Abstract
With climate change, streams and rivers are at increased risk of droughts and flow intermittency. The full implications of these conditions for fluvial carbon (C) processing and stream-atmosphere CO2 emissions are not well understood. We performed a controlled drought experiment in outdoor hyporheic flumes. We simulated small rain events that increase sediment moisture content, but do not cause streamflow in order to investigate how these events affect streambed dissolved organic C dynamics, biofilm respiration and enzyme activity, and bacterial community composition. Flumes were subject to a non-flow phase of one month with small rain events with varying frequency (weekly, 3 × weekly, and no rain). Sediment was sampled at the surface and from the hyporheic zone at the end of the non-flow phase. We quantified microbial respiration of the dry sediments and sediment DOC leaching after simulated flow resumption. We found that, at the surface, more frequent rain events significantly increased microbial respiration from 12.6 ± 0.25 µg CO2 g−1 DW h−1 to 26.5 ± 11.3 µg CO2 g−1 DW h−1 between the control and 3 × weekly rain events. The average amount of DOC leached from surface sediments during flow resumption was reduced by 0.813 ± 0.62 mg L−1 with more frequent rain events. More frequent rain events also resulted in the leaching of fresher DOM with increased tryptophan fluorescence and a higher BIX. This, along with higher glucosidase activity in the biofilms, indicates higher OC processing during the drought period with more frequent rain events. Small rain events also enhanced Shannon diversity of microbial communities, with a stronger presence of ‘terrestrial-like’ bacterial clades. We propose that rain events during drought, even those of small size, are highly relevant for fluvial organic C processing during the dry phase. Future research should explicitly consider small rain events when investigating C fluxes in intermittent streams to fully understand the C processing in these systems with climate change. We conclude that small rain events impact DOM dynamics during reflow and likely impact the cascading C processing in the downstream river network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streams and rivers play an important role in the global carbon (C) cycle. They receive, transform, and transport organic carbon (OC) from terrestrial sources to the oceans (Allen and Pavelsky 2018; Drake et al. 2018). As OC is mineralized by aquatic microorganisms during transport, it contributes to the emissions of carbon dioxide (CO2) from aquatic systems to the atmosphere (Hotchkiss et al. 2015). C cycling in streams is shaped by drying and rewetting commonly occurring during droughts (Fierer and Schimel 2002). Several studies have shown that dry streambeds may act as hotspots for CO2 emissions compared to perennial reaches (Boodoo et al. 2019; Gómez-Gener et al. 2016; Marcé et al. 2019). Rewetting creates further hot moments for both microbial respiration and dissolved organic C (DOC) leaching (Harjung et al. 2018), thereby increasing aquatic CO2 emissions (Gallo et al. 2013; von Schiller et al. 2019) and impacting the C cycle in downstream water bodies.
Microbial biofilms in streams are important sites for biotic OC transformation, storage, and release (Battin et al. 2016; Leff et al. 2016; Pusch et al. 1998). Biofilms occupy large surface areas of the streambed and the hyporheic zone (HZ). Droughts, drying, and rewetting can affect microbial OC processing and microbial community structure in multiple ways.
Structural changes of the stream microbiome include the loss of some heterotrophic organisms in coarser sediments (Timoner et al. 2012), while in some sediments, such as sand, enough moisture may be stored for maintaining the community structure and retaining some functionality (Coulson et al. 2021; McKew et al. 2011). Desiccation may decrease the microbial biomass and change the microbial community composition (Amalfitano et al. 2008; Timoner et al. 2014). Community shifts occur due to the fact that certain bacterial groups can resist desiccation better than others. Several authors have observed an increase in the proportion of alpha- and beta-Proteobacteria during the dry phase, probably due to losses in other more sensitive groups (Amalfitano et al. 2008; Gionchetta et al. 2020). Furthermore, Actinobacteria are more resistant to drought due to their gram-positive cell wall (Gionchetta et al. 2020; Timoner et al. 2014).
Functional changes of the stream microbiome include the reduction of OC mineralization during the dry phase which is tightly linked to a similar reduction of extracellular enzymatic activities (EEAs) (Amalfitano et al. 2008; Gionchetta et al. 2019; Pohlon et al. 2013; Zoppini and Marxsen 2010). The exudation of extracellular enzymes from bacteria embedded in fluvial biofilms are the first step in OC breakdown in streams (Marxsen and Fiebig 1993). Here, enzyme-based decomposition models have shown that terrestrial organic matter is hydrolyzed by a group of correlated C-acquiring enzymes whos activity can be expressed by a few representatives (e.g. glucosidase; Arnosti et al. 2014). Changes in microbial respiration depend on the remaining moisture content and oxygen availability, showing increases under moist and oxygen-rich conditions during the initial phase of drying (Arce et al. 2021; Coulson et al. 2021; Fromin et al. 2010), but decreases in most fluvial ecosystems during non-flow conditions (Acuña et al. 2015; Oprei et al. 2019; von Schiller et al. 2019).
Upon flow resumption, a short pulse of water with strongly enhanced DOC and inorganic nutrient concentrations is released (Arce et al. 2015; Gessner 1991; von Schiller et al. 2011), exceeding base flow and sometimes even flood flow concentrations (Bernal et al. 2005; Vazquez et al. 2018). This DOC and nutrient pulse originates from organic matter accumulated in the terrestrial ecosystem during drought, as well as in fluvial sediments during the dry phase. In sediments, the accumulation is caused by reduced decomposition rates and enhanced cell lysis due to osmotic stress (Kaiser et al. 2015; Marcé et al. 2019; Meisner et al. 2013; Schiller et al. 2019). While the precise DOC composition depends on the respective source in the sediments (e.g. Shumilova et al. 2019), many studies have reported a high proportion of low molecular weight DOM, thus of higher bioavailability, triggering events of high microbial respiration (Harjung et al. 2018, 2019b; Kaiser et al. 2015; Marcé et al. 2019; von Schiller et al. 2019). Consequently, several studies have observed high respiration rates upon rewetting of dry soils (Sponseller 2007), stream sediments (Arce et al. 2021; von Schiller et al. 2019; Zlatanović et al. 2018), and pond sediments (Fromin et al. 2010). Repeated dry-wet cycles resulting from, for example, frequent water level fluctuations on the sediment surface and in the hyporheic zone can also stimulate microbial respiration even over longer time periods (Gómez-Gener et al. 2021; Pinto et al. 2020). This reduced impact to the hyporheic zone can be attributed to changes in the physical habitat with depth, such has higher moisture content, reduced porosity, and decreasing organic matter content with depth (Boodoo et al. 2019; Coulson et al. 2021). Rain events during droughts can rewet sediments without reinstating flow, distinguishing them from other rewetting types, which has similar effects on microbial respiration. For example, Gallo et al. (2013) observed increased CO2 emissions from dry river beds during a rainfall simulation experiment of low intensity. Similarly, in laboratory drying-rewetting experiments with benthic stream sediments, simulated rainfall boosted sediment CO2 evasion depending on rainfall intensity and duration of the preceding desiccation phase (Arce et al. 2021). Yet despite these relevant first findings, there remains large uncertainty on the precise mechanisms and implications of small rain events occurring during the non-flow phase on sediment OC processing and mineralization.
The aim of this study was to evaluate the effects of short rain events on the microbial respiration in benthic and hyporheic sediments during the non-flow period and the DOC leaching after flow resumption with a rainfall simulation experiment. We wanted to know whether the effects of small rain events depended on the frequency of rainfall and whether the hyporheic communities responded differently to the rain events than the surface sediment communities. The simulated rain events were short and resulted only in an increase sediment moisture content without sediment saturation or flow resumption. We hypothesized that (1) an increase in the frequency of short rain events enhances sediment respiration during the non-flow period, reduces the amount of DOC leached, and alters the quality of DOM leached to fresher, lower MW compounds during flow resumption. We further expected that (2) the bioavailability of the DOM leached will increase with enhanced rainfall frequency due to the stimulation of microbial mineralization by rewetting. Finally, we expected that (3) these predicted effects are most pronounced near the sediment surface and decline with increasing sediment depth, due to a reduced impact of drought (Coulson et al. 2021) and surface rainfall in the deeper hyporheic zone (HZ).
Methods
Experimental design and sample collection
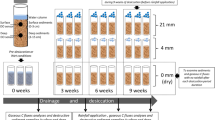
We addressed these hypotheses through a drought experiment in outdoor hyporheic flumes. The flumes were filled with natural gravel and pre-conditioned with sediment microbial communities from stream water. This provided us the exceptional opportunity to study sediment processes under nearly natural environmental conditions, in which the presence of water in the stream and sediments can be fully controlled. This experiment was conducted in six roofed outdoor flumes (5 m long, 0.6 m wide, 1.2 m deep) that were filled with coarse natural fluvial gravel (average diameter: 25 mm) to a depth of 60 cm to create a HZ (Coulson et al. 2021). The flumes are part of the experimental flume facilities at the WasserCluster Lunz Biological Station, Austria, located in the eastern European Alps (47° 15′ N, 15° 04′ E). In this pre-alpine region underlain by karstic calcareous rock, non-flow periods in streams can occur naturally during summer (May through September) and in cold winters (January through March). The average air temperature in summer 2020 was 16.5 °C.
In order to evaluate the temporal dynamics of sediment microbial processes during non-flow conditions, we used reusable sediment samplers as described in Coulson et al. (2021). In short, the samplers consist of four outer cores that are permanently installed in the HZ of each flume made of a perforated plastic tube (PVC, 7.5 cm diameter, holes every ~ 4 cm) placed in the sediment. In each outer core, a screened inner core composed of a 3 mm PVC mesh was installed. The inner cores were then filled with sediment inside nylon mesh bags (grain size < 4.0 mm, ~ 100 mL sediment per bag). For sampling, the inner core was removed from the outer core and sediment bags retrieved for further processing and analysis. Removed sediment bags were replaced with new bags to maintain the vertical position of the remaining bags within the core. Replacement bags were excluded from subsequent sampling to avoid uneven pre-conditioning bias.
The flumes, with the sediment cores and bags installed, were pre-conditioned for two months prior to the experiment with stream water from the Oberer Seebach (OSB) stream. The OSB is a pristine oligotrophic mountain stream providing well aerated, CaCO3-buffered (mean pH = 8.3 ± 0.2), low nutrient waters (Fasching et al. 2016). The OSB water has microbial abundance of 5 × 105–3.86 × 106 cells mL−1, with a Shannon diversity index of 7.6–7.9 using 16 S rRNA sequencing (Caillon et al. 2021; Caillon and Schelker 2020). The OSB water was allowed to flow through the flumes at the surface, and 5 and 10 cm below the sediment surface. We used a roof to exclude direct rainfall to the flumes during pre-conditioning and the experimental drought. Surface flow during pre-conditioning was approximately 60 mL per second. This would result in a theoretical turnover time of the entire gravel-filled volume of the hyporheic flumes of roughly 4 h.
After pre-conditioning, all flumes were drained and subjected to a non-flow period for one month. During this period, we used three sediment cores per flume for the different treatment. This amounted to 6 replicates per treatment, one from each flume. The treatments were as follows: One set of cores was allowed to dry naturally for the entire experimental period, a second set of cores experienced rain events once per week, and a third set of cores experienced rain events three times per week. This setup of treatments across flumes allowed us to statistically account for potential systematic variability originating from differences in pre-conditioning between the flumes.
Rain events were simulated by removing the inner cores from the sediment samplers and sprinkling artificial rainwater (stream water diluted 1:1 with ultra-pure deionized water (MQ)) onto the intact cores from above. Thus, similar to a potential natural short rain event during drought, surface sediments received more rainwater than deeper HZ sediments. We used 15 mL of artificial rainwater over 30 min per core, which would be roughly equivalent to a rain intensity of 10 mm per hour with a duration of 30 min. We chose this volume following prior experimental testing, which suggested that this amount can be considered as non-effective rainfall, as it did not exceed the field capacity of the sediment. The field capacity is the amount of water retained in soil or sediment at a matrix potential of − 33 kPa, which is approximately equivalent to the amount of water retained after excess water has drained by gravity for 2–3 days (Dingman 2002). We thus assumed, that this amount would not initiate streamflow in a dry streambed. For simplicity, we standardized the amount of artificial rainwater added per experimental rain event by pipetting. All differences in rain fluxes among treatments, thus, refer to the total amount added over the duration of the experiment.
Sediment was sampled from sediment bags at the end of the non-flow phase by removing bags from the inner cores. To avoid the direct effects of the rain events, samples were collected 6 days after the last rain event for the samples that experienced weekly rain events and 2 days after the last rain event for the samples that experienced 3 rain events per week. Samples were collected from the surface, the shallow HZ (~ 10 cm below the sediment surface), and the deep HZ (~ 40 cm below the surface). Six replicates for each treatment and the three depths (surface, shallow HZ, and deep HZ) were sampled (total n = 54 samples). Sediments were brought to the lab within two hours after sampling and a portion was used for the leaching experiment. The leaching experiment was performed in a lab-controlled environment to simulate the complete rewetting of the sediments upon flow resumption. For this purpose, 20 g of fresh sediment from each sample bag was incubated in 100 mL stream water in 250 mL glass bottles. The bottles were gently shaken in the dark for 4 h at 20 °C. After incubation, the leachate was filtered (0.7 μm, pre-combusted Whatmann GF/F filters) and analyzed for DOC concentrations. Values were then compared to concentrations of similarly treated sediment samples collected at the beginning of the drought experiment (after pre-conditioning). Samples were stored dark and cool. DOC and DOM spectroscopic characteristics were measured within 24 h using a Shimadzu TOC-L Analyzer (DOC; Shimadzu Scientific Instruments, Kyoto, Japan), a Hitachi fluorescence spectrophotometer F-7000 (fluorescence spectroscopy; Hitachi High-tech Corporation, Kyoto, Japan), and a Shimadzu UV-1700 spectrophotometer (absorbance; Shimadzu Scientific Instruments, Kyoto, Japan).
The remaining sediment from the experiment was immediately analyzed for bacterial respiration, moisture content, and organic matter (OM) content. To gain additional understanding about the conditions of the microbial biofilms and their OC processing ability, the sediments were also analyzed for EEA, bacterial abundances, and microbial community composition (surface samples only). Bacterial abundance samples (1 g sediment per sample) were fixed with formaldehyde (final concentration 2.5%) and stored at 4 °C until analysis. Sediment samples for 16s rRNA bacterial community analysis were filled into a sterile microcentrifuge tube under sterile condition and stored at − 20 °C until DNA extraction. Due to limited resources, only surface sediment samples were extracted for 16s rRNA community analysis.
Laboratory analyses
Increased respiration indicates an increase in stream ecosystem functions, that is, biofilm metabolism, and therefore an increased breakdown of OC in biofilm (Battin et al. 2003, 2008). Bacterial respiration was measured via the MicroResp™ system. This was done as described in the MicroResp technical manual (MicroResp™, James Hutton Ltd, Aberdeen, UK). The sediment was weighted into a deep 96-well microplate (approximately 0.7 g sediment per well, 3 analytical replicates per sample). The microplate was incubated for 6 h at 25 °C with CO2 detection gel and the absorbance was measured using a spectrophotometric microplate reader (Varioskan Flash, Thermo Fischer Scientific, Vaanta, Finland) at 570 nm. Moisture content (percent water over dry weight) of the sediment was determined by drying the sediment at 80 °C for 24 h. Ash free dry weight (AFDW) was determined by treating the sediments at 400 °C for 4 h and is expressed as percent OM over dry weight.
We used glucosidase activity as proxy for the activity of the entire group of terrestrial OC-degrading enzymes in freshwater systems (Arnosti et al. 2014). High glucosidase levels are indicative of an enhanced OC degradation in the biofilm (Romaní and Sabater 2001). EEA of β-D-1,4-glucosidase (hereafter, glucosidase) was measured in the sediment according to the method described by Romani and Sabater (2001). In short, 120 µL substrate (final concentration 0.3 mM) was added to 1.0 g of sediment suspended in 4 mL sterile filtered stream water (0.2 μm pore size, polyethersulfone (PES)). Gluc was measured using an enzyme specific 4-methylumbelliferyl (MUF)-substrate (MUF-β-d-glucopyranoside, Sigma-Aldrich, Germany). After 1 h, glycine buffer (2 mL, pH 10.4) was added to stop the reaction and fluorescence was measured with 365/455 nm excitation/emission wavelengths, that is specific for the MUF-substrate on a platereader (Varioskan Flash, Thermo Fischer Scientific, Vaanta, Finland).
Bacterial abundances were determined according to the protocol described by Duhamel and Jacquet (2006). Prokaryotic cells in the fixed samples were detached from the sediment, filtered, and diluted 100 times. The samples were then stained with SYBR Green II stain (200 × diluted in DMSO, Invitrogen Molecular Probes Inc., California, USA), and analyzed by flow cytometry (CytoFLEX, Beckman Coulter GmbH, Krefeld, Germany). An increase of bacterial abundances over time generally indicates overall bacterial growth in the environment (i.e. an increase in heterotrophic biomass) (Timoner et al. 2012).
Changes in microbial community composition refer to changes of ecosystem structure and can be indicative of ecosystem alterations, such as droughts (Amalfitano et al. 2008; Timoner et al. 2014). Moreover, changes in community composition may also alter ecosystem functioning, such as respiration (Bell et al. 2009; Langenheder et al. 2010; Peter et al. 2011). It should be noted, however, that ecosystem functioning of altered communities can also remain completely unchanged, due to extensive functional redundancy within a community (Frossard et al. 2012). In order to analyse microbial community composition, we used the Qiagen DNeasy Power Soil Kit (Hilden, Germany) with the manufacturer’s protocol to extract DNA from the sediments. We included experimental controls (n = 2) to account for any potential contamination. Extracted products were sent to LGC Biosearch Technologies (LGC Genomics Gmbh, Berlin, Germany). The laboratory first quantified the DNA using gel electrophoresis. The V3–V4 region of the 16s rRNA gene was then amplified with polymerase chain reactions using 341 F and 785R primers (Thijs et al. 2017). Resultant DNA were sequenced using the Illumina Mi-Seq platform (300 bp paired-end reads) for amplicon creation.
Data Analysis
Differences between treatments were evaluated using the non-parametric Kruskal–Wallis test (Kruskal and Wallis 1952) with pairwise Mann–Whitney U-tests (Mann and Whitney 1947) with a Bonferroni correction for multiple comparisons (Dunn 1961), as part of the standard stats package in R. This was chosen since the data was not normally distributed as tested by Shapiro Wilk tests. All statistical analyses was performed in R v.3.5.3 (R Core Development Team 2020).
In order to evaluate DOM quality in the leachate samples, excitation-emission matrices were analyzed by a PARAFAC model (Andersen and Bro 2003; Pucher et al. 2019). A blank sample was subtracted from each sample to reduce noise. Samples were normalized to Raman units and corrected for inner-filter effects. Raman and Rayleigh scatter-bands of first and second order were removed and interpolated. The model was calculated with a tolerance of 10−12 and 40 converging random initializations. Three samples were identified as outliers due to high leverage and excluded from the model, but their sample loadings were calculated after completion of the final model. The model was validated by half-split comparisons, a visual inspection of the spectra and components were compared with other studies using the OpenFluor database (Murphy et al. 2014). Five components were identified in the samples (Table 1). Other spectroscopic parameters were also evaluated, including the biological index (BIX; Gabor et al. 2014), specific ultraviolet absorbance at 254 nanometers (SUVA 254; Weishaar et al. 2003), and the ratio of absorption at 250 to 365 nanometers (E2:E3; Helms et al. 2008). Statistical analysis between treatments on the PARAFAC results were performed as described before DOC quantity and DOM quality give indications of the amount ofC produced by the biofilm during drought and the bioavailability of that DOM (Battin 1999; von Schiller et al. 2011). For example, an increased DOC quantity leached indicates increased microbial C degradation occurring in the biofilm during drought. Here we used different tools to describe DOM quality, such as the PARAFAC-components, the biological index (BIX), specific ultraviolet absorbance at 254 nm (SUVA 254), and the ratio of absorption at 250 to 365 nm (E2:E3). BIX, also known as the “freshness” index, correlates to microbially derived, autochthonous DOM and therefore, an increase in BIX indicates the presence of more microbially derived, “fresh”, DOM (Gabor et al. 2014; Wilson and Xenopoulos 2009). An increase in SUVA254 indicate a higher proportion of aromatic molecules (Weishaar et al. 2003). The E2:E3 ratio is a proxy for molecular weight. As the E2:E3 ratio increases, the relative size of the DOM molecules decreases (Helms et al. 2008).
To analyze sequence data, we used Mothur version 1.44.3 (Schloss et al. 2009), and the Mothur MiSeq SOP (Kozich et al. 2013) with some modification. Identification of operational taxonomic units (OTUs) was performed with the SILVA reference database (version 132), with a 97% similarity and above required for identification. Due to limitations of the reference database, a maximum homopolymer of 8 was chosen. Chimeric fragments were identified and removed with UCHIME (Edgar et al. 2011). NCBI’s BLAST was used to identify sequences that could not be identified through SILVA. We were able to identify all but one OTU with 97% similarity. OTU23 is therefore reported as Proteobacteria_unclassified. In order to ordinate the samples based on dissimilarity in community composition, nonmetric multidimensional scaling (NMDS) with Bray-Curtis distances were executed using the ‘vegan’ package (Oksanen et al. 2020). To test the differences between treatments, we used an ANOSIM analysis with 9999 permutations.
Relative abundances were calculated and visualized with the ‘phyloseq’ package (McMurdie and Holmes 2013). All statistical analyses was performed in R v.3.5.3 (R Core Development Team 2020). Alpha diversity was calculated using the Shannon diversity index in the ‘vegan’ package (Jost 2007). The differences between treatments for the Shannon diversity index were evaluated using the non-parametric Kruskal–Wallis test (Kruskal and Wallis 1952) with pairwise Mann–Whitney U-tests (Mann and Whitney 1947) with a Bonferroni correction for multiple comparisons (Dunn 1961), as part of the standard stats package in R.
To gain insight into the effects of short rain events during drought on respiration and DOC leaching for the total OC loss, we calculated an organic C mass balance for each treatment in our system. Organic C in the sediment was calculated from OM content via the Van Bemmelen factor (1.72) which is commonly used to convert soil organic matter into soil organic C (Ahn and Jones 2013). The mass balance was calculated as:
where Cstart is the sediment OC content at the beginning in the sediment, Cresp is the total C respired during the course of the experiment, Cleach is the amount of C lost through the artificial leaching at the end of the experiment, and Crainfall is the C added by artificial rain additions. It is important to note that the same amount of C input from rain events was calculated for the HZ samples as for the surface samples, even though the rain event was only applied to the surface of the sediment core and may thus not have reached the deeper HZ. Leached DOC and respiration were included in the calculation as means for each treatment and depth.
Results
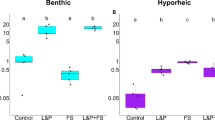
Rain events affected respiration, moisture content, bacterial abundances, and OM content at all depths, while glucosidase activity and DOC leaching were only affected at the sediment surface (Fig. 1). Respiration was significantly higher with increasing rain events at all depths (Kruskal–Wallis, p < 0.05, n = 6) but did not differ between depths within the same treatment (Fig. 1a). In contrast, leached DOC was significantly higher in the samples that did not experience any rain event (Kruskal-Wallis, p < 0.05, n = 6) (Fig. 1b). However, this pattern was only present in the surface samples, while the HZ samples showed no significant differences in the amount of leached DOC between treatments. Leached DOC amounts were significantly higher in surface sediment samples than in HZ samples for the dry treatment (Kruskal–Wallis, p < 0.05, n = 6). Moisture content increased with increasing rain events, however differences between treatments were only significant in the deeper HZ samples (Kruskal-Wallis, p < 0.05, n = 6) (Fig. 1c). As expected, the moisture content was higher in the HZ than on the surface for all treatments (Kruskal–Wallis, p < 0.05, n = 6). OM content appeared to increase (mostly non-significant) with increasing rain events with no clear differences between depths (Fig. 1d). Bacterial abundances appeared also higher in the samples that experienced more rain events, but differences were not statistically significant. We also did not observe differences between depths (Fig. 1e). Glucosidase activities significantly increased with rain frequency in the surface samples (Kruskal–Wallis, p < 0.05, n = 6) but not in the HZ (Fig. 1f). Glucosidase activities were generally higher in the surface samples than in the HZ (Kruskal–Wallis, p < 0.05, n = 6).
Various parameters measured at the end of the experiment across the different treatments and depths: a respiration, b dissolved organic carbon (DOC) leached from the sediment after the experiment, c moisture content, d ash free dry weight (AFDW), e bacterial abundances, and f glucosidase activity. p-values indicate the differences between treatments based on Kruskal–Wallis test (n = 6). The edges of the boxplots represent the interquartile range (25th−75th percentile), the middle line represents the median, the whiskers indicate the largest and smallest values within 1.5 × the interquartile range, and the dots indicate outside values greater than 1.5 × and less than 3 × the interquartile range
Frequent rain events also affected the quality of the DOM leached from the samples. Generally, the samples that experienced no rain events and weekly rain events had similar DOM qualities in their leachates, while samples that experienced 3 × weekly rain events were different (Fig. 2). No rain and weekly rain event samples showed higher fluorescence signals of humic-like, terrestrial material (components 1–3; Fig. 2a; Table 1), while the tryptophan-like component C4 was highest in the samples that experienced 3x weekly rain events (Fig. 2b; Table 1). Increased raining frequency also resulted in a higher BIX (Fig. 2d) corresponding to the higher tryp C4 fluorescence. Interestingly, component 5 described as tyrosine-like and thus also supposedly representing proteins was lowest in the 3 × weekly rain events (Fig. 2c). The E2:E3 ratio was usually higher in the rain treatments than in the dry treatment, indicating increased amounts of low-molecular components. We observed no consistent pattern of SUVA254 across treatments. Surface leachates contained generally more humic-like terrestrial material (components 1–3, Fig. 2a; Table 1) than deeper hyporheic layers, while SUVA254 increased with depth.
Dissolved organic matter quality parameters measured at the end of the experiment across the different treatments and depths: a component 1 (hum-terr C1) ; note that components 2 (hum rep) and 3 (hum-photo) had the same pattern, b component 4 (tryp C4), c component 5 (tyr C5), d biological index (BIX; Gabor et al. 2014), e specific ultraviolet absorbance at 254 nanometers (SUVA 254; Weishaar et al. 2003), and f the ratio of absorption at 250 to 365 nm (E2:E3; Helms et al. 2008). Letter annotations indicate differences between treatments based on Kruskal–Wallis test. The edges of the boxplots represent the interquartile range (25th−75th percentile), the middle line represents the median, the whiskers indicate the largest and smallest values within 1.5 × the interquartile range, and the dots indicate outside values greater than 1.5 × and less than 3 × the interquartile range
The microbial community in the surface sediments showed a significantly higher Shannon diversity in the treatment with 3 rain events per week than in the weekly rain event treatment (Mann–Whitney, p = 0.0092, n = 5; Table 2). Note that due to low DNA amounts obtained from the samples, only one sample from the dry treatment was analyzed. There were also significant differences in the microbial communities between the two treatments (ANOSIM, 9999 permutations, R = 0.9434). With increased rain frequency, the relative abundance of Actinobacteria increased, while those of Flavobacteria and Gamma-Proteobacteria decreased (Fig. 3).
We found differences in the sediment C mass balances among treatments and sediment depths (Fig. 4). The lowest estimated total C losses were found in the 3x weekly rain event treatments, whereby C loss rates were equal across depth layers (82 × 10−6 µg C g−1 DW h−1). The weekly rain event treatment showed the highest estimated C loss of all in the surface sediments (240 × 10−6 µg C g−1 DW h−1), while C losses in the shallow and the deep HZ amounted to less than half of the surface rates. In the dry treatment, estimated total C losses were around 150 × 10−6 µg C g−1 DW h−1 on the surface and in the deep HZ. Here, the shallow HZ showed the lowest C loss of all. Respiration was by far the dominant pathway for OC losses in all treatments and depths. Estimated average respiration rates (200–500 × 10−6 µg C g−1 DW h−1) often exceeded total C losses by far, probably due to inaccuracies and assumptions in the mass balance calculations. The largest differences were found in the 3 × weekly rain events treatment, with respiration rates approximately 5 times the total C loss rates. The smallest differences were observed in the dry treatment (except in the shallow HZ).
Mass balances for the three treatments (no rain events, weekly rain events, and 3x week rain events) and for the three depths (surface, shallow hyporheic zone (HZ) (10 cm below the surface), and deep HZ (40 cm below the surface). All values are 10−6 µg C g DW−1. Orange arrows indicate respiration, blue arrows indicate rainfall, and green arrows for DOC leaching after rewetting. Respiration and rainfall numbers are in µg C g DW−1 for the entire experiment. Leaching numbers are in µg C g DW−1 for 1 h. Note that the rain event may not have made it to the HZ, this value corresponds to the “rain” that was applied to the surface
Discussion
This experiment shows that mid-drought rain events can increase the amount of sediment OC respired by sediment biofilms, resulting in decreased DOC leaching from the sediments upon rewetting. This effect becomes stronger with increasing rain frequency. Although the leaching of DOC from dry stream sediments has been investigated previously (Shumilova et al. 2019), this is one of the first studies to show that the amount of DOC released into the stream water after rewetting can be altered by short rain events during drought. As these rain events do not directly contribute to stream runoff, they may be considered irrelevant from a stream water volume perspective. However, our results demonstrate that short rain events during drought may have profound effects on fluvial C cycling, as they modify sediment OC partitioning.
Short rain events during drought increase respiration and decrease DOC leaching
Several studies have demonstrated that rewetting may increase sediment respiration in lotic and lentic systems, where sediments exposed to frequent wet-dry cycles are among those with the highest respiration rates (Kosten et al. 2018; Pinto et al. 2020; Obrador et al. 2018). A global study of 200 intermittent stream reaches across different biomes yielded a 32-fold to 66‐fold increase in respiration upon sediment rewetting, with rates reaching up to 6.6 mg CO2 g−1 h−1 (von Schiller et al. 2019). In a previous desiccation study in our flumes (Coulson et al. 2021), we found respiration rates of 15–45 µg CO2 g−1 h−1 immediately upon rewetting compared to pre-drying rates of 30–35 µg CO2 g−1 h−1. Here, the increase was higher in sediments exposed to longer drought duration. While each of these studies focused on the complete inundation of the sediments, we know from soil science that the full saturation of sediment pore spaces with water is not necessarily required to achieve this respiration peak induced by rewetting and known as the Birch effect (Birch 1958; Sponseller 2007). In fact, our study showed an increase in respiration with increasing rain event frequency at all sediment depths, although moisture content remained far below saturation (rain events only approximated field capacity). Likewise, simulated rain events in ephemeral stream channels resulted in high CO2 peaks of up to 1.6 g C m−2 h−1 without reinstating stream flow in the sediments (Gallo et al. 2013). In a similar laboratory desiccation-rain study, Arce et al. (2021) observed CO2 peaks after rain events, the magnitude of which depended on the drought duration and the rain intensity. Thus, CO2 fluxes from dry streambeds to the atmosphere may still be underestimated especially in temperate climate regions where small rain events may occur frequently during the non-flow phase.
While respiration rates increased with increasing rain frequency, the amount of DOC leached from sediments after immersion in water decreased in the surface sediments. Furthermore, samples that experienced no rain and weekly rain events had similar DOM leachates with more humic-like, terrestrial material (C1–C3, Table 1). On the other hand, samples that experienced rain events 3 × per week had more protein-like components (C4) and fresher material (BIX). This clearly points towards higher microbial activity in these samples, and is fully congruent with the higher respiration rates, higher glucosidase activities, and lower amount of DOM leached (Tiefenbacher et al. 2020).
We assume that the repeated gentle rewetting by the small rain events and the subsequent slow drying kept the mineralization activity of the biofilms in the surface sediments high, thereby providing the microorganisms with labile DOC that was respired. Studies have shown that EEAs are rapidly activated in dry sediments upon rewetting (Pohlon et al. 2018; Zoppini and Marxsen 2010), potentially supporting substantial C mineralization. In contrast to the surface sediments, leaching and glucosidase activity remained low in the HZ sediments. It is possible that the higher moisture contents in the HZ buffered the effects of drying and rewetting caused by the rain events (Coulson et al. 2021). Furthermore, light rain initiates a weak, unidirectional water and solute transport through the unsaturated sediments from the surface to deeper layers. Thus, we suggest that the increased hyporheic respiration was largely fueled by labile DOC produced on the sediment surface or, to a minor degree added by our artificial rainwater. It must be mentioned here that no leaching from the sediment cores was observed during the rain simulations, that is no water (and thus no DOC) left the cores during or after the rain additions. Finally, it should be noted, that rain-induced vertical solute transport or translocation through unsaturated sediments clearly distinguishes the effects of short rain events during drought from those of other rewetting events occurring during flood either through surface flow resumption, or through groundwater level rise (Fig. 5).
We propose that more mechanistic studies are required both under controlled lab conditions and in the field to understand the consequences of different rewetting types on sediment OC cycling and CO2 production in intermittent streams on larger spatial scales. We want to stress the importance of considering dry stream sediments as 3-dimensional compartments, in which the term “rewetting” must not be restricted solely to the re-initiation of streamflow but should represent solute transport mechanism under unsaturated and saturated conditions across the 3-dimensional space.
Respiration as the dominant C loss regardless of depth
We found that respiration was by far the most dominant C flux in the calculated C mass balances for this experiment. High respiration has been measured in dry riverbeds (von Schiller et al. 2014), with CO2 fluxes often more than twice those of the studied streams during flowing conditions (Gómez-Gener et al. 2016). In our experiment, respiration was the dominant flux not only at the sediment surface, but also in the HZ. As drying is a continuous process, the initial drying has been observed to result in increased oxygen availability and thereby, can stimulate organic matter degradation (Marcé et al. 2019). Similarly, desiccated ponds showed an increased breakdown of OC and subsequent release of CO2 (Fromin et al. 2010), while previous flume experiments support the idea that moderate drought conditions enhance sediment OC degradation (Harjung et al. 2019a). This indicates that moderate drying (i.e. higher remaining moisture content) in the HZ can also stimulate high respiration in the subsurface during drought.
It is important to note that certain assumptions were made during the mass balance that limit the interpretation of our results. Respiration, for example, was only measured at the end of the experiment. This was assumed to be the rate for the entire duration of the experiment. However, respiration is expected to show some fluctuation with temperature and moisture content (i.e. immediate increases following rain events).
Rain events change the microbial community structure and functioning
Changes in sediment moisture affect the microbial community (Sponseller 2007; Zeglin et al. 2011) as different stages of drying and rewetting select different microbial species (Arce et al. 2018; Sabater et al. 2016). In general, lower moisture content can decrease microbial diversity. The remaining drought community is then assumed to represent a subset of the fluvial community during flowing conditions (Acuña et al. 2015; Timoner et al. 2012; Timoner et al. 2014). In our experiment, Shannon diversity was significantly higher in the samples that experienced three rain events per week as compared to the samples that experienced weekly rain. With increased rain events, the community composition also showed a shift towards a higher abundance of Actinobacteria which have, among others, also been associated with terrestrial communities (Aslam et al. 2016; Barnard et al. 2013; Schimel et al. 2007). Other drying experiments have demonstrated increased relative abundance of Actinobacteria with drought (Amalfitano et al. 2008; Gionchetta et al. 2019). The study by Amalfitano et al. (2008) also showed decreasing relative abundances of Flavobacteria and gamma-Proteobacteria with increasing drought. This clearly contrasts with our results; in our experiment the abundance of Flavobacteria and gamma-Proteobacteria increased with increasing rain events.
Overall, the community results indicate that while rain events maintain an intermediate moisture content with a matric potential between the permanent wilting point and field capacity, the microbial community of the stream sediment may shift towards a more terrestrial-like community. Such a shift can well be anticipated, as the physical habitat characteristics in dry sediments (unsaturated conditions, high oxygen availability; strong variation in microhabitat structure through small-scale variation in matric potential within pore-spaces) should ultimately cause a shift towards a more soil-like community under drought (Arce et al. 2019). Moreover, reoccurring small rain events may enhance the habitat diversity in some sediments, potentially enhancing microbial diversity and the prevalence of terrestrial bacteria—a pattern consistent with our data, while in other sediments (cf. Amalfitano et al. (2008)) the later may not be the case. It is also important to highlight that this shift in community composition and diversity is more pronounced with more frequent rain events. This indicates that dry streambeds shift more towards soil habitats during the non-flow phase even with additional sediment moisture content present.
Although we only analyzed community composition at the surface, we can see there was a shift towards OTUs that represent a terrestrial community in the samples that experienced more rain events, as highlighted above. This shift is also reflected in the glucosidase activities where the wetter sediments had higher levels. Higher glucosidase indicates higher OC processing in the biofilm (Romaní and Sabater 2001). This higher level of OC processing in wetter conditions is also reflected in the higher respiration rates and the more labile DOC leached after rewetting. Overall, we conclude that more frequent small rain events facilitate a shift in the community towards a more terrestrial composition as well as higher OC processing resulting in less but more labile DOC being leached.
Conclusions and implications
In this experiment, we found that short rain events during drought increased sediment respiration as compared to uninterrupted desiccation during drought. Respiration also dominated the C fluxes during drought, greatly exceeding the C entering the system with rainfall and leaving the sediment through leaching during rewetting. Rain events shifted the microbial community toward a more soil-like composition, while maintaining a higher microbial diversity as compared to the full drought. The less diverse communities in the drier sediments also demonstrated decreased C processing than in the wetter sediments. We also found that rain events decreased the DOC output after rewetting. Our results demonstrate the difference in rewetting caused by rain events versus the rewetting by increasing surface-flow or groundwater rise and highlight the relevance of even small rain events when investigating C fluxes in intermittent stream sediments.
The implications of small rain events identified in this study are likely to be of different relevance in different sections of a river network and in different climates. Firstly, headwater streams are generally considered the part of the river network that experiences flow intermittency most frequently (Datry et al. 2014). In humid catchments located along the northern European Alps, almost only headwater streams experience flow intermittency. This is because streamflow further downstream is supported by increasing groundwater inflows, while karstification enhances channel losses and groundwater recharge in high elevations (Hartmann et al. 2015).
Secondly, the spatiotemporal distribution of precipitation, such as the known increase with elevation (Smith 1979; Spreen 1947), may structure the occurrence of small rain events in fluvial networks. Here, we propose that small rain events during droughts are most likely to occur at mid-range elevations, while high elevations will experience either significantly higher, or no precipitation at all during dispersed convective events—the most common type of precipitation during summer drought along the northern Alps.
Thirdly, sediment C accumulation in fluvial sediments is spatially variable. Key drivers of high fluvial sediment and C accumulation (primarily as particulate organic C (POC)) are low channel slopes and reduced flow velocity (van Rijn 1993). Along the northern Alps, these conditions can be typically found in mid elevation valleys (elevation range ~ 400–1000 m a.s.l.). This compartment of the stream network can be defined as the lower upland zone (cf. Datry et al. 2017).
We note that our experimental setup well represents such a mountain system. Our experiment was performed in a side-channel of the Oberer Seebach, a 2nd order stream in the Ybbs river network in Lower Austria (Fasching et al. 2016). It is located at 600 m a.s.l. and has a slope of 0.41% (Battin 1999). The Oberer Seebach has also fallen dry in the past, for example during exceptionally dry summers (see Ejarque et al. 2017). Considering all factors, we expect our results to be most relevant for 2nd and 3rd order streams at mid-elevations, with low slopes. In our study river network of the Ybbs River, 2nd or 3rd order streams account for 37% of the total stream surface area of the entire stream network (Schelker et al. 2016).
Data availability
Data can be made available upon request.
References
Acuña V, Casellas M, Corcoll N, Timoner X, Sabater S (2015) Increasing extent of periods of no flow in intermittent waterways promotes heterotrophy. Freshw Biol 60(9):1810–1823. https://doi.org/10.1111/fwb.12612
Ahn C, Jones S (2013) Assessing organic matter and organic carbon contents in soils of created mitigation wetlands in Virginia. Environ Eng Res 18(3):151–156. https://doi.org/10.4491/eer.2013.18.3.151
Allen GH, Pavelsky TM (2018) Global extent of rivers and streams. Science 361(6402):585–588. https://doi.org/10.1126/science.aat0636
Amalfitano S, Fazi S, Zoppini A, Barra Caracciolo A, Grenni P, Puddu A (2008) Responses of benthic bacteria to experimental drying in sediments from Mediterranean temporary rivers. Microb Ecol 55(2):270–279. https://doi.org/10.1007/s00248-007-9274-6
Andersen CM, Bro R (2003) Practical aspects of PARAFAC modeling of fluorescence excitation-emission data. J Chemom 17(4):200–215. https://doi.org/10.1002/cem.790
Arce MI, del Sánchez-Montoya M, Gómez R (2015) Nitrogen processing following experimental sediment rewetting in isolated pools in an agricultural stream of a semiarid region. Ecol Eng 77:233–241. https://doi.org/10.1016/j.ecoleng.2015.01.035
Arce MI, von Schiller D, Bengtsson MM, Hinze C, Jung H, Alves RJE, Urich T, Singer G (2018) Drying and Rainfall Shape the Structure and Functioning of Nitrifying Microbial Communities in Riverbed Sediments. Frontiers in Microbiology, 9. https://doi.org/10.3389/fmicb.2018.02794
Arce MI, Mendoza-Lera C, Almagro M, Catalán N, Romaní AM, Martí E, Gómez R, Bernal S, Foulquier A, Mutz M, Marcé R, Zoppini A, Gionchetta G, Weigelhofer G, del Campo R, Robinson CT, Gilmer A, Rulik M, Obrador B, von Schiller D (2019) A conceptual framework for understanding the biogeochemistry of dry riverbeds through the lens of soil science. Earth Sci Rev 188:441–453. https://doi.org/10.1016/j.earscirev.2018.12.001
Arce MI, Bengtsson MM, von Schiller D, Zak D, Täumer J, Urich T, Singer G (2021) Desiccation time and rainfall control gaseous carbon fluxes in an intermittent stream. Biogeochemistry. https://doi.org/10.1007/s10533-021-00831-6
Arnosti C, Bell C, Moorhead DL, Sinsabaugh RL, Steen AD, Stromberger M, Wallenstein M, Weintraub MN (2014) Extracellular enzymes in terrestrial, freshwater, and marine environments: Perspectives on system variability and common research needs. Biogeochemistry 117(1):5–21. https://doi.org/10.1007/s10533-013-9906-5
Aslam SN, Dumbrell AJ, Sabir JS, Mutwakil MHZ, Baeshen MMN, Abo-Aba SEM, Clark DR, Yates SA, Baeshen NA, Underwood GJC, McGenity TJ (2016) Soil compartment is a major determinant of the impact of simulated rainfall on desert microbiota. Environ Microbiol 18(12):5048–5062. https://doi.org/10.1111/1462-2920.13474
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7(11):2229–2241. https://doi.org/10.1038/ismej.2013.104
Battin TJ (1999) Hydrologic flow paths control dissolved organic carbon fluxes and metabolism in an Alpine stream hyporheic zone. Water Resour Res 35(10):3159–3169. https://doi.org/10.1029/1999WR900144
Battin TJ, Kaplan LA, Newbold D, Hansen CME (2003) Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426(6965):439–442. https://doi.org/10.1038/nature02152
Battin TJ, Kaplan LA, Findlay S, Hopkinson CS, Marti E, Packman A, Newbold JD, Sabater F (2008) Biophysical controls on organic carbon fluxes in fluvial networks. Nat Geosci 1(2):95–100. https://doi.org/10.1038/ngeo101
Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14(4):251–263. https://doi.org/10.1038/nrmicro.2016.15
Bell T, Gessner MO, Griffiths RI, McLaren JR, Morin PJ, Heijden MVD, Putten WHVD (2009) Microbial biodiversity and ecosystem functioning under controlled conditions and in the wild. In: Naeem S, Bunker DE, Hector A, Loreau M, Perrings C (eds) Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199547951.003.0009
Bernal S, Butturini A, Sabater F (2005) Seasonal variations of dissolved nitrogen and DOC:DON ratios in an intermittent mediterranean stream. Biogeochemistry 75(2):351–372. https://doi.org/10.1007/s10533-005-1246-7
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10(1):9–31. https://doi.org/10.1007/BF01343734
Bittar TB, Vieira AAH, Stubbins A, Mopper K (2015) Competition between photochemical and biological degradation of dissolved organic matter from the cyanobacteria Microcystis aeruginosa. Limnol Oceanogr 60(4):1172–1194. https://doi.org/10.1002/lno.10090
Boodoo KS, Schelker J, Trauth N, Battin TJ, Schmidt C (2019) Sources and variability of CO2 in a prealpine stream gravel bar. Hydrol Process 33(17):2279–2299. https://doi.org/10.1002/hyp.13450
Caillon F, Schelker J (2020) Dynamic transfer of soil bacteria and dissolved organic carbon into small streams during hydrological events. Aquat Sci 82(2):41. https://doi.org/10.1007/s00027-020-0714-4
Caillon F, Besemer K, Peduzzi P, Schelker J (2021) Soil microbial inoculation during flood events shapes headwater stream microbial communities and diversity. Microb Ecol. https://doi.org/10.1007/s00248-021-01700-3
Chen M, Kim S-H, Jung H-J, Hyun J-H, Choi JH, Lee H-J, Huh I-A, Hur J (2017) Dynamics of dissolved organic matter in riverine sediments affected by weir impoundments: Production, benthic flux, and environmental implications. Water Res 121:150–161. https://doi.org/10.1016/j.watres.2017.05.022
Coulson LE, Schelker J, Attermeyer K, Griebler C, Hein T, Weigelhofer G (2021) Experimental desiccation indicates high moisture content maintains hyporheic biofilm processes during drought in temperate intermittent streams. Aquat Sci 83(3):46. https://doi.org/10.1007/s00027-021-00799-3
Datry T, Bonada N, Boulton AJ (2017) Chapter 1—general introduction. In: Datry T, Bonada N, Boulton A (eds) Intermittent rivers and ephemeral streams. Academic Press, Cambridge, pp 1–20. https://doi.org/10.1016/B978-0-12-803835-2.00001-2
Datry T, Larned ST, Tockner K (2014) Intermittent rivers: a challenge for freshwater ecology. BioScience 64(3):229–235. https://doi.org/10.1093/biosci/bit027
Dingman SL (2002) Physical hydrology. Prentice Hall, Hoboken
Drake TW, Raymond PA, Spencer RGM (2018) Terrestrial carbon inputs to inland waters: a current synthesis of estimates and uncertainty. Limnol Oceanogr Lett 3(3):132–142. https://doi.org/10.1002/lol2.10055
Du Y, Zhang Y, Chen F, Chang Y, Liu Z (2016) Photochemical reactivities of dissolved organic matter (DOM) in a sub-alpine lake revealed by EEM-PARAFAC: an insight into the fate of allochthonous DOM in alpine lakes affected by climate change. Sci Total Environ 568:216–225. https://doi.org/10.1016/j.scitotenv.2016.06.036
Duhamel S, Jacquet S (2006) Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. J Microbiol Methods 64(3):316–332. https://doi.org/10.1016/j.mimet.2005.05.008
Dunn OJ (1961) Multiple comparisons among means. J Am Stat Assoc 56(293):52–64. https://doi.org/10.1080/01621459.1961.10482090
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinf (Oxford England) 27(16):2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Ejarque E, Freixa A, Vazquez E, Guarch A, Amalfitano S, Fazi S, Romaní AM, Butturini A (2017) Quality and reactivity of dissolved organic matter in a Mediterranean river across hydrological and spatial gradients. Sci Total Environ 599–600:1802–1812. https://doi.org/10.1016/j.scitotenv.2017.05.113
Fasching C, Ulseth AJ, Schelker J, Steniczka G, Battin TJ (2016) Hydrology controls dissolved organic matter export and composition in an Alpine stream and its hyporheic zone. Limnol Oceanogr 61(2):558–571. https://doi.org/10.1002/lno.10232
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34(6):777–787. https://doi.org/10.1016/S0038-0717(02)00007-X
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Freeman C, Lock MA (1995) The biofilm polysaccharide matrix: a buffer against changing organic substrate supply? Limnol Oceanogr 40(2):273–278. https://doi.org/10.4319/lo.1995.40.2.0273
Fromin N, Pinay G, Montuelle B, Landais D, Ourcival JM, Joffre R, Lensi R (2010) Impact of seasonal sediment desiccation and rewetting on microbial processes involved in greenhouse gas emissions. Ecohydrology 3(3):339–348. https://doi.org/10.1002/eco.115
Frossard A, Gerull L, Mutz M, Gessner MO (2012) Disconnect of microbial structure and function: Enzyme activities and bacterial communities in nascent stream corridors. ISME J 6(3):680–691. https://doi.org/10.1038/ismej.2011.134
Gabor R, Baker A, McKnight DM, Miller M (2014) Fluorescence indices and their interpretation. Aquat Org Matter Fluorescence. https://doi.org/10.1017/CBO9781139045452.015
Gallo E, Lohse K, Ferlin C, Meixner T, Brooks P (2013) Physical and biological controls on trace gas fluxes in semi-arid urban ephemeral waterways. Biogeochemistry. https://doi.org/10.1007/s10533-013-9927-0
Gessner MO (1991) Differences in processing dynamics of fresh and dried leaf litter in a stream ecosystem. Freshw Biol 26(3):387–398. https://doi.org/10.1111/j.1365-2427.1991.tb01406.x
Gionchetta G, Oliva F, Menéndez M, Laseras PL, Romaní AM (2019) Key role of streambed moisture and flash storms for microbial resistance and resilience to long-term drought. Freshw Biol 64(2):306–322. https://doi.org/10.1111/fwb.13218
Gionchetta G, Romaní AM, Oliva F, Artigas J (2019) Distinct responses from bacterial, archaeal and fungal streambed communities to severe hydrological disturbances. Sci Rep 9(1):13506. https://doi.org/10.1038/s41598-019-49832-4
Gionchetta G, Oliva F, Romaní AM, Bañeras L (2020) Hydrological variations shape diversity and functional responses of streambed microbes. Sci Total Environ 714:136838. https://doi.org/10.1016/j.scitotenv.2020.136838
Gómez-Gener L, Obrador B, Marcé R, Acuña V, Catalán N, Casas-Ruiz J, Sabater S, Muñoz I, Von Schiller D (2016) When water vanishes: magnitude and regulation of carbon dioxide emissions from dry temporary streams. Ecosystems 19:1–14. https://doi.org/10.1007/s10021-016-9963-4
Gómez-Gener L, Siebers AR, Arce MI, Arnon S, Bernal S, Bolpagni R, Datry T, Gionchetta G, Grossart H-P, Mendoza-Lera C, Pohl V, Risse-Buhl U, Shumilova O, Tzoraki O, von Schiller D, Weigand A, Weigelhofer G, Zak D, Zoppini A (2021) Towards an improved understanding of biogeochemical processes across surface-groundwater interactions in intermittent rivers and ephemeral streams. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2021.103724
Harjung A, Sabater F, Butturini A (2018) Hydrological connectivity drives dissolved organic matter processing in an intermittent stream. Limnologica 68:71–81. https://doi.org/10.1016/j.limno.2017.02.007
Harjung A, Ejarque E, Battin T, Butturini A, Sabater F, Stadler M, Schelker J (2019a) Experimental evidence reveals impact of drought periods on dissolved organic matter quality and ecosystem metabolism in subalpine streams. Limnol Oceanogr 64(1):46–60. https://doi.org/10.1002/lno.11018
Harjung A, Perujo N, Butturini A, Romaní AM, Sabater F (2019b) Responses of microbial activity in hyporheic pore water to biogeochemical changes in a drying headwater stream. Freshw Biol 64(4):735–749. https://doi.org/10.1111/fwb.13258
Hartmann A, Gleeson T, Rosolem R, Pianosi F, Wada Y, Wagener T (2015) A large-scale simulation model to assess karstic groundwater recharge over Europe and the Mediterranean. Geosci Model Dev 8(6):1729–1746. https://doi.org/10.5194/gmd-8-1729-2015
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53(3):955–969. https://doi.org/10.4319/lo.2008.53.3.0955
Hotchkiss ER, Hall RO Jr, Sponseller RA, Butman D, Klaminder J, Laudon H, Rosvall M, Karlsson J (2015) Sources of and processes controlling CO2 emissions change with the size of streams and rivers. Nature Geosci 8(9):696–699. https://doi.org/10.1038/ngeo2507
Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88(10):2427–2439. https://doi.org/10.1890/06-1736.1
Kaiser M, Kleber M, Berhe AA (2015) How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol Biochem 80:324–340. https://doi.org/10.1016/j.soilbio.2014.10.018
Kosten S, Berg S, van den, Mendonça R, Paranaíba JR, Roland F, Sobek S, Hoek JVD, Barros N (2018) Extreme drought boosts CO2 and CH4 emissions from reservoir drawdown areas. Inland Waters 8(3):329–340. https://doi.org/10.1080/20442041.2018.1483126
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microbiol 79(17):5112–5120. https://doi.org/10.1128/AEM.01043-13
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621. https://doi.org/10.1080/01621459.1952.10483441
Langenheder S, Bulling MT, Solan M, Prosser JI (2010) Bacterial biodiversity-ecosystem functioning relations are modified by environmental complexity. PLoS ONE 5(5):e10834. https://doi.org/10.1371/journal.pone.0010834
Leff L, Gray JBV, Martí E, Merbt SN, Romaní AM (2016) Aquatic biofilms and biogeochemical processes. In: Romaní AM, Guasch H, Balaguer MD (eds) Aquatic biofilms: ecology, water quality and wastewater treatment. Caister Academic Press, Cambridge, pp 89–108. https://doi.org/10.21775/9781910190173.05
Lin H, Guo L (2020) Variations in colloidal DOM composition with molecular weight within individual water samples as characterized by flow field-flow fractionation and EEM-PARAFAC analysis. Environ Sci Technol 54(3):1657–1667. https://doi.org/10.1021/acs.est.9b07123
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18(1):50–60
Marcé R, Obrador B, Gómez-Gener L, Catalán N, Koschorreck M, Arce MI, Singer G, von Schiller D (2019) Emissions from dry inland waters are a blind spot in the global carbon cycle. Earth Sci Rev 188:240–248. https://doi.org/10.1016/j.earscirev.2018.11.012
Marxsen J, Fiebig DM (1993) Use of perfused cores for evaluating extracellular enzyme activity in stream-bed sediments. FEMS Microbiol Ecol 13(1):1–11. https://doi.org/10.1111/j.1574-6941.1993.tb00045.x
McKew BA, Taylor JD, McGenity TJ, Underwood GJC (2011) Resistance and resilience of benthic biofilm communities from a temperate saltmarsh to desiccation and rewetting. ISME J 5(1):30–41. https://doi.org/10.1038/ismej.2010.91
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
Meisner A, Bååth E, Rousk J (2013) Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol Biochem 66:188–192. https://doi.org/10.1016/j.soilbio.2013.07.014
Murphy KR, Stedmon CA, Wenig P, Bro R (2014) OpenFluor—an online spectral library of auto-fluorescence by organic compounds in the environment. Anal Methods 6(3):658–661. https://doi.org/10.1039/C3AY41935E
Obrador B, von Schiller D, Marcé R, Gómez-Gener L, Koschorreck M, Borrego C, Catalán N (2018) Dry habitats sustain high CO2 emissions from temporary ponds across seasons. Sci Rep 8(1):3015. https://doi.org/10.1038/s41598-018-20969-y
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community Ecology Package (2.5-7) [Computer software]. https://doi.org/https://CRAN.R-project.org/package=vegan
Oprei A, Zlatanović S, Mutz M (2019) Grazers superimpose humidity effect on stream biofilm resistance and resilience to dry-rewet stress. Sci Total Environ 659:841–850. https://doi.org/10.1016/j.scitotenv.2018.12.316
Osburn CL, Boyd TJ, Montgomery MT, Bianchi TS, Coffin RB, Paerl HW (2016) Optical proxies for terrestrial dissolved organic matter in estuaries and coastal waters. Front Mar Sci 2:127. https://doi.org/10.3389/fmars.2015.00127
Peter H, Ylla I, Gudasz C, Romaní AM, Sabater S, Tranvik LJ (2011) Multifunctionality and diversity in bacterial biofilms. PLoS ONE 6(8):e23225. https://doi.org/10.1371/journal.pone.0023225
Pinto MS, Weigelhofer R, Diaz-Pines G, Guerreiro Brito E, Zechmeister-Boltenstern A, Hein T (2020) River-floodplain restoration and hydrological effects on GHG emissions: Biogeochemical dynamics in the parafluvial zone. Sci Total Environ 715:136980. https://doi.org/10.1016/j.scitotenv.2020.136980
Pohlon E, Fandino AO, Marxsen J (2013) Bacterial community composition and extracellular enzyme activity in temperate streambed sediment during drying and rewetting. PLoS ONE 8(12):e83365. https://doi.org/10.1371/journal.pone.0083365
Pohlon E, Rütz NK, Ekschmitt K, Marxsen J (2018) Recovery dynamics of prokaryotes and extracellular enzymes during sediment rewetting after desiccation. Hydrobiologia 820(1):255–266. https://doi.org/10.1007/s10750-018-3662-4
Pucher M, Wünsch U, Weigelhofer G, Murphy K, Hein T, Graeber D (2019) staRdom: versatile software for analyzing spectroscopic data of dissolved organic matter in R. Water 11(11):2366. https://doi.org/10.3390/w11112366
Pusch M, Fiebig D, Brettar I, Eisenmann H, Ellis BK, Kaplan LA, Lock MA, Naegeli MW, Traunspurger W (1998) The role of micro-organisms in the ecological connectivity of running waters. Freshw Biol 40(3):453–495. https://doi.org/10.1046/j.1365-2427.1998.00372.x
Queimaliños C, Reissig M, Pérez GL, Soto Cárdenas C, Gerea M, Garcia PE, García D, Diéguez MC (2019) Linking landscape heterogeneity with lake dissolved organic matter properties assessed through absorbance and fluorescence spectroscopy: Spatial and seasonal patterns in temperate lakes of Southern Andes (Patagonia, Argentina). Sci Total Environ 686:223–235. https://doi.org/10.1016/j.scitotenv.2019.05.396
R Core Development Team (2020) The R project for statistical computing. https://www.r-project.org/. Accessed 6 Apr 2020
Romaní AM, Sabater S (2001) Structure and activity of rock and sand biofilms in a mediterranean stream. Ecology 82(11):3232–3245. https://doi.org/10.1890/0012-9658(2001
Sabater S, Timoner X, Borrego C, Acuña V (2016) Stream biofilm responses to flow intermittency: from cells to ecosystems. Front Environ Sci. https://doi.org/10.3389/fenvs.2016.00014
Schelker J, Singer GA, Ulseth AJ, Hengsberger S, Battin TJ (2016) CO2 evasion from a steep, high gradient stream network: Importance of seasonal and diurnal variation in aquatic pCO2 and gas transfer. Limnol Oceanogr 61(5):1826–1838. https://doi.org/10.1002/lno.10339
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88(6):1386–1394. https://doi.org/10.1890/06-0219
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541. https://doi.org/10.1128/AEM.01541-09
Shumilova O, Zak D, Datry T, von Schiller D, Corti R, Foulquier A, Obrador B, Tockner K, Altermatt F, Arce MI, Arnon S, Banas D, Banegas-Medina A, Beller E, Blanchette ML, Blanco‐Libreros JF, Blessing J, Boëchat IG, Boersma K, Zarfl C (2019) Simulating rewetting events in intermittent rivers and ephemeral streams: a global analysis of leached nutrients and organic matter. Glob Change Biol. https://doi.org/10.1111/gcb.14537
Shutova Y, Baker A, Bridgeman J, Henderson RK (2014) Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: from PARAFAC analysis to online monitoring wavelengths. Water Res 54:159–169. https://doi.org/10.1016/j.watres.2014.01.053
Smith RB (1979) The influence of mountains on the atmosphere. In: Saltzman B (ed) Advances in geophysics, vol 21. Elsevier, New York, pp 87–230. https://doi.org/10.1016/S0065-2687(08)60262-9
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob Change Biol 13(2):426–436. https://doi.org/10.1111/j.1365-2486.2006.01307.x
Spreen W (1947) A determination of the effect of topography upon precipitation. Eos Trans Am Geophys Union 28(2):285–290. https://doi.org/10.1029/TR028i002p00285
Thijs S, Beeck MOD, Beckers B, Truyens S, Stevens V, Hamme JDV, Weyens N, Vangronsveld J (2017) Comparative evaluation of four bacteria-specific primer pairs for 16S rRNA gene surveys. Front Microbiol 4:5. https://doi.org/10.3389/fmicb.2017.00494
Tiefenbacher A, Weigelhofer G, Klik A, Pucher M, Santner J, Wenzel W, Eder A, Strauss P (2020) Short-term effects of fertilization on dissolved organic matter in soil leachate. Water 12(6):1617. https://doi.org/10.3390/w12061617
Timoner X, Acuña V, Schiller DV, Sabater S (2012) Functional responses of stream biofilms to flow cessation, desiccation and rewetting. Freshw Biol 57(8):1565–1578. https://doi.org/10.1111/j.1365-2427.2012.02818.x
Timoner X, Acuña V, Frampton L, Pollard P, Sabater S, Bunn SE (2014) Biofilm functional responses to the rehydration of a dry intermittent stream. Hydrobiologia 727(1):185–195. https://doi.org/10.1007/s10750-013-1802-4
van Rijn LC (1993) Principles of sediment transport in rivers, estuaries and coastal seas. Aqua Publications, Amsterdam
Vazquez E, Amalfitano S, Fazi S, Butturini A (2018) (n.d.). Dissolved organic matter composition in a fragmented Mediterranean fluvial system under severe drought conditions | SpringerLink. Available (July 3) from https://doi.org/10.1007/s10533-010-9421-x
von Schiller D, Acuña V, Graeber D, Martí E, Ribot M, Sabater S, Timoner X, Tockner K (2011) Contraction, fragmentation and expansion dynamics determine nutrient availability in a Mediterranean forest stream. Aquat Sci 73(4):485. https://doi.org/10.1007/s00027-011-0195-6
von Schiller D, Marcé R, Obrador B, Gómez-Gener L, Casas-Ruiz J, Acuña V, Koschorreck M (2014) Carbon dioxide emissions from dry watercourses. Inland Waters 4(4):377–382. https://doi.org/10.5268/IW-4.4.746
von Schiller D, Datry T, Corti R, Foulquier A, Tockner K, Marcé R, García-Baquero G, Odriozola I, Obrador B, Elosegi A, Mendoza‐Lera C, Gessner MO, Stubbington R, Albariño R, Allen DC, Altermatt F, Arce MI, Arnon S, Banas D, Zoppini A (2019) Sediment respiration pulses in intermittent rivers and ephemeral streams. Glob Biogeochem Cycles 33(10):1251–1263. https://doi.org/10.1029/2019GB006276
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37(20):4702–4708. https://doi.org/10.1021/es030360x
Wilson HF, Xenopoulos MA (2009) Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat Geosci 2(1):37–41. https://doi.org/10.1038/ngeo391
Yamashita Y, Kloeppel BD, Knoepp J, Zausen GL, Jaffé R (2011) Effects of watershed history on dissolved organic matter characteristics in headwater streams. Ecosystems 14(7):1110–1122. https://doi.org/10.1007/s10021-011-9469-z
Zeglin LH, Dahm CN, Barrett JE, Gooseff MN, Fitpatrick SK, Takacs-Vesbach CD (2011) Bacterial community structure along moisture gradients in the parafluvial sediments of two ephemeral desert streams. Microb Ecol 61(3):543–556. https://doi.org/10.1007/s00248-010-9782-7
Zlatanović S, Fabian J, Premke K, Mutz M (2018) Shading and sediment structure effects on stream metabolism resistance and resilience to infrequent droughts. Sci Total Environ 621:1233–1242. https://doi.org/10.1016/j.scitotenv.2017.10.105
Zoppini A, Marxsen J (2010) Importance of extracellular enzymes for biogeochemical processes in temporary river sediments during fluctuating dry–wet conditions. Soil Enzymol. https://doi.org/10.1007/978-3-642-14225-3_6
Acknowledgements
We would like to thank Martin Tschikof and Damiano Baldan for assistance in the lab and with experimental design, as well as Matthias Pucher for assistance with DOM data analysis. We would also like to thank Michael Mayr and Annette Puritscher for assistance in constructing the flumes.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The study was funded by a research opportunity ‘STONE’ of the City of Vienna, MA31-Wiener Wasser (MA31-985621/17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: J.M. Melack
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coulson, L.E., Weigelhofer, G., Gill, S. et al. Small rain events during drought alter sediment dissolved organic carbon leaching and respiration in intermittent stream sediments. Biogeochemistry 159, 159–178 (2022). https://doi.org/10.1007/s10533-022-00919-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00919-7