Abstract
Green sea turtles (Chelonia mydas) face many threats from anthropogenic activity and are listed as Endangered on the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species. They spend the majority of their lives in coastal foraging areas, and show strong fidelity to narrow foraging ranges. Understanding the foraging ecology of green turtles is therefore important for their management and conservation. Using stable isotopes analysis (n = 200) and identification of stomach contents (n = 21), this study investigated the foraging ecology of four distinct green turtle foraging aggregations (Goro, Isle of Pines, Ouen Island and Uo/Mato Islands) within the Grand Lagon Sud. This World Heritage Area located in New Caledonia supports regionally important nesting and foraging green turtle populations. The δ13C and δ15N values of skin samples ranged from − 19.3 to − 7.3‰ and 2.8–15.9‰, respectively. Metrics of isotopic niche and regression analyses were used to assess the degree of omnivory, trophic diversity and potential ontogenetic diet shifts in the different aggregations of foraging green turtles in this location. Each of the four sites and four age-classes had distinct isotopic niches, with evidence of omnivory and ontogenetic shifts, particular in new recruits and juveniles, and at Goro and Uo/Mato Islands. This was generally supported by the stomach contents analyses of a small number of green turtles opportunistically sampled from traditional ceremonies. This study provides important information on the foraging ecology of green turtles in New Caledonia, supporting management outcomes in this region. This collaboration with the tribes of the Grand Lagon Sud is also the first step towards the integrated management of the resource.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The green sea turtle (Chelonia mydas) faces multiple threats from human activities, including global warming, habitat loss, incidental capture in fishing gear and overharvesting (Donlan et al. 2010), and is currently listed as ‘Endangered’ on the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (Seminoff 2004). Green turtles have unique life cycles, spending the majority of their lives in shallow coastal areas foraging primarily on seagrass and algae (Bjorndal 1997), although more recent studies indicate significant omnivory in some populations (Esteban et al. 2020). They recruit to these foraging grounds at a mean size ranging from 40 to 50 cm CCL (Limpus 2008, Piovano et al. 2020), and show strong fidelity to very narrow foraging ranges as they grow to maturity (Makowski et al. 2006; Seminoff et al. 2002; Shimada et al. 2016). Even when green turtles begin breeding, which may involve long-distance migrations to breeding and nesting areas (Hays and Scott 2013; Luschi et al. 1996; Read et al. 2014), they return to their home foraging ground between breeding seasons (Shimada et al. 2020). Understanding the foraging ecology of green turtles in these habitats will contribute for implementing an effective conservation strategy. Whilst early studies on sea turtle foraging ecology were limited to investigating stomach contents of deceased animals (Bjorndal 1985; Garnett et al. 1985; Mortimer 1981; Ross 1985), more recent development of minimally invasive techniques, such as skin sampling for stable isotope analyses, have allowed for more hypothesis-driven and comprehensive assessments of green turtle foraging ecology.
Stable isotope analysis (SIA) involves the measurement of the ratio of different isotopes of the same element, which change in a predictable way as elements cycle through the environment (Peterson and Fry 1987). SIA, typically of carbon, nitrogen and sulphur, can be performed on plant and animal (e.g. skin) samples to investigate the interconnectedness of ecosystem components. Generally, the stable isotope ratios of these elements in consumers reflect the isotope ratios of their prey items, which can be traced down to the baseline isotope value of primary producers, adjusting for trophic discrimination, or enrichment between trophic levels (Fry 2006; Peterson and Fry 1987). Further, ratios of nitrogen isotopes can be used to predict the trophic position of consumers as they are enriched, via metabolic processes, between trophic levels in a predictable way (DeNiro and Epstein 1978; Hobson 1999). SIA has been used widely to study green turtle foraging ecology, including spatial and temporal differences in diet composition (Arthur et al. 2009; Burkholder et al. 2011; Lemons et al. 2011; Williams et al. 2014), ontogenic diet shifts (Arthur et al. 2008; Cardona et al. 2009; Reich et al. 2007), intrapopulation variation in feeding habitat (Hatase et al. 2006; Vander Zanden et al. 2013), and trophic niches (Di Beneditto et al. 2016).
Despite widespread use of SIA to investigate foraging ecology of C. mydas, very little is known about the foraging ecology of this species in New Caledonian waters. The Grand Lagon Sud is considered one of the major feeding grounds for C. mydas in New Caledonia (Read et al. 2020). This foraging ground supports adult green turtles that nest in locations such as the d’Entrecasteaux atolls (Chimmo 1856; Pritchard 1982), and the southern Great Barrier Reef (Limpus et al. 1992), as well as significant numbers of juvenile and sub-adult green turtles (Read et al. 2020). The Grand Lagon Sud also supports an independent genetic stock of nesting green turtles (Dethmers et al. 2006), including turtles that migrate from foraging grounds as far away as Australia (Dethmers et al. 2010; Jensen 2010; Read et al. 2014).
This study used SIA analyses of free ranging green turtles, complemented with the opportunistic examination of stomach contents of green turtles harvested by Indigenous communities for tribal ceremonies, to assess the foraging ecology of the Grand Lagon Sud foraging green turtle population. Specifically, differences in isotopic niche width between different foraging aggregations and age-classes, as well as relationships between stable isotope values and turtle size (a proxy for age), were investigated to understand the degree of omnivory, trophic diversity and potential ontogenetic diet shifts in different aggregations of foraging green turtles in this location. This study will facilitate the understanding of the foraging ecology and habitat use of green sea turtles in this regionally important area for green turtles, whilst promoting links between science and Indigenous culture in New Caledonia.
Materials and methods
Study site
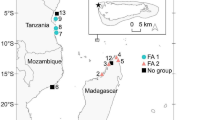
This study was conducted in the Grand Lagon Sud (GLS) (Fig. 1) of New Caledonia (22º 30″ S, 167º 57ʹ E), which supports a major foraging population of C. mydas. GLS is a World Heritage site that is characterized by a large reef diversity and the presence of many endangered species (Menu and Hebert 2006). Sea turtles are a protected species in New Caledonia (No. 4-2009/APS), but permits to hunt C. mydas are granted to Indigenous communities for traditional ceremonies such as weddings, funerals and yam celebrations.
Turtle capture
A total of 200 C. mydas were captured using the turtle rodeo method (Limpus and Reed 1985) in four areas of the GLS: Goro (n = 68), Isle of Pines (n = 26), Ouen Island (n = 47), and Mato/Uo Islands (n = 59), between August 2012 and March 2013 (Fig. 1). Goro is a town located on the northern part of the main island, and is in close proximity to an important seagrass meadow and a large river inlet. Ouen Island is located 1 km from the southern coastline of the main island. The Mato/Uo Islands are located approximately 16 km from the southern coastline of the main island and are in close proximity to the barrier reef. Isle of Pines is the largest island of the south province and is located 55 km to the south-east of the main island, surrounded by the barrier reef. All turtles were tagged with a titanium tag (Limpus 1992), their midline curved carapace length (CCL) was measured, and their sex determined (when possible) using external morphology or gonad examination. The turtles were released in the vicinity of their capture site following processing. In addition to the rodeo-captured turtles, 21 C. mydas that were caught and killed by tribes in the GLS for traditional ceremonies Goro (n = 13), Ouen Island (n = 3) and Isle of Pines (n = 5) between March 2013 and March 2014 were also used in this study. A small number of the turtles used for the ceremonies (n = 12) could not be measured precisely (due to access restrictions during tribal ceremonies), but an age-class was assigned based on an estimate of size.
The captured turtles were divided into four age-class categories, according to Limpus et al. (1994) and Limpus and Chaloupka (1997): new recruits (20–65 cm CCL, with a very white plastron and very low algae presence on the carapace), juveniles (25–65 cm CCL, with a yellow/discoloured plastron), sub-adults (65–90 cm CCL) and adults (> 90 cm CCL). The juveniles, sub-adults and adults were presumed to be long term residents at their capture sites.
Skin sample collection and stable isotope analysis
Epidermal (skin) tissue (~ 1 cm3) was collected from the hind flipper of all C. mydas (n = 200), using a sterilised scalpel, and immediately placed in 90% NaCl solution for storage (Arthur et al. 2008). Before stable isotopes analysis (δ13C and δ15N), samples were rinsed with distilled water and dried at 50 °C for 48 h, then ground into a fine powder using a mortar and pestle. The analysis was undertaken at the Griffith University Stable Isotope Analysis Laboratory using a sample preparation system (Sercon Europa EA-GSL), and an isotope ratio mass spectrometer (Sercon Hydra 20-22). This analysis provided δ13C and δ15N isotope values as a relative deviation (‰) from conventional standards (Pee-Dee Belemnite for carbon, atmospheric air for nitrogen), and expressed in delta (δ) notation in parts per thousand:’
where Rsample is the ratio of heavy to light isotopes in the sample, and Rstandard is the isotope ratio of the corresponding standard. Bovine liver (n = 18) was used for quality control, with the standard deviation of the mean bovine liver samples assessing the precision of analysis (0.03‰ for δ13C and 0.13‰ for δ15N). Lipid extraction was not conducted on the skin samples as previous studies consider this process unnecessary for sea turtles due to the low lipid composition of sea turtle epidermis (Vander Zanden et al. 2012; Turner Tomaszewicz et al. 2017).
Stomach content collection and diet identification
Stomach contents were collected from the green turtles harvested by Indigenous communities (n = 21). The samples were collected as the turtles were being sacrificed, which was up to 5 days following capture. The collection and analysis of the stomach contents followed methods described by Garnett et al. (1985). Briefly, the entire stomach sample was removed from each turtle and homogenised before a sub-sample was collected and dried at 70 °C for 48 h. The samples were then further sub-sampled (~ 5% of the dry weight) into a petri dish for identification of individual diet items under a microscope, using methods described by Lanyon (1986). Animal matter was identified to phylum (Porifera or Cnidaria), algae to genus (or sometimes family), seagrass to species, and any diet items that could not be identified were assigned ‘unidentified’. In each stomach sample, each forage group (animal phylum, algal genus/family, seagrass species and ‘unidentified’) was weighed and the percentage of each forage group in each stomach sample was calculated.
It is important to note that sample numbers for stomach contents were low, and the exact capture locations of the turtles used in the tribal ceremonies were difficult to know precisely. So, for example, a turtle used in an Ouen Island ceremony may not necessarily have been captured in the Ouen Island foraging grounds.
Statistical analyses
As food items typically consumed by green turtles were not collected for stable isotope analyses in this study, the skin stable isotope data were not used to infer diet composition. Literature stable isotope data for forage material and trophic enrichment in green turtles could have been used to assess diet composition via stable isotope mixing model analyses (e.g. Piovano et al. 2020). However, due to significant temporal and spatial variation in stable isotope values of basal resource item isotopic values (Montoya et al. 2002), even over very small geographic ranges (Gillis et al. 2018), as well as the wide range in trophic enrichment values for green turtles in the literature (Seminoff et al. 2006; Turner Tomaszewicz et al. 2017; Vander Zanden et al. 2012), diet composition assessments were deemed inappropriate for the data collected in this study.
Instead, stable isotope data from green turtles (skin samples) were used to compare trophic width-between sites (all age-classes combined) and between age-classes (all sites combined). Standard metrics of isotopic niche width were calculated from skin δ13C and δ15N measurements using the Stable Isotope Bayesian Ellipses in R (SIBER) package (Jackson et al. 2011) of R version 4.3.2. Briefly, the six metrics used are based on the position of samples in the δ13C–δ15N bivariate space (Jackson et al. 2011; Layman et al. 2007), and are as follows: (1) δ15N range (NR), to represent trophic diversity within each group (site or age class); (2) δ13C range (CR), a measure of basal food source variability—groups with multiple basal resources can be expected to have higher CR values; (3) standard ellipse area (SEA), a measure of isotopic niche width; (4) mean distance to centroid (CD), a measure of tropic diversity in the group; (5) mean nearest neighbour distance (MNND), a measure of diet variability derived from the level of clustering within a group (mean Euclidean distances to the nearest neighbour in the δ13C–δ15N bi-plot); and (6) standard deviation of nearest neighbour distance (SDNND), a measure of evenness that is less influenced by sample size than MNND.
SEAs were corrected for differences in sample sizes by using a Bayesian approach (Jackson et al. 2011). Bayesian SEAs were further compared probabilistically, both between sites and between age-classes, using the posterior Bayesian distributions of simulated ellipses. To further investigate potential ontogenetic shifts in diet of these green turtles, the relationships between the CCL (a proxy for age) and green turtle skin δ13C and δ15N values were assessed via a series of regressions. All statistical analyses were run in RStudio version 1.4.1106 (2009–2021 RStudio, PBC).
Due to low and variable replication of stomach contents samples, and the uncertainty in the precise locations of the foraging habitats of these turtles, no statistical analyses were performed on the stomach contents data. These data were, however, discussed in the context of the stable isotope data.
Results
Stable isotope summary
The majority of green turtles sampled in this study were juveniles (159, 80% of turtles sampled), with smaller numbers of the other age-classes (Table 1). The δ13C and δ15N values of green turtle skin samples ranged from − 19.3 to − 7.3‰ and 2.8–15.9‰, respectively, and there was generally more similarity in δ13I values within sites compared to within age-classes (Fig. 2, Table 1). However, both juveniles and adults had very similar mean δ15N values within their age-classes, regardless of which site they were foraging in.
Niche width-between sites
The standard ellipse areas (SEAs) were similar for Goro, Isle of Pines and Ouen Island, but much smaller for Uo/Mato Islands (Fig. 3, Table 2). The mean overlap between SEAs, based on 10 simulations of their Bayesian posterior distributions, was relatively low (24–35%) and similar for all pair-wise site comparisons (Table 3). Goro (high NR) had the highest tendency for omnivory and highest trophic diversity, whilst Ouen Island had high levels of herbivory and lowest trophic diversity. Isle of Pines (high CR) had the highest diversity of basal food sources, which was much lower in Uo/Mato Islands. The CD and MNND values were similar between sites, indicating similar trophic ecology at all sites. However, higher SDNND values at Isle of Pines indicated that there was a less even distribution of these turtles in the tropic niche at this site.
The δ13C and δ15N values of green turtles foraging within the Grand Lagon Sud, New Caledonia: Goro (black circles), Isle of Pines (red triangles), Ouen Island (green pluses) and Uo/Mato Islands (blue crosses). Lines depict the standard ellipse (the 40% confidence interval) for the site-specific isotopic niches
Niche width-between age-classes
SEAs were similar in size for new recruits, juveniles and adults, and much larger for sub-adults (Fig. 4, Table 4). Similar to the site comparisons, the mean overlap between SEAs, based on 10 simulations of their Bayesian posterior distributions, was relatively low (22–35%) and similar for all pair-wise age-class comparisons (Table 5). New recruits and juveniles (high NR) had the highest tendency for omnivory, while sub-adults and adults had high levels of herbivory and low range of trophic levels. New recruits (low CR) had the lowest diversity of food sources, which was much higher in the other age-classes. The CD and MNND values were similar between new recruits, sub-adults and adults, indicating similar trophic diversity and redundancy in these age-classes. However, juveniles had lower MNND and SDNND values, indicating this age-class has greater trophic redundancy, and a more even distribution of individuals in their isotopic niche.
The δ13C and δ15N values of green turtles foraging within the Grand Lagon Sud, New Caledonia, separated by age-class: new recruit (black circles), juvenile (red triangles), sub-adult (green pluses) and adult (blue crosses). Lines depict the standard ellipse (the 40% confidence interval) for the age-class-specific isotopic niches
The regression analyses revealed significant but weak positive relationships between CCL and δ13C at Goro (p < 0.05, slope = 0.10, R2 = 0.11). In addition, significant but weak negative relationships were identified between CCL and δ15N for Goro (p < 0.05, slope = − 0.14, R2 = 0.11) and Uo/Mato Islands (p < 0.05, slope = − 0.12, R2 = 0.07) (Fig. 5). There were no other significant relationships between CCL and δ13C or δ15N.
Stomach contents
The per cent contribution of different forage types in stomach contents varied greatly, both within and between sites (Table 6), although some trends could be observed. The stomach contents of green turtles foraging in Goro contained, on average, 90.4% algae, predominantly the red alga, Hypnea sp., which was present in all 13 stomach contents at an average of 44% of the total stomach contents. Green algae (mostly Caulerpa sp. and Ulva sp.) were present in 12 out of the 13 Goro green turtle stomach content samples, with an average of 28% each. Only four of the 13 Goro green turtle stomach content samples contained animal matter, although when they did, it was usually a substantial proportion (mean = 15%). Only one of the 13 Goro green turtle stomach samples contained seagrass, just 9.5% of Cymodocea serrulata. In contrast, the stomach contents of green turtles foraging in Ouen Island contained, on average, 82% seagrass (67% Halodule uninervis and 15% Cymodocea serrulata) with the remainder mostly animal matter. Finally, the stomach contents of two green turtles foraging in Isle of Pines contained 100% seagrass, mostly Cymodocea serrulata (69%), and stomach contents of the remaining three turtles contained 100% algae (or unidentified forage), mostly green and red species.
Discussion
The stable isotope niche width analyses presented here show that green turtles foraging in the Grand Lagon Sud (GLS) of New Caledonia have diverse foraging ecology, both within and between sites. The Uo/Mato Islands had the smallest isotopic niche width (smallest SEA), which combined with a relatively low diversity of food sources (low CR value, Table 2), indicates that green turtles foraging at Uo/Mato Islands have higher fidelity to a narrow range of foraging resources, and may therefore be more sensitive to potential habitat change at this site (Di Beneditto et al. 2016). However, it is important to note that this site included only one age-class (juveniles), which all seem to have similar trophic ecology in this region (low MNND value, Table 4). The remaining three sites had larger niche widths, and higher diversity of food sources, also reflecting the higher diversity of age-classes present, but possibly also indicating more resilience to future habitat impacts.
The minimal overlap (< 35%) of the SEAs between sites indicates that green turtles foraging at these four foraging sites have distinct isotopic niches. This is supported by the stomach contents analyses, which illustrated that Goro turtles feed predominantly on algae, Ouen Island turtles feed predominantly on seagrass, and Isle of Pines turtles have a mixture of both seagrass and algae (Table 6). Taking a closer look at individual sites, high NR values (Table 2) and negative correlations between δ15N and CCL at both Goro and Uo/Mato Islands (Fig. 5) indicated that there is a higher tendency for omnivory at these sites. This is supported by the dominance of new recruits and juveniles at these sites, and the higher tendency of these two age-classes for omnivory (high NR values; Table 4). In addition, although there were no stomach contents analyses for Uo/Mato Islands, the turtles sampled at Goro showed significant levels of animal matter in their stomach contents.
Green turtles are known to sometimes consume large quantities of animal matter, mainly macroplankton (e.g. jellyfish), especially when these animals are in abundance, and when abundance and quality of seagrasses and algae are low (Burkholder et al. 2011; González Carman et al. 2014; Lemons et al. 2011). The ingestion of animal material by green turtles was originally hypothesised to be incidental (Brand-Gardner et al. 1999; Mortimer 1981), although more recent studies have reported voluntary ingestion (Arthur et al. 2007; González Carman et al. 2014; Williams et al. 2014). Regardless if voluntary or involuntary, a certain amount of animal material is frequently found in stomach contents of green turtle populations globally, and it could be a more important diet source to this species than currently understood. Due to the high digestibility of animal material compared to plant material, the percentages found are usually low compared to seagrass or algae (Arthur et al. 2007; Heithaus et al. 2002). Certainly, the SIA and the stomach content data in this study indicate that green turtles in New Caledonia feed at more than one trophic level, and with significant consumption of animal matter, thus supporting the hypothesis for an omnivorous diet.
The tendency of new recruits and juveniles towards omnivory (high NR values and negative regression between δ15N at Goro and Uo/Mato Islands), in particular, supports findings from Moreton Bay (Australia) in which new recruits had significantly higher δ15N, compared to the older life stages that had been in the foraging ground for longer periods (Arthur et al. 2008). This was explained by an ontogenetic shift in diet, where oceanic juveniles feed mainly on a range of mostly carnivorous food items, compared to when they recruit to coastal habitats and switch to a diet of primarily herbivorous food items. This is further supported in the present study by the lower NR values in sub-adult and adult age-classes (Table 4), which implies high herbivory in these age-classes. Using SIA of different scute layers, this ontogenetic shift in diet was also found, in green turtles recruiting to coastal habitats in the Bahamas (Reich et al. 2007).
Globally, the diet of neritic foraging green turtle populations can vary greatly. The diet of some populations is dominated by one particular food source, such as seagrass (e.g. Green Island, Australia; Fuentes et al. 2006), algae (e.g. Moreton Bay, Australia; Brand-Gardner et al. 1999), or jellyfish (e.g. Samborombón Bay, Argentina; González Carman et al. 2014). Other green turtle populations (e.g. Fiji; Piovano et al. 2020), including the populations studied in GLS here, have more diverse diets, feeding on different proportions of a range of food items. From a management perspective, it is therefore important to understand the resilience of foraging areas to impact, and the environmental and anthropogenic processes that may drive the abundance and quality of food resources. Data from this study suggest that, even within a relatively small geographic area like the GLS, each of the distinct green turtle foraging aggregations, and even different age-classes within these aggregations, are supported by different food webs, and must therefore be managed individually.
Conclusion
This is the first study to investigate the feeding ecology of green turtles in New Caledonia. Combined SIA and stomach content analyses revealed that, even within a relatively small geographic area of the Grand Lagon Sud, the trophic ecology of green turtles differed significantly between foraging locations and age-classes, suggesting customised management is required at each location. This study therefore provides important information on the foraging ecology of green turtles in New Caledonia and ramifications for how to approach conservation of the species in this region. Further, this collaboration with the different tribes of the Grand Lagon Sud is also the first step towards the integrated management of green turtles as a resource and could be used as an example for the rest of the South Pacific.
Data availability
Data is available upon request.
References
Arthur KE, O’Neil JM, Limpus CJ, Abernathy K, Marshall G (2007) Using animal-borne imaging to assess green turtle (Chelonia mydas) foraging ecology in Moreton Bay. Aust Mar Technol Soc J 41(4):9–13
Arthur KE, Boyle MC, Limpus CJ (2008) Ontogenetic changes in diet and habitat use in green sea turtle (Chelonia mydas) life history. Mar Ecol-Prog Ser 362:303–311
Arthur KE, McMahon KM, Limpus CJ, Dennison WD (2009) Feeding ecology of green turtles (Chelonia mydas) from Shoalwater Bay, Australia. Mar Turt Newsl 123:6–12
Bjorndal KA (1985) Nutritional ecology of sea turtles. Copeia 1985:736–751
Bjorndal KA (1997) The biology of sea turtles. CRC Press, London, pp 199–231
Brand-Gardner SJ, Lanyon JM, Limpus CJ (1999) Diet selection by immature green turtles, Chelonia mydas, in subtropical Moreton Bay, south-east Queensland. Aust J Zool 47(2):181–191
Burkholder DA, Heithaus MR, Thomson JA, Fourqurean JW (2011) Diversity in trophic interactions of green sea turtles Chelonia mydas on a relatively pristine coastal foraging ground. Mar Ecol Prog Ser 439:277–293
Cardona L, Aguilar A, Pazos L (2009) Delayed ontogenic dietary shift and high levels of omnivory in green turtles (Chelonia mydas) from the NW coast of Africa. Mar Biol 156(7):1487–1495
Chimmo W (1856) Narrative of the loss of the Chinese Junk Ningpo on d'Entrecasteaux Reefs, near New Caledonia, with an account of the reefs. Nautical Magazine and Naval Chronicle March 1856, pp 113–121
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42(5):495–506
Dethmers KEM, Broderick D, Moritz C, Fitzsimmons NN, Limpus CJ, Lavery S, Whiting S, Guinea M, Prince RIT, Kennett R (2006) The genetic structure of Australasian green turtles (Chelonia mydas): exploring the geographical scale of genetic exchange. Mol Ecol 15(13):3931–3946
Dethmers KEM, Jensen MP, FitzSimmons NN, Broderick D, Limpus CJ, Moritz C (2010) Migration of green turtles (Chelonia mydas) from Australasian feeding grounds inferred from genetic analyses. Mar Freshw Res 61(12):1376–1387
Di Beneditto APM, Siciliano S, Monteiro LR (2016) Herbivory level and niche breadth of juvenile green turtles (Chelonia mydas) in a tropical coastal area: insights from stable isotopes. Mar Biol 164(1):13
Donlan CJ, Wingfield DK, Crowder LB, Wilcox C (2010) Using expert opinion surveys to rank threats to endangered species: a case study with sea turtles. Conserv Biol 24(6):1586–1595
Esteban N, Mortimer JA, Stokes HJ, Laloë J-O, Unsworth RKF, Hays GC (2020) A global review of green turtle diet: sea surface temperature as a potential driver of omnivory levels. Mar Biol 167(12):183
Fry B (2006) Stable isotope ecology. Springer Science and Business Media, New Yory
Fuentes M, Lawler IR, Gyuris E (2006) Dietary preferences of juvenile green turtles (Chelonia mydas) on a tropical reef flat. Wildl Res 33(8):671–678
Garnett ST, Price IR, Scott FJ (1985) The diet of the green turtle, Chelonia mydas (L) Torres Strait. Aust Wildl Res 12(1):103–112
Gillis AJ, Ceriani SA, Seminoff JA, Fuentes M (2018) Foraging ecology and diet selection of juvenile green turtles in the Bahamas: insights from stable isotope analysis and prey mapping. Mar Ecol Prog Ser 599:225–238
González Carman V, Botto F, Gaitán E, Albareda D, Campagna C, Mianzan H (2014) A jellyfish diet for the herbivorous green turtle Chelonia mydas in the temperate SW Atlantic. Mar Biol 161(2):339–349
Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K (2006) Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia 149(1):52–64
Hays GC, Scott R (2013) Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Funct Ecol 27(3):748–756
Heithaus MR, McLash JJ, Frid A, Dill LM, Marshall G (2002) Novel insights into green sea turtle behaviour using animal-borne video cameras. J Mar Biol Assoc UK 82(6):1049–1050
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120(3):314–326
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J Anim Ecol 80(3):595–602
Jensen MP (2010) Assessing the composition of green turtle (Chelonia mydas) foraging grounds in Australasia using mixed stock analyses. University of Canberra
Lanyon J (1986) Guide to the Identification of Seagrasses in the Great Barrier Reef Region, Great Barrier Reef Marine Park Authority, Townsville
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88(1):42–48
Lemons G, Lewison R, Komoroske L, Gaos A, Lai C-T, Dutton P, Eguchi T, LeRoux R, Seminoff JA (2011) Trophic ecology of green sea turtles in a highly urbanized bay: insights from stable isotopes and mixing models. J Exp Mar Biol Ecol 405(1):25–32
Limpus CJ (1992) Estimation of tag loss in marine turtle research. Wildl Res 19(4):457–469
Limpus C, Chaloupka M (1997) Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 149(1–3):23–34
Limpus CJ, Reed PC (1985) The biology of Australasian frogs and reptiles. Surrey Beatty and Sons, Sydney, pp 343–351
Limpus CJ, Miller JD, Parmenter CJ, Reimer D, McLachlan N, Webb R (1992) Migration of green (Chelonia mydas) and loggerhead (Caretta caretta) turtles to and from eastern Australian rookeries. Wildlife Res 19(3):347
Limpus CJ, Couper PJ, Read MA (1994) Memoirs of the Queensland Museum, Brisbane, pp. 139–154
Luschi P, Papi F, Liew HC, Chan EH, Bonadonna F (1996) Long-distance migration and homing after displacement in the green turtle (Chelonia mydas): a satellite tracking study. J Comp Physiol a Sens Neural Behav Physiol 178(4):447–452
Makowski C, Seminoff JA, Salmon M (2006) Home range and habitat use of juvenile Atlantic green turtles (Chelonia mydas L.) on shallow reef habitats in Palm Beach, Florida, USA. Mar Biol 148(5):1167–1179
Montoya JP, Carpenter EJ, Capone DG (2002) Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol Oceanogr 47(6):1617–1628
Mortimer JA (1981) The feeding ecology of the west Caribbean green turtle (Chelonia mydas) in Nicaragua. Biotropica 13(1):49–58
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 1:293–320
Piovano S, Lemons GE, Ciriyawa A, Batibasaga A, Seminoff JA (2020) Diet and recruitment of green turtles in Fiji, South Pacific, inferred from in-water capture and stable isotope analysis. Mar Ecol Prog Ser 640:201–213
Pritchard P (1982) Marine turtles of the South Pacific Biology and Conservation of Sea Turtles. Smithsonian Institution Press, Washington, pp 253–262
Read TC, Wantiez L, Werry JM, Farman R, Petro G, Limpus CJ (2014) Migrations of green turtles (Chelonia mydas) between nesting and foraging grounds across the Coral Sea. PLoS ONE 9(6):e100083
Read TC, Wantiez L, Werry JM, Farman R, Chateau O, Keller F, Limpus CJ (2020) Where are the adults? First results of a study on C. mydas foraging in New Caledonia. Oceanogr Fish. https://doi.org/10.19080/OFOAJ.2020.11.555815
Reich KJ, Bjorndal KA, Bolten AB (2007) The 'lost years’ of green turtles: using stable isotopes to study cryptic lifestages. Biol Let 3(6):712–714
Ross JP (1985) Biology of the green turtle, Chelonia mydas, on an Arabian feeding ground. J Herpetol 1:459–468
Seminoff JA (2004) Chelonia mydas. The IUCN Red List of Threatened Species 2004:2022. https://doi.org/10.2305/IUCN.UK.2004.RLTS.T4615A11037468.en.Downloadedon23February
Seminoff JA, Resendiz A, Nichols WJ (2002) Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California, Mexico. Mar Ecol Prog Ser 242:253–265
Seminoff JA, Jones TT, Eguchi T, Jones DR, Dutton PH (2006) Stable isotope discrimination (δ13C and δ15N) between soft tissues of the green sea turtle Chelonia mydas and its diet. Mar Ecol Prog Ser 308:271
Shimada T, Jones R, Limpus C, Groom R, Hamann M (2016) Long-term and seasonal patterns of sea turtle home ranges in warm coastal foraging habitats: implications for conservation. Mar Ecol Prog Ser 562:163–179
Shimada T, Limpus CJ, Hamann M, Bell I, Esteban N, Groom R, Hays GC (2020) Fidelity to foraging sites after long migrations. J Anim Ecol 89(4):1008–1016
Turner Tomaszewicz CN, Seminoff JA, Price M, Kurle CM (2017) Stable isotope discrimination factors and between-tissue isotope comparisons for bone and skin from captive and wild green sea turtles (Chelonia mydas). Rapid Commun Mass Spectrom 31(22):1903–1914
Vander Zanden HB, Bjorndal KA, Mustin W, Ponciano JM, Bolten AB (2012) Inherent variation in stable Isotope values and discrimination factors in two life stages of green turtles. Physiol Biochem Zool 85(5):431–441
Vander Zanden HB, Arthur KE, Bolten AB, Popp BN, Lagueux CJ, Harrison E, Campbell CL, Bjorndal KA (2013) Trophic ecology of a green turtle breeding population. Mar Ecol Prog Ser 476:237–249
Williams NC, Bjorndal KA, Lamont MM, Carthy RR (2014) Winter diets of immature green turtles (Chelonia mydas) on a northern feeding ground: integrating stomach contents and stable isotope analyses. Estuaries Coasts 37(4):986–994
Acknowledgements
We would like to thank the tribes of Isle of Pines, Ouen Island and Goro for letting us participate in their traditional ceremonies, and allowing us to collect the samples included in this study. We also extend our gratitude to the volunteers that helped us collect the skin samples for SIA (particularly the boys in blue from the Aquarium des Lagons). Rene Diocares from Griffith University was instrumental in processing the samples, and Dr Claude Payri was very helpful for the identification of the stomach contents. We would like to also acknowledge our funders.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was funded by the Tribal Council for the Environment (CCCE) and Vale Inco, through the Biodiversity Convention, signed with the South Province of New Caledonia.
Author information
Authors and Affiliations
Contributions
The project was conceived by TCR, LW, FK, RF, OC, JMW. Samples were collected by TCR and AdL. Analysis was done by LW, TCR, JPV, JMW, JJM. The manuscript was written by TCR, LW, OC, JPV, JJM, JMW. All authors contributed to editing drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest or competing interests for all authors.
Ethical approval
Samples were collected under New Caledonian permits 1139-2012/ARR/DENV, 1517-2013/ARR/DENV, 1656-2014/ARR/DENV, 2012-3253/GNC and Griffith University animal care and ethics approval ENG/01/12/AEC. Samples were exported from New Caledonia under CITES FR1398800027-E and imported into Australia with AQIS permit IP1209572. Data is available upon request from TCR.
Additional information
Responsible Editor: L. Avens .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Read, T.C., Wantiez, L., Keller, F. et al. The foraging ecology of green turtles (Chelonia mydas) in New Caledonia. Mar Biol 171, 81 (2024). https://doi.org/10.1007/s00227-023-04385-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04385-z