S., W.C. Burnett, J. Chanton, B. Mwashote, I.G. Suryaputra, and T. Dittmar. 2008. Nutrient biogeochemistry in a Gulf of Mexico subterranean estuary and groundwater-derived fluxes to the coastal ocean. Limnology and Oceanography 53: 705–718.
Serrano, O., C.E. Lovelock, T.B. Atwood, et al. 2019. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nature Communications 10: 1–10.
Short, F.T., and S. Wyllie-Echeverria. 1996. Natural and human-induced disturbance of seagrasses. Environmental Conservation 23: 17–27.
Smith, V.H., G.D. Tilman, and J.C. Nekola. 1999. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100: 179–196.
Solomon S., D. Qin, M. Manning, K. Averyt, and M. Marquis. 2007. Climate change 2007—The physical science basis: Working group I contribution to the fourth assessment report of the IPCC. Cambridge University Press.
Spivak A.C., E.A. Canuel, J.E. Duffy, and J.P. Richardson. 2009. Nutrient enrichment and food web composition affect ecosystem metabolism in an experimental seagrass habitat. PLoS One 4: e7473.
Staehr, P.A., and J. Borum. 2011. Seasonal acclimation in metabolism reduces light requirements of eelgrass (Zostera marina). Journal of Experimental Marine Biology and Ecology 407: 139–146.
Terrados, J., C.M. Duarte, L. Kamp-Nielsen, et al. 1999. Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquatic Botany 65: 175–197.
Thayer, G.W., P.L. Parker, M.W. LaCroix, and B. Fry. 1978. The stable carbon isotope ratio of some components of an eelgrass, Zostera marina, bed. Oecologia 35: 1–12.
Tokoro, T., S. Hosokawa, E. Miyoshi, et al. 2014. Net uptake of atmospheric CO 2 by coastal submerged aquatic vegetation. Global Change Biology 20: 1873–1884.
Touchette, B.W. 2007. Seagrass-salinity interactions: Physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology 350: 194–215.
Udy, J.W., and W.C. Dennison. 1997. Growth and physiological responses of three seagrass species to elevated sediment nutrients in Moreton Bay Australia. Journal of Experimental Marine Biology and Ecology 217: 253–277.
Walker D., and W.J. Woelkerling. 1988. Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia. Marine Ecology Progress Series 71–77.
Wanninkhof, R. 2014. Relationship between wind speed and gas exchange over the ocean revisited. Limnology and Oceanography: Methods 12: 351–362.
Waycott, M., C.M. Duarte, T.J. Carruthers, et al. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106: 12377–12381.
Webb, J.R., D.T. Maher, and I.R. Santos. 2016. Automated, in situ measurements of dissolved CO2, CH4, and δ13C values using cavity enhanced laser absorption spectrometry: Comparing response times of air-water equilibrators. Limnology and Oceanography: Methods 14: 323–337.
Weiss, R. 1974. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Marine Chemistry 2: 203–215.
Weiss, R., and B. Price. 1980. Nitrous oxide solubility in water and seawater. Marine Chemistry 8: 347–359.
Welsh, D.T., M. Bartoli, D. Nizzoli, G. Castaldelli, S.A. Riou, and P. Viaroli. 2000. Denitrification, nitrogen fixation, community primary productivity and inorganic-N and oxygen fluxes in an intertidal Zostera noltii meadow. Marine Ecology Progress Series 208: 65–77.
Wiesenburg, D.A., and N.L. Guinasso Jr. 1979. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. Journal of Chemical and Engineering Data 24: 356–360.
Xia, Y., Y. Li, X. Li, M. Guo, D. She, and X. Yan. 2013. Diurnal pattern in nitrous oxide emissions from a sewage-enriched river. Chemosphere 92: 421–428.
Acknowledgements
Thank you to Alice Gavoille for helping during the sample collection. This research was in‐part funded by a Holsworth Wildlife Research Endowment, and we thank the Holsworth foundation for its ongoing support of critical ecological research. We thank the Corangamite Catchment Management Authority for their funding and collaborative support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. We thank Deakin University’s Centre for Integrative Ecology and Department of Life and Environmental Sciences for ongoing funding support provided to DTM via the Researcher in Residence programme. We also thank the Australian Research Council for their funding support, LP160100242 and DE150100581.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Just Cebrian
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ollivier, Q.R., Maher, D.T., Pitfield, C. et al. Net Drawdown of Greenhouse Gases (CO2, CH4 and N2O) by a Temperate Australian Seagrass Meadow. Estuaries and Coasts 45, 2026–2039 (2022). https://doi.org/10.1007/s12237-022-01068-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01068-8