Abstract
Sea turtles spend the majority of their lives at foraging grounds. These areas are important for population persistence but generally occur in coastal habitats, which are under increasing human pressure. Identifying key foraging areas is therefore an important step to understanding critical sea turtle habitats, and therefore their threats. Isotope ratios (δ15N, δ13C) from skin tissues of 90 green turtles (Chelonia mydas) nesting at the regionally important rookery of Aldabra Atoll, Seychelles, were analyzed with samples collected during two periods: 51 samples from March to September 2021, and 39 samples from January to April 2022. These analyses were complemented by nine satellite tracks to identify key foraging areas. Hierarchical clustering and discriminant function analysis identified two main foraging areas: the East African coast (Tanzania and Somalia) and northern Madagascar. Isotopic values indicated that the majority of green turtles nesting at Aldabra forage at one of these two areas, with slightly more green turtles visiting the East African coast. Geographical isotopic patterns were not found latitudinally, suggesting a potential limit to this approach for coastal areas in the Western Indian Ocean. However, we found longitudinal variation of δ13C and δ15N that few studies have reported. This is the first study in this region to combine satellite telemetry and stable isotope analysis to assign likely foraging areas of green turtles and advances understanding of the complex migration patterns and foraging ecology of this species. We demonstrate the potential and the limitations of stable isotope analysis for identifying/characterizing green turtle foraging areas in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migration is an integral part of the life histories of numerous species. For long-lived animals, it often involves seasonal round-trip movements between foraging and breeding grounds (Dingle and Drake 2007). Sea turtles, for example, exhibit philopatry, returning to genetically distinct nesting areas, but have mixed foraging grounds (Jensen et al. 2013). Understanding the migration patterns of sea turtles is essential for comprehending habitat connectivity and implementing effective, long-term conservation measures. Protecting sea turtles at or near their nesting beaches has been instrumental to the recovery of previously declining nesting populations (Mazaris et al. 2017; Pritchard et al. 2022). However, reproductive females likely spend > 75% of their time in their foraging areas (discussed in Shimada et al. 2020), and these regions can have a significant influence on body size and fecundity (e.g. in loggerhead turtles Caretta caretta; Ceriani et al. 2015). Declines in the number of juvenile green turtles at foraging aggregations have been recorded (e.g. green turtles in Florida; Long et al. 2021) despite increasing trends in the nearby nesting female populations (Chaloupka et al. 2008). Since many foraging aggregations are composed of sea turtles from multiple nesting regions (Bjorndal and Bolten 2008), decreases in nesting populations due to threats in foraging areas (e.g., habitat degradation, fisheries, etc.) may not be detected by nesting beach monitoring for several years (e.g., Ceriani et al. 2019). Identifying and accessing foraging areas remains challenging, hindering the assessment of threats and prioritization of conservation efforts. Nonetheless, tools, such as satellite telemetry, have helped to identify key conservation areas by identifying crucial foraging habitats and migratory pathways in various ocean regions (e.g., Ferreira et al. 2021; Cerritelli et al. 2022; Lamont et al. 2023).
Stable isotope analysis (SIA) is a powerful complementary tool to satellite telemetry for understanding the spatial and foraging ecology of sea turtles (Ceriani et al. 2017; Bradshaw et al. 2017). SIA relies on variations in the ratio of heavy to light isotopes of common elements, which exhibit spatial and temporal variability, leading to natural and predictable isotope gradients within the environment (DeNiro and Epstein 1978, 1981). Analyzing animal tissues with slower turnover rates such as skin or muscle enables inferences to be made on the animal’s foraging location over a longer period of time, ranging from several weeks to months, depending on the species and tissue type (Hobson 1999; Rubenstein and Hobson 2004). This can reveal the isotopic values of the non-breeding foraging areas when samples are collected at breeding grounds, if the animal remains long enough in a non-breeding area to assimilate a local isotopic signature and moves between isotopically distinct areas (Hobson 1999; Rubenstein and Hobson 2004; Hobson and Wassenaar 2019). SIA provides coarser geographic information than satellite telemetry but allows for a larger sample size when the two techniques are combined (Newsome et al. 2007). Commonly used stable isotopes in sea turtle research are carbon (δ13C) and nitrogen (δ15N) (Ceriani et al. 2012; Seminoff et al. 2012; Vander Zanden et al. 2014). The δ13C of a consumers’ tissues reflects that of the primary producer, since there is minimal fractionation (~ 1‰) with successive trophic levels (DeNiro and Epstein 1978). δ13C has generally been used to identify location, because it decreases with increasing latitude, and benthic environments tend to be more enriched than pelagic (Rubenstein and Hobson 2004). δ15N is more indicative of trophic position (DeNiro and Epstein 1978, 1981) because the δ15N of a consumer is enriched by its diet. δ15N values increase when eating prey with higher trophic positions in the food web (DeNiro and Epstein 1981). Using SIA to determine foraging areas requires knowledge of the spatial distribution of isotopic values in potential areas, through large-scale isotopic maps or regional isoscapes (Graham et al. 2010; McMahon et al. 2013), although such studies are limited (but see Lorrain et al. 2015). Some sea turtle studies have bypassed this limitation by using isotopic values of tissue samples with lower turnover rates (i.e., skin has a 3‒4 month turnover in fast growing juvenile sea turtles; Reich et al. 2008) (Seminoff et al. 2007; Reich et al. 2008; Ceriani et al. 2014a) from satellite tracked females to characterize isotopically distinct regions. Isotopic values of satellite tracked females are then used to develop models to assign the likely origin of non-satellite tracked individuals that are sampled for SIA, allowing for larger sample sizes (Ceriani et al. 2012, 2014b; Seminoff et al. 2012; Bradshaw et al. 2017).
The Western Indian Ocean (WIO) hosts globally important rookeries for Endangered green turtles (Chelonia mydas) (Seminoff et al. 2015), mostly on islands (Mortimer et al. 2020). Satellite telemetry has been used to investigate foraging areas in the region with nesting green turtles in the Chagos islands (Hays et al. 2020) and in the French territories (Dalleau et al. 2019). North-west Madagascar, South Madagascar, central Tanzania, and North and South Mozambique have been identified as green turtle foraging hotspots in this region, which is supported by satellite tracked green turtles within the Mozambique Channel (Dalleau et al. 2019). These modeled foraging hotspots had some overlap (such as along East Africa and Madagascar) with those used by satellite tagged females from the Chagos islands to their foraging grounds (Hays et al. 2020). One study so far has looked at adult green turtle diet in the WIO, with seagrass as the dominant prey item for non-breeding females (Stokes et al. 2019). Otherwise, very little is known about adult green turtle diet in this region (Esteban et al. 2020).
Despite their vast spatial range, green turtles in the WIO continue to face threats, including from fisheries interactions and direct take (Williams et al. 2016; Temple et al. 2018; van de Geer et al. 2022). In South-west Madagascar, it is estimated that at least 10,000 green turtles are caught and sold annually (Humber et al. 2011), emphasizing the importance of identifying and protecting critical foraging habitats in the region to ensure conservation efforts are effective and sustained. To do so, a sufficiently large sample size to ensure representation of the nesting populations is needed, which can be achieved with SIA.
The second largest green turtle rookeries in the WIO is Aldabra Atoll (> 15,000 clutches annually), which is a large, remote atoll in the southern Seychelles (Pritchard et al. 2022). Aldabra falls within the Southwest Indian Ocean regional management unit (RMU) for green turtles (Wallace et al. 2023). The RMU has an estimated 103,000‒144,000 green turtle clutches laid annually (Mortimer et al. 2020) and over 10% are laid at Aldabra (Pritchard et al. 2022). Therefore, there is a lot of conservation value to understanding what foraging grounds green turtles nesting at Aldabra use. In the WIO, SIA has been evaluated for co-occurring large marine megafauna surrounding La Reunion (including foraging green turtles) (Chandelier et al. 2023), but it has not been evaluated in the region for linking nesting green turtles to their foraging areas. Also, the Indian Ocean has been recognized as having a lack of SIA research for contributing to and advancing ecological knowledge and making effective conservation measures for sea turtles in the region (Pearson et al. 2017; Haywood et al. 2019).
Our overall aim was to investigate the use of bulk carbon and nitrogen stable isotope analyses in identifying the foraging areas used by green turtles nesting at Aldabra, within the broader WIO. Using preliminary information on foraging areas obtained by satellite tracking (Seychelles Islands Foundation, unpubl. data), we investigated (i) whether nesting green turtles at Aldabra utilize isotopically distinct foraging areas; and (ii) where they primarily forage.
Methods
Study site and sampling
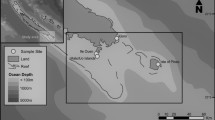
Aldabra Atoll, managed by the Seychelles Islands Foundation, is a large raised atoll and UNESCO World Heritage site in the Western Indian Ocean (WIO; 9°25’S, 46°20’E; Fig. 1). It is ca. 34 × 14 km with a total land area of 155 km2 with four main islands that surround a large, mostly shallow lagoon (< 3 m) (Hamylton et al. 2018). There are 52 beaches (total beach length ca. 5.2 km) around the outer coastline of the islands which are used by nesting green turtles (Pritchard et al. 2022).
Foraging areas (FA)/end points of the 13 satellite tracked green turtles after nesting on Aldabra (shown in insert; research station/Settlement beach represented with a star) (SIF, unpubl. data). Green turtle numbers 1–5 coincide with the endpoints in Madagascar (FA2; filled triangles). Numbers 6–11 are endpoints along the East African coastline (FA1; filled circles) (Table S1, supplementary material). Green turtles 12 and 13 had no isotopic data, and green turtles 1 and 6 were not considered to belong to neither FA1 nor FA2 (filled boxes)
A subset of nesting females was encountered and sampled for stable isotope analysis (SIA) on Settlement Beach during night patrols and morning track count surveys in three periods; from March to October 2020 (n = 39), March to September 2021 (n = 51) and January to April 2022 (n = 39), hereafter referred to by the year of sampling. Curved carapace length notch-to-tip (CCLn-t) was recorded (Bolten 1999), and as part of a long-term monitoring program, unique metal tags were applied to both front flippers (Titanium Turtle Tags, Stockbrands Pty Ltd) (Balazs 1999) after the green turtle started covering her eggs. In 2020, epidermis (0.3 cm2) was sampled from the soft skin on the trailing edge of the rear flipper (originally sampled for a separate genetic study), while in 2021 and 2022, the top epidermal layer (‘skin sample’; 0.3 cm2) was sampled from the neck (between the neck and front flipper). All skin samples were collected with sterilized razor blades. In 2020, samples were originally stored in 99.8% ethanol (ETOH) for 372–567 days, then a small piece was subsampled and transferred to 75% ETOH for ca. 9 months until analyzed. In 2021 and 2022, skin samples were stored in iodized salt.
Additionally, in 2022, 13 green turtles were satellite tagged (SIF, unpubl. Data). Eleven of those satellite tagged green turtles were also skin sampled for SIA; therefore the 2022 skin sample total for SIA (n = 39) had 28 non-satellite tagged green turtle tissues and 11 satellite tagged green turtle tissues. For the 13 satellite tagged green turtles, Wildlife computers Splash 10-224D (n = 5), Lotek Kiwisat K237E (n = 5), and Telonics SeaTrkr units (n = 3) were applied to the green turtles’ carapaces using standard procedures (Coyne et al. 2008): nesting females were approached after laying or while returning to the sea after an unsuccessful nesting attempt, and were restrained in a wooden box. The carapace was cleaned and sanded, then cleaned with acetone. Epoxy resin (DeWalt Pure 150-Pro) was applied to the highest, flattest part of the carapace along the vertebral scutes and to the bottom of the tag (also roughened with sandpaper and cleaned with acetone). The tag was attached to the carapace, additional epoxy applied, and smoothed along the shell. Green turtles were released once the epoxy had dried, generally 2–3 h after the tag was applied (Fig. S1, supplementary material).
The three different tag types might theoretically affect the length of transmissions. However, no such evidence exists (Hart et al. 2021), and for our aim, the tags just need to transmit long enough to identify their wide foraging areas, which could be achieved for all tags.
Sample preparation and SIA
Samples were prepared for SIA following standard procedures (Ceriani et al. 2014b, 2015). Each sample was put in successive distilled water baths to remove NaCl, then surface epidermis and any dermal tissue underneath was separated. The epidermis was dried in the drying oven overnight (> 12 h) at 60 °C. Each tissue was cut into several small pieces, and lipids were extracted using an accelerated solvent extractor (Model 200, Dionex) with petroleum ether. Each batch underwent 5 min of heating followed by 5 min of static purging, three times.
Subsamples of prepared tissues (0.2–0.6 mg) were weighed with a microbalance and sealed into sterile 3 mm x 5 mm tin capsules and analyzed for % carbon, % nitrogen, ẟ13C, and ẟ15N values. Analyses were performed at the Marine Environmental Chemistry Laboratory at the University of South Florida College of Marine Science (St. Petersburg, FL, USA), where samples were converted to N2 and CO2 using a Carlo-Erba NA EA1108 Elemental Analyzer (Thermoquest Italia, S.p.A., Rodano, Italy) and analyzed with a continuous flow isotope ratio mass spectrometer (Delta PlusXP, Thermo Finnigan, Bremen, Germany). Stable isotope ratios were expressed in conventional notation as parts per thousand (‰) according to the following equation:
where X is 15N or 13C, and R represents the corresponding ratios of heavy to light isotopes (15N:14N and 13C:12C) in the sample and international standard, respectively. Raw measurements were calibrated relative to VPDB (δ13C) and AT-Air (δ15N) with certified reference materials NIST 8574 (δ13C = + 37.63 ± 0.10‰, δ15N = + 47.57 ± 0.22‰, N = 9.52%, C = 40.81%, C: N [molar] = 5.0) and NIST 8573 (ẟ13C = -26.39 ± 0.09‰, δ15N = -4.52 ± 0.12‰ N = 9.52%, C = 40.81%, C: N [molar] = 5.0). Estimates of analytical precision were obtained by replicate (n = 58) measurements of an internal laboratory reference material (NIST1577b Bovine liver, ẟ13C = -21.69 ± 0.14‰, δ15N = 7.83 ± 0.16‰, %N = 9.95 ± 0.22%, %C = 48.04 ± 0.90%, C: N [molar] = 5.63 ± 0.08) and yielded a precision (reflecting ± 1 SD) ≤ 0.13‰ for ẟ13C and ≤ 0.11‰ for δ15N.
Tissue storage treatment comparison
Due to concerns in the literature about preservation method and effect on isotopic values (Barrow et al. 2008; Bradshaw et al. 2017), we compared tissue treatments, following Bradshaw et al. (2017). Twenty-two of the 39 skin samples collected in 2022 were large enough to be subdivided in three parts with a razor blade. They were placed into three treatments: salt, 75% ETOH, 99.8% ETOH. Tissues (between individuals) were in treatments from 105 to 151 days. A Friedman test was used to analyze the repeated treatments.
Satellite tagged green turtles and foraging areas
To designate if the green turtle’s reached their foraging ground, apparent minimum distances and time differences between successive locations were calculated and the ratio between the two was used to calculate the apparent minimum speeds between locations. These were used to filter out locations with speeds > 5 km h− 1. Displacement plots were made with difference in time and straight-line distance between the first location and successive locations, and the plots were visually inspected for migratory stage and foraging areas (Cerritelli et al. 2022). For our purpose, we needed to identify broad foraging areas rather than the exact foraging location of each individual. We considered a green turtle had reached their foraging area once their displacement gradient effectively reached zero. For the 13 satellite tagged green turtles, two main foraging areas were identified but two green turtles migrated elsewhere (one to South Madagascar, one to Mozambique). The displacement gradient of the green turtle that last transmitted in Mozambique did not reach zero, but due to the general pattern of Aldabra satellite tagged green turtles not switching between the East African coastline and Madagascar, Mozambique was considered her general foraging area. The 12 other green turtles were considered to have reached their respective general foraging areas (Fig S2; Table S1, supplementary material).
Data analyses
All analyses were performed in R (v4.3.0; R Core Team 2023) using RStudio (v1.2.5; Posit team 2022). The relationship between δ15N and δ13C and size or latitude and longitude was explored using the Pearson correlation coefficient. The relationship between year (2021, 2022) and δ15N and δ13C was investigated with a Wilcoxon rank sum test. Data were tested for normality and equal variance (Shapiro-Wilk and Levene’s tests), and since the results were not significant, a multivariate analysis of variance (MANOVA) with Pillai’s trace test and post-hoc Games-Howell (multiple comparison tests) were used to determine differences in isotopic values and foraging areas.
Two methods were used to group foraging areas and assign non-satellite tagged green turtles to a foraging area: (1) Ward’s hierarchical cluster analysis (Ward and Hook 1963), and (2) discriminant function analysis (DFA). For the first, the package “stats” (R Core Team 2023) was used with function “hclust” and method “ward.D2” using a Euclidean distance. For DFA, the package “MASS” (Venables and Ripley 2002) with function “lda” was used to take the stable isotope values of the training dataset (satellite tagged green turtles) to make predictions, using “predict()”, for the untagged green turtles. Function “lda” uses the ratio of between-class variance to within-class variance to ensure the best class separability (Venables and Ripley 2002). Nine of the 13 satellite tagged individuals were used as the training set with two foraging areas: FA1 (East Africa: n = 5) and FA2 (northern Madagascar; n = 4). The other four satellite tagged individuals were not included because two did not have skin tissues taken and two went to areas other than the two main areas above (a sample of one for each of these additional areas was too small for the analysis). The more conservative posterior probability ≥ 0.8 was employed since there is little information available about green turtles and stable isotopes in the region; this threshold gives a fourfold improvement in odds for assignment compared to random allocation (Wunder 2012) and has been used in similar studies (Haywood et al. 2020; Pfaller et al. 2020). Equal priors were used since the satellite tagged sample was too small to confidently use the observed proportions as priors. A leave-one-out cross-validation for individual assignment to a foraging area was carried out. The proportions of green turtles frequenting the two foraging areas were estimated through two approaches: (i) as the proportions of assigned individuals and (ii) as the average posterior probabilities of all individuals (Ceriani et al. 2023). Size (CCLn-t) was compared between the two groups with Welch’s t-test (incl. satellite tagged females).
Results
Sample preservation
For the comparison of skin sample storage method experiment for the salt, 75% ETOH and 99.8% ETOH, δ15N values differed between the three treatments (df = 2, p = 0.006; Table S2, supplementary material), while δ13C values did not (df = 2, p = 0.23). These effects, in addition to the magnitude of variation between the treatments being greater than the mass spectrometer’s precision, led to the exclusion of the 39 samples collected in 2020 from further analyses. Further support for excluding the 2020 samples includes that the samples were exposed to two different preservatives at two different times (99.8% ETOH then into 75% ETOH), and the skin tissue samples for SIA from 2020 were taken from the trailing edge of the rear flipper, while the samples from 2021 to 2022 were taken from the neck. Whether SI skin values differ among regions of the body has not been investigated in the literature. Therefore, only the 90 green turtles sampled in 2021 and 2022 and preserved with the same method (iodized salt) were analyzed.
General summary
Stable isotope values were 5.7–14.0‰ (mean 9.6 ± 1.7‰) for δ15N and from − 19.4 to -6.1‰ (-11.8 ± 3.50‰) for δ13C (n = 90). The average green turtle size was 109.2 ± 5.3 SD cm CCLn-t (range 95.7–128.0 cm; n = 90). No relationship was found between green turtle size and δ15N or δ13C (p > 0.05). In the 11 satellite tracked green turtles with isotopic values, δ15N decreased (t = -3.52, df = 9, p = 0.007) and δ13C increased (t = 2.38, df = 9, p = 0.041) with longitude (from west to east), while no relationship was detected with latitude for δ15N (t = 0.43, df = 9, p = 0.68) or δ13C (t = 0.47, df = 9, p = 0.65). There was no difference between year and δ15N (W = 1104.5, p = 0.37) and δ13C (W = 1009, p = 0.91).
Foraging areas
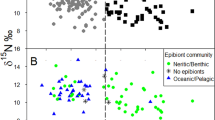
Hierarchical cluster analysis showed foraging areas in two clear foraging area clusters. One cluster (Cluster 1) included satellite tagged individuals who went to Tanzania (n = 3), Somalia (n = 2), Mozambique (n = 1), South Madagascar (n = 1), and North Madagascar (n = 1). The other cluster (Cluster 2) included three satellite tagged individuals that went to northern Madagascar (Figs. 1 and 2; Fig. S3, supplementary material). Two main foraging areas were identified: Somalia and Tanzania (Foraging area 1; hereafter ‘FA1’) and northern Madagascar (Foraging area 2; hereafter ‘FA2’). Only one satellite tagged green turtle, that migrated to north-east Madagascar was assigned to the ‘incorrect’ cluster (Cluster 1 instead of 2) while two green turtles assigned to Cluster 1 went to South Madagascar and Mozambique. Assuming that Cluster 1 and 2 indicate FA1 and FA2, respectively, of the total 90 green turtles sampled for SIA, 47 (52.2%) would be assigned to FA1 and 43 (47.8%) to FA2.
The isospace of untagged and satellite tagged green turtles nesting at Aldabra when split into two clusters (Cluster 1 = blue; Cluster 2 = red; Ward clustering). Satellite tagged individuals are shown with bold dots, with the number representing the foraging area group each green turtle was assigned to based on the three migration patterns detected by satellite tracking (1 = East Africa: Tanzania/ Somalia, 2 = northern Madagascar, 3 = other: South Madagascar + Mozambique). Labels indicate the region the satellite tagged green turtles went to: Mozambique (Moz), Madagascar (Mad), Tanzania (Tan)
The isotopic signatures of the nine satellite tagged green turtles assigned to FA1 and FA2 were different (MANOVA, Pillai = 0.85, approx. F = 17.39, num df = 2, den df = 6, p = 0.003) for both δ15N (p < 0.001) and δ13C (p = 0.044; Fig. 3). The DFA results assigned 100% of the nine satellite tagged green turtles with a posterior probability ≥ 0.8 and seven (77.8%) were assigned correctly. The two incorrectly assigned satellite tagged green turtles went to NE Madagascar (FA2, green turtle #5) and Tanzania (FA1, green turtle #8). For green turtles with unknown foraging areas, 73 of 81 (90%) were assigned to a foraging group with a posterior probability ≥ 0.8. Forty-five green turtles were assigned to FA1 (East Africa) and 28 to FA2 (northern Madagascar). For the whole dataset of satellite tagged and non-satellite tagged females with a posterior probability ≥ 0.8 (n = 82), 50 (61.0%) were assigned to FA1 and 32 (39.0%) to FA2. The two satellite tracked green turtles with isotopic values that went to minor foraging areas and the two that were treated as unknowns (i.e., excluded from the training dataset) were both assigned to FA1 with the DFA. The alternative approach based on the average posterior probabilities of all individuals (n = 90) estimated that 58.9% of sampled green turtles foraged in FA1 and 41.1% in FA2. The size of green turtles with posterior probabilities ≥ 0.8 was similar at the two FAs (Welch’s t-test, t = 0.6, df = 57.2, p = 0.5; n = 82).
Discussion
We identified two main foraging areas, the East African coast (Tanzania and Somalia) and northern Madagascar, and two other potential foraging areas that should be investigated (Mozambique and South Madagascar). Our results indicate that the majority of the sampled green turtles nesting at Aldabra forage in one of these two main areas, with slightly more foraging along the East African coast. Although green turtle populations are recovering in the region after protection of major nesting areas (Lauret-Stepler et al. 2007; Mortimer et al. 2020; Pritchard et al. 2022), threats remain in their foraging areas, including poaching and fisheries bycatch (Bourjea 2015; Temple et al. 2018). The similar contribution of both foraging areas to the Aldabra nesting population highlights the importance of these large regional areas, and further investigation would determine if finer differentiation is possible. Worldwide, only a few studies on green turtles have used satellite telemetry combined with SIA to assign likely foraging areas (e.g., Hatase et al. 2006; Bradshaw et al. 2017), and this is the first such published study in the Western Indian Ocean.
Our sample size of satellite tagged females was small, but they covered a large geographic range (ca. 2800 km latitudinally) when migrating from Aldabra to their foraging areas. Other studies that tracked females from nesting rookeries to foraging areas have also found Mozambique and the East African coast to be key areas for foraging sea turtles. Loggerhead turtles (Caretta caretta) nesting in iSimangaliso Wetland Park in southeast Africa generally migrate along the coast of Mozambique or cross the Mozambique Channel to Madagascar and travel north up the western coast of Madagascar (Robinson et al. 2018). Green turtles nesting in the Chagos Islands forage widely in Seychelles, the East African coast (Mozambique to Somalia), North Madagascar, Tromelin and the Maldives (Hays et al. 2020). Foraging area modeling for green turtles from oceanic island rookeries in the Mozambique Channel identified the coastal waters of northern Madagascar and Africa, bordering the north Mozambique channel to be the most used (Dalleau et al. 2019), and the two main foraging areas identified for green turtles leaving Aldabra overlapped with the predicted hotspot area. Further SIA research linking nesting rookeries and foraging grounds will improve these models to give a better understanding of spatial use and population dynamics in this region.
We found no difference in body size between green turtles using the two foraging areas. Previous studies in other ocean basins have found mixed results: some found size differences based on neritic/oceanic foraging areas or latitude (Zbinden et al. 2011; Ceriani et al. 2014b; Özdilek et al. 2023), while others did not (Hatase et al. 2002; Tucker et al. 2014). The lack of size difference in our study could suggest that the sampled green turtles have a similar foraging strategy across their geographic range and/or that the foraging habitats are similar.
Isotopic patterns
This is the first study from the Western Indian Ocean using carbon and nitrogen bulk stable isotopes to infer foraging grounds of nesting green turtles, therefore we do not have regional comparisons. We found general low isotopic structure for Aldabra nesting green turtles in comparison to sea turtle species in other regions, such as loggerhead turtles in the northern hemisphere (Ceriani et al. 2012; Vander Zanden et al. 2015a). Although a latitudinal gradient in δ13C has been documented elsewhere (e.g. Southern Ocean; Quillfeldt et al. 2005), and even within the pelagic realm of the Western Indian Ocean (Lorrain et al. 2015), we found no latitudinal variation in δ15N and δ13C for green turtles that migrated from Aldabra to their potential foraging grounds. This may represent a limit of this approach in the Western Indian Ocean with this species, and possibly the wider Indian Ocean.
The two satellite tracked green turtles that went to areas (Mozambique and South Madagascar green turtles) other than the two main foraging areas identified (FA1 and FA2) and their isospace values suggest that other minor foraging areas of Aldabra nesting green turtles may exist in the region. Identifying more isotopically distinct areas with a larger sample size of individuals that are both satellite tracked and sampled for SIA would facilitate more accurate, fine-scale assignment of non-tracked green turtles to specific foraging grounds, allowing more precise identification of critical foraging habitats. Additionally, there would be higher confidence in assigning green turtles to specific foraging grounds, increasing the reliability of the inferences.
No other published studies have used both SIA and telemetry to infer post-nesting green turtle foraging areas in the Western Indian Ocean, so no direct comparisons can be made within the region. However, there have been similar studies on different taxa using coastal areas in the region. For example, whale shark foraging areas were found to isotopically overlap (δ13C and δ15N) in Mozambique and Tanzania, but were isotopically different between Mozambique and Qatar (Prebble et al. 2018). Prebble et al. (2018) compared whale sharks with goose barnacles and two species of tuna in the region (from Lorrain et al. 2015; Sardenne et al. 2016), and found that the δ13C and δ15N values generally increased with decreasing latitude, but the δ13C trend was less clear. The enriching latitudinal trend of δ15N was proposed to be driven by the δ15N baseline in the local environment. Our study, however, did not find any relationship with isotopic values and latitude. The lack of δ13C pattern in our study concurred with Lorrain et al.’s (2015) study on tuna, as well as results from loggerhead turtles in southeast Queensland, Australia, where there was no correlation between latitude and δ13C or δ15N. This was attributed to wide variation in individual diet, and to the southwest dynamic current patterns and environmental factors of the Pacific Ocean, which prevented the formation of unique isoscapes (Coffee et al. 2020). This emphasizes the need to investigate each region separately.
We found longitudinal variation of δ13C and δ15N that few studies have reported; with δ13C becoming more enriched and δ15N becoming more depleted with increasing longitude (from west to east). In the South Atlantic Ocean, variations in δ13C and δ15N were detected longitudinally (with lower values for both in the west compared to the east) and vertically in deep-sea copepods (higher δ15N with increased depth) and were attributed to regional differences in hydrography and sea surface temperature (Laakmann and Auel 2010). The oceanography of the Western Indian Ocean is dynamic: the Mozambique Channel and East African coast have strong currents and eddies: the South Equatorial Current flows east to west across the Indian Ocean and diverges north and south off northern Mozambique. The northern split becomes the East African Coast Current, where Tanzanian reefs and islands have similar coral communities to those in the northern Mozambique Channel (Obura 2012), while the southern split forms the Mozambique Channel, where various eddies and dipoles (100–300 km across) along the Mozambique coast drive primary production by bringing dissolved nutrients towards the surface (Schott et al. 2009; Obura et al. 2018). Additionally, upwelling events occur off NW Madagascar (Pripp et al. 2014). The complex oceanography of the WIO (Schott et al. 2009) is likely contributing to the patterns seen in our study.
Overall, further research is needed into the isotopic ranges of the foraging areas identified and other potential confounding or influencing factors, such as dietary shifts, regional prey, and individual feeding strategies (Thomson et al. 2018). Additionally, green turtle foraging ecology is strongly related to sea surface temperatures (Esteban et al. 2020), therefore individuals at lower latitudes may consume different prey, such as more animal matter, than green turtles at warmer sites (Esteban et al. 2020). The variation in δ13C and δ15N at the base of the food web can also vary across small spatial gradients (Post 2002; Magozzi et al. 2017). Contrastingly, foraging grounds of nesting green turtles in the Mediterranean show several geographically distinct areas with the same isotopic profile (Bradshaw et al. 2017). While the isotopic patterns of the WIO are not yet clear, analyzing additional isotopes or trace elements may help delineate the carbon and nitrogen, and identify more distinct sites (Bradshaw et al. 2017; Pearson et al. 2019).
An incidental finding of our study was that isotopic values differed depending on sample treatment. Although this led to the exclusion of one year of samples from our analyses, it is an important finding to inform how preservation methods (salt, 75% ETOH, and 99% ETOH) affected bulk isotope nitrogen values. A similar experiment on green turtles in the Mediterranean (using 96% ETOH and 70% ETOH) found no difference between treatments (Bradshaw et al. 2017), but our results indicate that the tissue preservation technique (e.g., salt or different concentrations of ethanol) can significantly influence the results. Therefore, where different storage methods are used, a comparison of the tissues preserved with different methods should be done to ensure confidence in the results.
Method limitations
Several limitations of our study warrant consideration, including the relatively small sample size of green turtles, which may prevent a fuller understanding of foraging area contribution, or not include all foraging areas used by green turtles from Aldabra’s rookery. The dynamic nature of Western Indian Ocean oceanography means that additional satellite tracking combined with SIA throughout the region is needed to determine whether more distinct foraging areas exist.
As green turtles were tracked after nesting, we assumed that the isotopic values of samples were representative of foraging area prior to the nesting season as has been a commonly used assumption in other sea turtle studies (Ceriani et al. 2012, 2023; Seminoff et al. 2012; Pajuelo et al. 2012). The isotopic turnover rate of skin is not known for green turtles, but for captive, fast-growing juvenile loggerhead turtles (Caretta caretta) it is ca. 3–4 months (Reich et al. 2008). Using ectotherm predictive equations (from Vander Zanden et al. 2015b); Prior et al. (2016) estimated the δ13C of large juvenile and adult green turtle skin samples to reflect their diet > 9 months before sampling. This strongly supports the likelihood that isotope ratios reflect foraging ground diets. We also assumed that green turtles had fidelity to their foraging areas, which many other studies have found (Evans et al. 2019; Pilcher et al. 2020; Shimada et al. 2020). Studies using both satellite telemetry and SIA of skin tissues to infer sea turtle post-nesting foraging areas have had either empirical evidence of site fidelity or have assumed site fidelity based on other studies (Ceriani et al. 2012; Vander Zanden et al. 2015a; Bradshaw et al. 2017; Haywood et al. 2019).
SIA will reflect a combination of signatures from different locations in sea turtles with low fidelity; therefore, if the sampled green turtles exhibit low foraging area fidelity, the SIA results may represent a composite of isotopic signatures from multiple foraging areas they have visited, potentially leading to misidentification of specific foraging locations. This emphasizes the need to verify if green turtles from this region have high site fidelity; there is also a need to know more about the regional isotopic structure of primary producers/sea turtle prey items, including precise information on the identified and other potential foraging areas, to either support or refute the findings here.
Conclusions and recommendations
The regional structure of stable isotopes from foraging areas of green turtles nesting at Aldabra is less clear than in other regions, but the combined SIA/satellite-tracking approach indicated that these green turtles migrate to at least two main foraging areas after nesting. More distinct and/or additional foraging areas could potentially be determined with larger sample sizes from Aldabra, and also from other potential foraging grounds (e.g., Mozambique and southern Madagascar) which highlights the need for international cooperation. Understanding the spatial resolution of SIA for sea turtles in this region would help evaluate the use and practicality of SIA. The lower cost and larger sample size that SIA allows, compared to satellite tracking, is beneficial for understanding the relative importance of these foraging grounds, for managing critical habitats, and for identifying threats to green turtles in this region, which are not well known. For further SIA research in this region, including additional tracers may be necessary (Bradshaw et al. 2017; Haywood et al. 2019). Additionally, research on the isotopic values of primary producers, such as seagrasses, in sea turtle foraging areas is currently lacking and would provide valuable insight into isotopic regional patterns. Continued research on green turtles at Aldabra, and other green turtle rookeries and foraging areas in the region, will shed further light on green turtle connectivity and will help inform effective conservation actions for foraging regions, where green turtles spend the majority of their lives.
Data availability
Data are available upon request.
References
Balazs GH (1999) Factors to consider in the tagging of sea turtles. In: Eckert KL, Bjorndal KA, Abreu-Grobois A, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group, Washington, pp 101–109
Barrow LM, Bjorndal KA, Reich KJ (2008) Effects of preservation method on stable carbon and nitrogen isotope values. Physiol Biochem Zool 81:688–693. https://doi.org/10.1086/588172
Bjorndal KA, Bolten AB (2008) Annual variation in source contributions to a mixed stock: implications for quantifying connectivity. Mol Ecol 17:2185–2193. https://doi.org/10.1111/j.1365-294X.2008.03752.x
Bolten AB (1999) Techniques for measuring sea turtles. KL Eckert, KA Bjorndal, FA Abreu-Grobois, M Donnelly Res Manag Tech Conserv Sea Turtles IUCN/SSC Mar Turt Spec Gr Publ No 4 1–5
Bourjea J (2015) Sea turtles; a review of status, distribution and interaction with fisheries in the Southwest Indian Ocean. In: van der Elst R, Everett B (eds) Offshore fisheries of the Southwest Indian Ocean: their Status and the impact on vulnerable species. Oceanic Research Institute, Durban, South Africa, pp 325–349
Bradshaw P, Broderick A, Carreras C, Inger R, Fuller W, Snape R, Stokes K, Godley B (2017) Satellite tracking and stable isotope analysis highlight differential recruitment among foraging areas in green turtles. Mar Ecol Prog Ser 582:201–214. https://doi.org/10.3354/meps12297
Ceriani SA, Roth JD, Evans DR, Weishampel JF, Ehrhart LM (2012) Inferring foraging areas of nesting loggerhead turtles using satellite telemetry and stable isotopes. PLoS ONE. https://doi.org/10.1371/journal.pone.0045335
Ceriani SA, Roth JD, Ehrhart LM, Quintana-Ascencio PF, Weishampel JF (2014a) Developing a common currency for stable isotope analyses of nesting marine turtles. Mar Biol 161:2257–2268. https://doi.org/10.1007/s00227-014-2503-x
Ceriani SA, Roth JD, Sasso CR, McClellan CM, James MC, Haas HL, Smolowitz RJ, Evans DR, Addison DS, Bagley DA, Ehrhart LM, Weishampel JF (2014b) Modeling and mapping isotopic patterns in the Northwest Atlantic derived from loggerhead sea turtles. Ecosphere 5:1–24. https://doi.org/10.1890/ES14-00230.1
Ceriani SA, Roth JD, Tucker AD, Evans DR, Addison DS, Sasso CR, Ehrhart LM, Weishampel JF (2015) Carry-over effects and foraging ground dynamics of a major loggerhead breeding aggregation. Mar Biol 162:1955–1968. https://doi.org/10.1007/s00227-015-2721-x
Ceriani SA, Weishampel JF, Ehrhart LM, Mansfield KL, Wunder MB (2017) Foraging and recruitment hotspot dynamics for the largest Atlantic loggerhead turtle rookery. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-17206-3
Ceriani SA, Casale P, Brost M, Leone EH, Witherington BE (2019) Conservation implications of sea turtle nesting trends: elusive recovery of a globally important loggerhead population. Ecosphere. https://doi.org/10.1002/ecs2.2936
Ceriani SA, Murasko S, Addison DS, Anderson D, Curry G, Desjardin NA, Eastman SF, Evans DR, Evou N, Fuentes MMPB, Godfrey MH, Hart KM, Hillbrand P, Hirsch SE, Mott CR, Mansfield KL, Mazzarella KT, Norris SV, Pate SM, Phillips KF, Rusenko KW, Shamblin BM, Stevenson A, Sloan KA, Tucker AD, Welsh RC, Casale P (2023) Monitoring population-level foraging distribution of a marine migratory species from land: strengths and weaknesses of the isotopic approach on the Northwest Atlantic loggerhead turtle aggregation. Front Mar Sci 10:1–22. https://doi.org/10.3389/fmars.2023.1189661
Cerritelli G, Casale P, Sözbilen D, Hochscheid S, Luschi P, Kaska Y (2022) Multidirectional migrations from a major nesting area in Turkey support the widespread distribution of foraging sites for loggerhead turtles in the Mediterranean. Mar Ecol Prog Ser 683:169–177. https://doi.org/10.3354/meps13946
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304. https://doi.org/10.1111/j.1466-8238.2007.00367.x
Chandelier G, Kiszka JJ, Dulau-Drouot V, Jean C, Poirout T, Estrade V, Barret M, Fayan J, Jaquemet S (2023) Isotopic niche partitioning of co-occurring large marine vertebrates around an Indian Ocean tropical oceanic island. Mar Environ Res 183:105835. https://doi.org/10.1016/j.marenvres.2022.105835
Coffee O, Booth D, Thia J, Limpus C (2020) When isotopes fail: importance of satellite telemetry and multi-site validation when estimating the foraging grounds of migratory species. Mar Ecol Prog Ser 633:197–206. https://doi.org/10.3354/meps13179
Coyne M, Godfrey MH, Godley BJ, Lay K (2008) Hard shell sea turtle PTT attachment protocol. On-line manual- http://www.seaturtle.org/documents/PTT_Attachment_Protocol.pdf. 1–6
Dalleau M, Kramer-Schadt S, Gangat Y, Bourjea J, Lajoie G, Grimm V (2019) Modeling the emergence of migratory corridors and foraging hot spots of the green sea turtle. Ecol Evol 10317–10342. https://doi.org/10.1002/ece3.5552
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506. https://doi.org/10.1016/0016-7037(78)90199-0
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. https://doi.org/10.1016/0016-7037(81)90244-1
Dingle H, Drake AV (2007) What is migration? Bioscience 57:113–121. https://doi.org/10.1641/B570206
Esteban N, Mortimer JA, Stokes HJ, Olivier J, Richard L, Hays GC (2020) A global review of green turtle diet: sea surface temperature as a potential driver of omnivory levels. Mar Biol 1–17. https://doi.org/10.1007/s00227-020-03786-8
Evans DR, Carthy RR, Ceriani SA (2019) Migration routes, foraging behavior, and site fidelity of loggerhead sea turtles (Caretta caretta) satellite tracked from a globally important rookery. Mar Biol 166:134. https://doi.org/10.1007/s00227-019-3583-4
Ferreira LC, Thums M, Fossette S, Wilson P, Shimada T, Tucker AD, Pendoley K, Waayers D, Guinea ML, Loewenthal G, King J, Speirs M, Rob D, Whiting SD (2021) Multiple satellite tracking datasets inform green turtle conservation at a regional scale. Divers Distrib 27:249–266. https://doi.org/10.1111/ddi.13197
Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D (2010) Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In: West J, Bowen G, Dawson T, Tu K (eds) Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping. Springer, Berlin, p 29. https://doi.org/10.1007/978-90-481-3354-3
Hamylton S, Hagan A, Bunbury N, Fleischer-Dogley F, Spencer T (2018) Mapping the lagoon at Aldabra Atoll, Western Indian Ocean. Atoll Res Bull 45–59. https://doi.org/10.17863/CAM.38477
Hart KM, Guzy JC, Smith BJ (2021) Drivers of realized satellite tracking duration in marine turtles. Mov Ecol 9:1–14. https://doi.org/10.1186/s40462-020-00237-3
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281. https://doi.org/10.3354/meps233273
Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K (2006) Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia 149:52–64. https://doi.org/10.1007/s00442-006-0431-2
Hays GC, Koldewey HJ, Andrzejaczek S, Attrill MJ, Barley S, Bayley DTI, Benkwitt CE, Block B, Schallert RJ, Carlisle AB, Carr P, Chapple TK, Collins C, Diaz C, Dunn N, Dunbar RB, Eager DS, Engel J, Embling CB, Esteban N, Ferretti F, Foster NL, Freeman R, Gollock M, Graham NAJ, Harris JL, Head CEI, Hosegood P, Howell KL, Hussey NE, Jacoby DMP, Jones R, Sannassy Pilly S, Lange ID, Letessier TB, Levy E, Lindhart M, McDevitt-Irwin JM, Meekan M, Meeuwig JJ, Micheli F, Mogg AOM, Mortimer JA, Mucciarone DA, Nicoll MA, Nuno A, Perry CT, Preston SG, Rattray AJ, Robinson E, Roche RC, Schiele M, Sheehan EV, Sheppard A, Sheppard C, Smith AL, Soule B, Spalding M, Stevens GMW, Steyaert M, Stiffel S, Taylor BM, Tickler D, Trevail AM, Trueba P, Turner J, Votier S, Wilson B, Williams GJ, Williamson BJ, Williamson MJ, Wood H, Curnick DJ (2020) A review of a decade of lessons from one of the world’s largest MPAs: conservation gains and key challenges. Mar Biol 167:1–22. https://doi.org/10.1007/s00227-020-03776-w
Haywood JC, Fuller WJ, Godley BJ, Shutler JD, Widdicombe S, Broderick AC (2019) Global review and inventory: how stable isotopes are helping us understand ecology and inform conservation of marine turtles. Mar Ecol Prog Ser 613:217–245. https://doi.org/10.3354/meps12889
Haywood JC, Fuller WJ, Godley BJ, Margaritoulis D, Shutler JD, Snape RTE, Widdicombe S, Zbinden JA, Broderick AC (2020) Spatial ecology of loggerhead turtles: insights from stable isotope markers and satellite telemetry. Divers Distrib 26:368–381. https://doi.org/10.1111/ddi.13023
Hobson KA (1999) Tracing origins and migration. Oecologia 120:314–326
Hobson KA, Wassenaar LI (2019) Tracking Animal Migration with Stable Isotopes (2nd Edition). Academic Press. Elsevier
Humber F, Godley BJ, Ramahery V, Broderick AC (2011) Using community members to assess artisanal fisheries: the marine turtle fishery in Madagascar. Anim Conserv 14:175–185. https://doi.org/10.1111/j.1469-1795.2010.00413.x
Jensen MP, FitzSimmons M, Dutton N, Wyneken III, Lohmann J, Musick KJ (2013) JA. 135–162. Boca Raton, FL: CRC
Laakmann S, Auel H (2010) Longitudinal and vertical trends in stable isotope signatures (δ13C and δ15N) of omnivorous and carnivorous copepods across the South Atlantic Ocean. Mar Biol 157:463–471. https://doi.org/10.1007/s00227-009-1332-9
Lamont MM, Benscoter AM, Hart KM (2023) Green turtle movements in the Gulf of Mexico: Tracking reveals new migration corridor and habitat use suggestive of MPA expansion. Glob Ecol Conserv 42:e02380. https://doi.org/10.1016/j.gecco.2023.e02380
Lauret-Stepler M, Bourjea J, Roos D, Pelletier D, Ryan PG, Ciccione S, Grizel H (2007) Reproductive seasonality and trend of Chelonia mydas in the SW Indian Ocean: a 20 year study based on track counts. Endanger Species Res 3:217–227
Long CA, Chabot RM, El-Khazen MN, Kelley JR, Mollet‐Saint Benoît C, Mansfield KL (2021) Incongruent long‐term trends of a marine consumer and primary producers in a habitat affected by nutrient pollution. Ecosphere 12:e03553. https://doi.org/10.1002/ecs2.3553
Lorrain A, Graham BS, Popp BN, Allain V, Olson RJ, Hunt BPV, Potier M, Fry B, Galván-Magaña F, Menkes CER, Kaehler S, Ménard F (2015) Nitrogen isotopic baselines and implications for estimating foraging habitat and trophic position of yellowfin tuna in the Indian and pacific oceans. Deep Res Part II Top Stud Oceanogr 113:188–198. https://doi.org/10.1016/j.dsr2.2014.02.003
Magozzi S, Yool A, Vander Zanden HB, Wunder MB, Trueman CN (2017) Using ocean models to predict spatial and temporal variation in marine carbon isotopes. Ecosphere 8:e01763. https://doi.org/10.1002/ecs2.1763
Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC (2017) Global sea turtle conservation successes. Sci Adv. https://doi.org/10.1126/sciadv.1600730
McMahon KW, Hamady LL, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58:697–714. https://doi.org/10.4319/lo.2013.58.2.0697
Mortimer JA, Esteban N, Guzman AN, Hays GC (2020) Estimates of marine turtle nesting populations in the south-west Indian Ocean indicate the importance of the Chagos Archipelago. Oryx 54:332–343. https://doi.org/10.1017/S0030605319001108
Newsome SD, del Rio CM, Bearhop S, Philips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436. https://doi.org/10.1890/060150.01
Obura D (2012) Evolutionary mechanisms and diversity in a western Indian Ocean center of diversity. Proc 12th Int Coral Reef Symp 9–13
Obura DO, Bandeira SO, Bodin N, Burgener V, Braulik G, Chassot E, Gullström M, Kochzius M, Nicoll M, Osuka K, Ralison HO, Richmond M, Samoilys MA, Scheren P, Ternon JF (2018) The Northern Mozambique Channel. World seas an Environ Eval. Indian Ocean Pac II:75–99. https://doi.org/10.1016/B978-0-08-100853-9.00003-8
Özdilek ŞY, Sönmez B, Mestav B (2023) Body size-related polymorphic foraging strategy in adult green turtles. Ocean Coast Manag 237:106538. https://doi.org/10.1016/j.ocecoaman.2023.106538
Pajuelo M, Bjorndal KA, Reich KJ, Vander Zanden HB, Hawkes LA, Bolten AB (2012) Assignment of nesting loggerhead turtles to their foraging areas in the Northwest Atlantic using stable isotopes. Ecosphere 3:1–18. https://doi.org/10.1890/ES12-00220.1
Pearson RM, Van De Merwe JP, Limpus CJ, Connolly RM (2017) Realignment of sea turtle isotope studies needed to match conservation priorities. Mar Ecol Prog Ser 583:259–271. https://doi.org/10.3354/meps12353
Pearson RM, van de Merwe JP, Gagan MK, Limpus CJ, Connolly RM (2019) Distinguishing between sea turtle foraging areas using stable isotopes from commensal barnacle shells. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-42983-4
Pfaller JB, Pajuelo M, Vander Zanden HB, Andrews KM, Dodd MG, Godfrey MH, Griffin DB, Ondich BL, Pate SM, Williams KL, Shamblin BM, Nairn CJ, Bolten AB, Bjorndal KA (2020) Identifying patterns in foraging-area origins in breeding aggregations of migratory species: loggerhead turtles in the Northwest Atlantic. PLoS ONE 15:1–22. https://doi.org/10.1371/journal.pone.0231325
Pilcher NJ, Rodriguez-Zarate CJ, Antonopoulou MA, Mateos-Molina D, Das HS, Bugla IA (2020) Combining laparoscopy and satellite tracking: successful round-trip tracking of female green turtles from feeding areas to nesting grounds and back. Glob Ecol Conserv 23:e01169. https://doi.org/10.1016/j.gecco.2020.e01169
Posit team (2022) RStudio: integrated Development Environment for R. Posit Software. PBC, Boston, MA. http://www.posit.co/
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Prebble C, Rohner C, Pierce S, Robinson D, Jaidah M, Bach S, Trueman C (2018) Limited Latitudinal ranging of juvenile whale sharks in the Western Indian Ocean suggests the existence of regional management units. Mar Ecol Prog Ser 601:167–183. https://doi.org/10.3354/meps12667
Prior B, Booth DT, Limpus CJ (2016) Investigating diet and diet switching in green turtles (Chelonia mydas). Aust J Zool 63:365. https://doi.org/10.1071/ZO15063
Pripp T, Gammelsrød T, Krakstad JO (2014) Physical influence on biological production along the western shelf of Madagascar. Deep Res Part II Top Stud Oceanogr 100:174–183. https://doi.org/10.1016/j.dsr2.2013.10.025
Pritchard A, Sanchez C, Bunbury N, Burt A, Currie J, Doak N, Fleischer-Dogley F, Metcalfe K, Mortimer JA, Richards H, van de Crommenacker J, Godley B (2022) Green turtle population recovery at Aldabra Atoll continues after 50 year of protection. Endanger Species Res 47:205–215. https://doi.org/10.3354/esr01174
Quillfeldt P, McGill R, Furness R (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304. https://doi.org/10.3354/meps295295
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Reich KJ, Bjorndal KA, Martínez del Rio C (2008) Effects of growth and tissue type on the kinetics of 13 C and 15 N incorporation in a rapidly growing ectotherm. Oecologia 155:651–663. https://doi.org/10.1007/s00442-007-0949-y
Robinson NJ, Anders D, Bachoo S, Harris L, Hughes GR, Kotze D, Maduray S, Mcgue S, Meyer M, Oosthuizen H, Paladino FV, Luschi P (2018) Satellite tracking of leatherback and loggerhead sea turtles on the southeast African coastline. Indian Ocean Turt Newsl 28:3–7
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19:256–263. https://doi.org/10.1016/j.tree.2004.03.017
Sardenne F, Bodin N, Chassot E, Amiel A, Fouché E, Degroote M, Hollanda S, Pethybridge H, Lebreton B, Guillou G, Ménard F (2016) Trophic niches of sympatric tropical tuna in the Western Indian Ocean inferred by stable isotopes and neutral fatty acids. Prog Oceanogr 146:75–88. https://doi.org/10.1016/j.pocean.2016.06.001
Schott FA, Xie S, McCreary JP (2009) Indian Ocean circulation and climate variability. Rev Geophys 47:1–46. https://doi.org/10.1029/2007RG000245
Seminoff JA, Bjorndal KA, Bolten AB (2007) Stable carbon and nitrogen isotope discrimination and turnover in pond sliders Trachemys scripta: insights for trophic study of freshwater turtles. Am Soc Ichthyol Herpetol 534–542
Seminoff JA, Benson SR, Arthur KE, Eguchi T, Dutton PH, Tapilatu RF, Popp BN (2012) Stable isotope tracking of endangered sea turtles: validation with satellite telemetry and δ15N analysis of amino acids. PLoS ONE 7:e37403. https://doi.org/10.1371/journal.pone.0037403
Seminoff JA, Camryn AD, Balazs GH, Dutton PH, Eguchi T, Haas HL, Hargrove SA, Jensen M, Klemm DL, Lauritsen AM, MacPherson SL, Opay P, Possardt EE, Pultz S, Seney E, Van Houtan KS, Waples RS (2015) Status review of the green turtle (Chelonia mydas). under the U.S. Endangered Species Act
Shimada T, Limpus CJ, Hamann M, Bell I, Esteban N, Groom R, Hays GC (2020) Fidelity to foraging sites after long migrations. J Anim Ecol 89:1008–1016. https://doi.org/10.1111/1365-2656.13157
Stokes HJ, Mortimer JA, Hays GC, Unsworth RKF, Laloë JO, Esteban N (2019) Green turtle diet is dominated by seagrass in the Western Indian Ocean except amongst gravid females. Mar Biol. https://doi.org/10.1007/s00227-019-3584-3
Temple AJ, Kiszka JJ, Stead SM, Wambiji N, Brito A, Poonian CNS, Amir OA, Jiddawi N, Fennessy ST, Pérez-Jorge S, Berggren P (2018) Marine megafauna interactions with small-scale fisheries in the southwestern Indian Ocean: a review of status and challenges for research and management. Rev Fish Biol Fish 28:89–115. https://doi.org/10.1007/s11160-017-9494-x
Thomson JA, Whitman ER, Garcia-Rojas MI, Bellgrove A, Ekins M, Hays GC, Heithaus MR (2018) Individual specialization in a migratory grazer reflects long-term diet selectivity on a foraging ground: implications for isotope-based tracking. Oecologia 188:429–439. https://doi.org/10.1007/s00442-018-4218-z
Tucker A, MacDonald B, Seminoff J (2014) Foraging site fidelity and stable isotope values of loggerhead turtles tracked in the Gulf of Mexico and northwest Caribbean. Mar Ecol Prog Ser 502:267–279. https://doi.org/10.3354/meps10655
van de Geer C, Bourjea J, Broderick A, Dalleau M, Fernandes R, Harris L, Inteca G, Kiponda F, Louro C, Mortimer J, Msangameno D, Mwasi L, Nel R, Okemwa G, Olendo M, Pereira M, Rees A, Silva I, Singh S, West L, Williams J, Godley B (2022) Marine turtles of the African east coast: current knowledge and priorities for conservation and research. Endanger Species Res 47:297–331. https://doi.org/10.3354/esr01180
Vander Zanden H, Tucker AD, Bolten AB, Reich KJ, Bjorndal KA (2014) Stable isotopic comparison between loggerhead sea turtle tissues. Rapid Commun Mass Spectrom 28:2059–2064. https://doi.org/10.1002/rcm.6995
Vander Zanden H, Tucker AD, Hart KM, Lamont MM, Fujisaki I, Addison DS, Mansfield KL, Phillips KF, Wunder MB, Bowen GJ, Pajuelo M, Bolten AB, Bjorndal KA (2015a) Determining origin in a migratory marine vertebrate: a novel method to integrate stable isotopes and satellite tracking. Ecol Appl 25:320–335. https://doi.org/10.1890/14-0581.1.sm
Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC (2015b) Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10:e0116182. https://doi.org/10.1371/journal.pone.0116182
Venables W, Ripley B (2002) Modern applied statistics with S, Fourth edition. Springer, New York. ISBN 0-387-95457-0, https://www.stats.ox.ac.uk/pub/MASS4/
Wallace B, Posnik Z, Hurley B, DiMatteo A, Bandimere A, Rodriguez I, Maxwell S, Meyer L, Brenner H, Jensen M, LaCasella E, Shamblin B, Abreu Abreu-Grobois F, Stewart K, Dutton P, Barrios-Garrido H, Dalleau M, Dell’amico F, Eckert K, FitzSimmons N, Garcia-Cruz M, Hays G, Kelez S, Lagueux C, Madden Hof C, Marco A, Martins S, Mobaraki A, Mortimer J, Nel R, Phillott A, Pilcher N, Putman N, Rees A, Rguez-Baron J, Seminoff J, Swaminathan A, Turkozan O, Vargas S, Vernet P, Vilaça S, Whiting S, Hutchinson B, Casale P, Mast R (2023) Marine turtle regional management units 2.0: an updated framework for conservation and research of wide-ranging megafauna species. Endanger Species Res 52:209–223. https://doi.org/10.3354/esr01243
Ward JH, Hook ME (1963) Application of an hierarchical grouping procedure to a problem of grouping profiles. Educ Psychol Meas 23:69–81. https://doi.org/10.1177/001316446302300107
Williams J, Pierce S, Fuentes M, Hamann M (2016) The tradition of take: sea turtle consumption in Dovela, Mozambique. Afr Sea Turt Newsl 5:27–31
Wunder MB (2012) Determining geographic patterns of migration and dispersal using stable isotopes in keratins. J Mammal 93:360–367. https://doi.org/10.1644/11-MAMM-S-182.1
Zbinden JA, Bearhop S, Bradshaw P, Gill B, Margaritoulis D, Newton J, Godley BJ (2011) Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Mar Ecol Prog Ser 421:291–302. https://doi.org/10.3354/meps08871
Acknowledgements
We thank the Seychelles Islands Foundation for support to and on the atoll, and for assistance with the data and sample collection. Special thanks go to Bernadette Julie, Corianna Julie, Elsa Pool, Christina Quanz, Ronny Rose, and Julio Agricole from Head Office, and to Alvin Bonnelame, Frances Benstrong, Jude Brice, Sebastian Cowin, Esthel Didon, Mikael Esparon, Anna Koester, Emma Mederic, Gilly Mellie, Jessica Moumou, Michelle Risi and Marvin Roseline for assistance on Aldabra. Collaboration with Michael Jensen allowed us to analyze samples from 2020. Germano Soru provided assistance in acquiring supplies on Mahé for Aldabra. Holly Edwards provided logistic support during laboratory work in St. Petersburg, FL (USA). Susan Murasko and Kasey Wade provided laboratory assistance, Ernst Peebles provided access to lab space and Ethan Goddard provided assistance with stable isotope analysis. Paolo Luschi provided three of the satellite tags, and the other ten satellite tags were from Seychelles Islands Foundation from the STORM project, which was supported by the European Union, the Regional Council of Reunion Island and the French State under INTERREG-V Indian Ocean project STORM-IO and by the CNES under STORM-SAT. Special thanks to Olivier Bousquet and Anne Barat for coordinating and including Aldabra in the STORM project.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: CLS, SAC. Data Curation: CLS, LA. Methodology: CLS, SAC, PC. Investigation: CLS, MCvR, BS, MB, LA, CWK. Formal Analysis: CLS. Supervision: PC, SAC, NB. Administration: NB, FFD. Funding Acquisition: PC, SAC, FFD. Writing- Original Draft: CLS, SAC, PC. Writing- Review & Editing: NB, FFD, LA, CJ, EM.
Corresponding author
Ethics declarations
Ethical approval
The research was conducted following national guidelines for the care and use of animal, and under all the necessary permits from Seychelles Bureau of Standards (A0157) and CITES (Seychelles export A1523/ Florida import 22US21494E/9).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Vinicius Giglio.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanchez, C.L., Casale, P., Bunbury, N. et al. Regional foraging areas of green turtles (Chelonia mydas) nesting at Aldabra Atoll: insights from stable isotopes and satellite telemetry. Biodivers Conserv (2024). https://doi.org/10.1007/s10531-024-02899-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10531-024-02899-6