Abstract
Drainage ditches play a key role in the conservation of fragmented landscapes by providing refuge sites and secondary habitats for many terrestrial and aquatic organisms across various taxa. Species richness of ditches can exceed that of adjacent natural habitats, but here, we looked further and assessed the role of drainage ditches in shaping the community structure of true bugs aiming to better estimate ditches’ conservation value from the point of their species and trait composition. We tested the effects of the ditch substrate (saline, sandy or fen), landscape matrix (agrarian or grassland) and vegetation (species richness of all plants and invasive plants, and abundance of woody plants) on the true bug communities of 60 drainage ditches in the lowland of East-Central Europe. We found that substrate and landscape matrix contributed the most in determining true bug communities. Based on species composition, different substrates and landscape matrix types had distinct communities, but the trait composition showed differentiation according to the landscape matrix in saline habitats only. The trait composition in true bug communities was more diverse in grassland ditches than in agrarian ones, which hosted more habitat generalists associated with invasive vegetation. We concluded that a pronounced gradient in habitat stress, originating in substrate salinity and aridity, causes the differentiation of the true bug communities based on their trait composition. Additionally, intense habitat stress increases the number of habitat specialists and the conservation value of a drainage ditch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Man-made and modified ecosystems have been attracting the attention of conservationists because they can harbour a significant proportion of local biodiversity (Kowarik 2011; Chester and Robson 2013; Dorotovičová 2013; Kantsa et al. 2013; Simaika et al. 2016). In this sense, farmlands and agricultural areas are often the focus of conservation efforts (Bignal and McCracken 1996; Kleijn and Sutherland 2003; Tscharntke et al. 2005; DeClerck et al. 2010; Queiroz et al. 2014), where anthropogenic landscape elements (e.g. field margins, road verges, burial mounds, power line corridors, river dikes) have an important role in providing secondary habitats for numerous organisms including red-listed species (Deák et al. 2016; Torma et al. 2018; Phillips et al. 2020; Dániel-Ferreira et al. 2023).
Ditch banks or slopes can have more or less structured and dense vegetation, which depends on the applied management practices (Marja and Herzon 2012; Dollinger et al. 2015; Tölgyesi et al. 2022). Shrubs and woody plants along the ditches may increase habitat complexity, provide additional food sources, shelter, resting and nesting places for birds and invertebrates (Arnold 1983; Herzon and Helenius 2008; Marja and Herzon 2012). Well-preserved grassy strips on ditch slopes have the role of biodiversity hubs in intensively used croplands (Herzon and Helenius 2008; Dollinger et al. 2015; Torma et al. 2018), accumulate a great number of species (Tölgyesi et al. 2022), serve as flower strips for pollinators (Jansen et al. 2012; Königslöw et al. 2021) and provide a natural source of biocontrol agents (Decleer et al. 2015; Dollinger et al. 2017). Ditch-related communities predominantly consist of habitats generalists with limited conservation value (Herzon and Helenius 2008; Decleer et al. 2015), however, the quality of the landscape matrix surrounding a drainage ditch and the availability of natural habitat fragments can increase the conservation potential of ditches (Marja and Herzon 2012; Decleer et al. 2015).
In the Pannonian lowland of East-Central Europe, the drainage system of canals and ditches is interconnected with the remnants of the high-value grasslands which are traditionally used as extensive pastures. The best-preserved grassland fragments of the region are recognized as conservation priority in Europe, belonging to several categories in the Habitats Directive (e.g. 1530 *Pannonic salt steppes and salt marshes, 6260 *Pannonic sand steppes, 6410 Molinia meadows on calcareous, peaty or clayey-silt-laden soils (Molinion caeruleae)) (Council Directive 92/43/EEC). The Pannonian lowland was a mosaic of wetlands, grasslands and forest patches in the past, but it changed markedly in the middle of the 20th century when a dense network of drainage canals and ditches was constructed to make more land for intensive agriculture. In a few decades, most of the wetlands disappeared or have been spontaneously turned into drier habitats, mostly grasslands (Biró et al. 2007). Remnants of the Pannonian grasslands still preserve many important species, including endemics among plants (Riezing 2023) and arthropods (Varga 1995; Szinetár et al. 2005; Pokluda et al. 2012; Kenyeres and Bauer 2021).
Our previous multi-taxa study on the drainage ditch system in this landscape has revealed that ditches have higher numbers of plants and terrestrial arthropods (i.e. butterflies, spiders and true bugs) than the nearby semi-natural grasslands (Tölgyesi et al. 2022). However, many of these ditches also hosted a great amount of ruderal and invasive plants, which have the potential to increase the number of habitat generalists, but also to homogenize communities (Gallé et al. 2023). In our study, we also showed that an increase in habitat stress enhances the conservation value of drainage ditches (Tölgyesi et al. 2022). It is well-known that great habitat stress leads to species-poor and specialized plant communities, and a good example of this phenomenon is saline habitats, some of the most stressful environments in the region (Molnár and Borhidi 2003; Šefferová-Stanová et al. 2008; Deák et al. 2014). Therefore, the potential of ditches to support local biodiversity is unquestionable, but we still lack important information. From the conservation perspective, it is necessary to find out which ditches support habitat generalists and which ones promote more specialized communities. It is also important to explore what features of the ditches can indicate/predict the presence of organisms with a specific combination of functional traits.
True bugs are especially suitable for capturing the trait-based assembly mechanisms of complex secondary habitats like drainage ditches. In the Pannonian lowland, true bugs are highly diverse and well-studied with a growing literature on the ecology of their communities (Kőrösi et al. 2012; Torma and Császár 2013; Torma et al. 2017, 2019). They are good biodiversity indicators and have been frequently used in conservation assessments (Duelli and Obrist 1998; Fauvel 1999; Achtziger et al. 2007; Rabitsch 2008; Gerlach et al. 2013), while their functional traits have been adopted to estimate habitat quality and the success of habitat management (Birkhofer et al. 2015; Simons et al. 2016; Torma et al. 2019; Korányi et al. 2023). Having in mind the high responsiveness of true bug communities to vegetation structure and species composition of plants (Zurbrügg and Frank 2006; Torma and Császár 2013; Klimm et al. 2024), we expected that ditches with different vegetation properties have true bug communities of distinctive combination of traits. Also, we assumed that an increase in the abundance of invasive plants in ditches would promote habitat generalists among true bugs, in contrast to nearby semi-natural grasslands, where pronounced habitat stress, caused by salinity and dryness, prevents non-native vegetation from establishing (Perelman et al. 2007; Kelemen et al. 2012) and preserves original true bug fauna.
In this study, we investigated drainage ditches in the Pannonian lowland by comparing trait-based assembly mechanisms of their terrestrial true bug communities to those of surrounding high-value grasslands. Our main goal was to understand how the environment drives the true bugs’ traits and how it reflects to the conservation value of drainage ditches. Specifically, (i) we assessed the effect of the surrounding landscape matrix (agrarian vs. grassland), substrate type (saline vs. sandy vs. fen) and vegetation (species richness of all plants and invasive plants, and abundance of woody plants) of ditches on the species composition and trait composition of true bugs, (ii) we identified characteristic relationships between species traits or trait combinations (i.e. trait syndromes) and environmental predictors, and (iii) we assessed the effects of habitat stress on the presence of habitat specialists.
Materials and methods
Study area and sampling sites
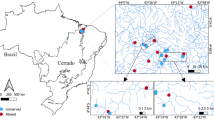
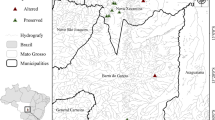
The study was carried out in the Danube-Tisza Interfluve of central Hungary, mostly within the Kiskunság National Park (Fig. 1). The region has dry continental climate, with cold winters and hot summers where the mean annual precipitation is 550–600 mm and the mean temperature is 10–11℃ (Tölgyesi et al. 2016). The region is characterized by a diversity of substrates and zonation of soil types—sand occupies the central part of the region, salt-affected soils are distributed along the former floodplains of the Danube and Tisza rivers, and peaty loam (fen substrate) is embedded between the previous two zones (Biró et al. 2007). Extrazonal patches of saline habitats and fens can also be found in the depressions of the central sandy zone (Pásztor et al. 2018). Three different substrates (sandy, saline and peaty loam) reflect gradients in humidity and salinity in a habitat; fens have medium humidity and low salinity, sandy habitats are seasonally extremely dry and saline habitats have similar drought periods to sandy ones but the salinity adds to the habitat stress. Considering that the interfluve has a narrow elevation gradient (mostly lying between 90 and 120 m a.s.l.) and continental climate, substrate primarily determines the grasslands formed (Biró et al. 2007, 2008; Molnár et al. 2008; Tölgyesi et al. 2022).
We selected 200 m long sections in 60 drainage ditches for sampling sites. Ditches varied according to landscape matrix (30 agrarian and 30 grassland ditches) and substrate type (20 fen, 20 saline and 20 sandy ditches), in a full-factorial balanced design (Fig. 1). Agrarian ditches were bordered by annual croplands on both sides and with no grassland fragments in a one km buffer, where grassland ones were embedded in semi-natural grasslands, without any crop fields in a same one km buffer. Sampled ditches were dry for most of the year, usually having open water only temporarily in early spring and after heavy rains. We selected ditches which were 1–5 m wide and up to 2 m deep. Large regional canals and ditches with constant water cover were avoided, as they are usually intensively managed due to their regional importance in water management. As a reference, we selected 200 m long transects in the semi-natural grassland (hereafter reference grassland) parallel to every grassland ditch and approximately 50 m from the ditch. In total, we sampled 30 grasslands (10 fens, 10 saline and 10 sandy grasslands), which correspond to the number of grassland ditches.
True bug and plant sampling
We surveyed plants in eight evenly spaced 1 m2 plots along the ditch sections and reference transects by identifying all vascular species that were rooted within the plots. We used only species richness data (presence–absence data) in this study as a possible environmental driver of true bug communities. In total, 480 plots for plant species were processed in ditches and 240 in reference transects. Additionally, we assessed the abundance of woody vegetation, i.e. small trees and bushes, in the ditches. The measure was the cumulative length of the covered ditch bank with a resolution of 1 m. Each bank was measured separately, leading to a maximum coverage of 400 m by woody vegetation. The plant sampling was done during the second half of June 2018.
We sampled true bugs by standard sweep-netting method alongside the 200 m ditch sections and the reference transects. One sample contained specimens swept from four evenly spaced 25 m long sub-transects (i.e. 4 × 25 one-direction sweeps by a 35 cm diameter sweep net) per ditch section or reference transect. All material collected by sweep-netting was placed in plastic bags filled with 70% ethanol and stored in a freezer until identification. Only records of adult true bugs were used for this study and they were identified to species level in our laboratory. To account for seasonal changes in true bug communities, we applied three sampling periods: May 15–24, July 9–17 and September 5–18, 2018.
True bug traits
We selected six traits to describe the trait composition in true bug communities (Table 1): (i) body size, defined as the mean length of an adult body; (ii) dispersal ability, based on forewing length, where apterous individuals lack wings or they are extremely reduced, brachypterous individuals have shortened wings, and macropterous are those with full (maximum) wing length; (iii) herbivory category, defined by the taxonomic diversity of host plants (species of predatory or mixed feeding strategies, i.e. zoophytophagous, were excluded from the trait analysis); (iv) overwintering stage, matching the number of the developmental stages of hemimetabolous true bugs and reflecting different survival strategies; (v) humidity preference; and (vi) shade tolerance described along a continuum of true bugs’ preferences of humid and shaded, forest-like habitats up to completely open and dry grassland-like habitat types. To maximize the informativeness of qualitative data, we applied numeric coding ranging from 0 to 1 on ordinal traits of true bugs (Table 1). All details on true bug traits were taken from the available literature (Wagner and Weber 1964; Péricart 1972, 1983, 1984, 1987, 1998; Wachmann et al. 2004, 2006, 2007, 2008; Gossner et al. 2015a, b).
Data analysis
Data collected in a single sampling site (eight plots for plants and 4 × 25 sweeps for true bugs) were pooled making a single statistical sample each. Also, samples of true bugs collected in different seasons were merged to create a joined species composition matrix.
We analyzed the species composition of true bug communities using permutational multivariate analysis of variance (PERMANOVA). The total species composition matrix (90 sampling sites, including 60 ditches and 30 reference grasslands) was used as a dependent variable, where the substrate (sandy, saline and fen) and landscape matrix (agrarian ditch, grassland ditch and reference grassland) were independent variables. We also ran six separate single-predictor models for each of the substrate and landscape matrix types. PERMANOVA tests were run in R version 4.3.0 (R Core Team 2023) with the adonis2 function of the ‘vegan’ package (Oksanen et al. 2017), whereas pairwise comparisons of the single-predictor models were done by pairwise.adonis function of the ‘pairwiseAdonis’ package (Martinez Arbizu 2020). The significance of all PERMANOVA models was tested using 999 permutations on Bray-Curtis distance matrices.
To reveal what habitat and landscape attributes are associated with trait syndromes of true bugs, we applied RLQ and fourth-corner analyses following Kleyer et al. (2012) and Dray et al. (2014). Both methods are based on the analysis of the fourth matrix, which crosses species traits weighted by species abundances and environmental variables. The input matrices for the resulting fourth matrix are the R-table (samples × environmental variables), the L-table (samples × species) and the Q-table (species × species traits). These two methods complement each other; RLQ is a multivariate ordination technique that summarizes the joint structure among the three tables, whereas the fourth-corner tests individual trait–environment relationships.
We performed RLQ and the fourth-corner analyses separately for different substrates. The R-table for a single substrate type had 10 agrarian ditches, 10 grassland ditches and 10 reference grasslands for ‘samples’, whereas, landscape matrix, the abundance of woody vegetation, total species richness of plants and species richness of invasive plants were used as ‘environmental variables’. The L-table was the standard species composition matrix (i.e. sampling sites × true bug species). The Q-table was based on true bug trait values given in Table 1. We used the ‘ade4’ package (Dray and Dufour 2007) to carry out the RLQ and the fourth-corner analyses. To evaluate the significance of the trait-environment relationships in the RLQ and fourth-corner, we followed Dray et al. (2014) and set the ‘modeltype’ argument to 6, the combination of Model 2 (permuted site vectors, i.e. rows of the L-table) and Model 4 (permuted species vectors, i.e. columns of the L-table). The significance of RLQ and fourth-corner models was tested using 9,999 permutations.
To differentiate species groups according to their trait syndromes, we applied hierarchical cluster analysis, which was followed by the analysis of variance (ANOVA) and Tukey’s tests to validate the distinction of trait values among species clusters. Functions hclust and aov of the basic ‘stats’ package in the R were used (R Core Team 2023).
The commonness index (CI) was calculated for the total true bug dataset and each substrate separately to test what species were common and which ones were rare, whether there was a difference in commonness of shared species in different substrates and what species could indicate specific substrates based on their CI values. The commonness index estimates the probability for each species to be common (1) or rare (0) based on abundance–occupancy information from the species composition matrix. Commonness indices were calculated using the ‘FuzzyQ’ package and its fuzzyq function (Balbuena et al. 2021a, b).
From the literature and available red lists of the Pannonian countries, we selected habitat specialists of characteristic semi-natural grasslands (Štepanovičová and Bianchi 2001; Wachmann et al. 2004, 2006, 2007, 2008; Rabitsch 2012; Kment et al. 2017; Šeat and Nadaždin 2021) to check what substrates promote more specialists and how specialists are distributed among landscape matrix types.
Results
Species composition shaped by landscape matrix and substrate
Results of PERMANOVA for the full dataset showed that the substrate (F = 6.061, R2 = 0.100, p < 0.001), landscape matrix (F = 6.601, R2 = 0.109, p < 0.001) and their interaction (F = 3.097, R2 = 0.102, p < 0.001) significantly affected the true bug communities. Results of the single-predictor PERMANOVAs indicated distinct true bug composition among the landscape matrix types of each substrate and among the substrate types of grassland ditches and grasslands. Regarding agrarian ditches in pairwise comparisons, we could not detect any difference between fen ditches and the other two substrates, although saline and sandy ditches still differed (Table 2).
Species trait–environment relationships
The total inertia in every one of the three RLQ ordinations was explained by five axes, and the first two axes described more than 95% of the variation in each of the three ordinations. Models were significant for saline habitats (p = 0.025), but not for sandy habitats (p = 0.089) nor fens (p = 0.350). The tests which provide separate results for Model 2 and Model 4 showed significant results for saline habitats (pmod2 = 0.013, pmod4 = 0.030), but only the Model 2 component was significant for sandy habitats (pmod2 < 0.001, pmod4 = 0.085) and fens (pmod2 < 0.001, pmod4 = 0.342), which implies that in sandy habitats and fens the trait composition of true bug communities of different landscape matrices do not significantly differ from each other.
In saline habitats, the ordination biplot of RLQ analysis showed a certain grouping of true bug traits and environmental variables along both main axes (Fig. 2a), and resulted in significant species trait–environment correlations by the fourth-corner analysis (Tab. S2). In sandy habitats, species traits were grouped and positively associated with environmental attributes only along the horizontal axis (Fig. 2c). In fens, no grouping of true bug traits and environment was detected, and only one negative correlation could be observed on the ordination biplot (Fig. 2e).
Results of RLQ, the fourth-corner analyses and species clustering. (a, c, e) True bug trait–environment ordination biplots are complemented with correlation lines of fourth-corner analysis (Table S2). True bug traits are given in italics, environmental variables are in boldface. Red lines refer to significantly positive correlations and blue lines to significantly negative correlations (p < 0.05). Dashed lines refer to correlations significant for 0.05 < p < 0.1. Arrows point towards high values of true bug traits (SR.plants: species richness of plants, SR.invas.plants: species richness of invasive plants, Ref.grassland: reference grasslands, Grass.ditch: grassland ditches, Agr.ditch: agrarian ditches). (b, d, f) The ordination plots of species scores are based on the values of species traits (RLQ results). Species clusters A, B and C were defined by hierarchical cluster analysis of species trait values
The results of hierarchical cluster analysis distinguished three species clusters or trait syndromes (Fig. 2b, d and f) for all three substrates according to trait partitioning (Fig. 3). Species clusters of different substrates do not correspond to each other and should not be compared, however, a similarity of trait syndromes is present in communities of saline and sandy habitats (Fig. 3). Only in saline habitats defined trait syndromes correspond to three landscape categories—cluster A is associated with grassland ditches, cluster B with reference grasslands and cluster C with agrarian ditches (Fig. 2b). In sandy habitats, there is a certain relatedness of cluster B with reference grasslands and cluster C with both types of ditches (Fig. 2d), but in fens, defined trait syndromes do not correspond to any of the three landscape categories (Fig. 2f).
Distribution of habitat specialists
Saline habitats came up as the richest in habitat specialists among substrates, and saline specialists Conostethus hungaricus and Henestaris halophilus were the most common out of nine recorded specialist species (Table 3). Antheminia varicornis was the only specialist that was not recorded in reference grasslands but exclusively in ditches. In saline habitats, most of the habitat specialists were associated with cluster B which corresponds to the reference grasslands (Fig. 4).
The ordination plots of species scores based on values of functional and life-history traits (RLQ analysis). True bug habitat specialists are given for (a) saline and (b) sandy habitats. Dot size indicates the value of the species commonness index (CI). Dot colour indicates the affiliation of specialists to a species cluster (hierarchical cluster analysis)
Discussion
Based on the species composition, true bugs from various substrates and landscape matrix types formed distinct communities. Trait composition confirmed the results of species composition in the case of saline habitats, but we could not identify clear trademark trait syndromes and communities exclusive for ditches or reference semi-natural grasslands in fens and sandy habitats. For instance, no specific relationship between true bug traits and any of the environmental attributes was detected in fens. It means that true bug communities of reference grasslands and ditches with fen vegetation are relatively trait-uniform and their trait composition is independent of the landscape matrix. These habitats appear similar to each other and the resemblance originates in their structure and resources (Tölgyesi et al. 2022). On the other hand, well-defined trait syndromes in saline habitats were correlated with specific habitat and landscape attributes.
Communities of semi-natural grasslands
To some degree, true bug communities of all reference grasslands, regardless of the substrate, belong to the same trait syndrome. Combination of traits of grassland communities refers to the presence of species of medium size (ca. 5 mm) with good dispersal abilities, species that are oligophagous, overwinter in early developmental stages (i.e. as eggs or nymphs) and have high preferences for dry and open habitats. This characterisation is strongly supported by the results from sandy and saline grasslands. In these grasslands, species of clusters B match the previously described trait syndrome. In fens, there was no clear association of any of the species clusters to reference grasslands, however, the species of cluster C are the closest to what can be considered as a community of a reference fen.
The combination of traits in communities of semi-natural grasslands was expected, because studied reference grasslands shared several grass-feeding species that had high commonness indices in all substrates (e.g. Acetropis carinata, Amblytylus nasutus, Megaloceroea recticornis, Stenodema calcarata, Trigonotylus caelestialium and T. pulchellus) (Tab. S5). A previous study on the species composition of true bugs from saline Artemisia steppes showed that those communities encompass a great number of oligophagous grass-feeding Mirids (Šeat et al. 2021), which typically overwinter as eggs oviposited in plant tissues. Many specialized grass-feeding species are shared among different types of dry grasslands in the region and represent the most abundant group here (Torma and Császár 2013; Torma et al. 2014, 2017, 2019).
Even having many species in common and communities with similar trait combinations, what differentiate reference grasslands of saline and sandy substrates are unique subsets of habitat specialists. Low abundance of most of habitat specialists could not differentiate saline and sandy communities according to their trait composition, but the presence of specialists affected species composition results. Out of nine habitat specialists recorded in our study, eight species were present in reference grasslands, making saline and sandy semi-natural grasslands irreplaceable habitats for the conservation of characteristic true bugs.
Communities of drainage ditches
Sandy ditches are considerably more humid than the related reference grasslands, which increases the number of plant species in ditches, but also the encroachment of some invasive ones (Tölgyesi et al. 2022). In our study, both types of sandy ditches had more complex vegetation structures than the adjacent grasslands, which was likely an important driver of true bug communities in these habitats. Our results also revealed the trait syndrome of true bugs associated with ditches covered by abundant herbaceous and woody vegetation regardless of the substrate. These ditches were typically inhabited by large true bug species (> 7 mm) with good flying abilities, which were polyphagous and overwinter in the adult stage. These species also had preferences for medium humidity and partly shaded habitats. The aforementioned description of the trait syndrome fits a combination of traits in cluster C from sandy habitats, but also cluster C from saline habitats. Both clusters shared several common habitat generalists (e.g. Adelphocoris lineolatus, Carpocoris purpureipennis, Eurydema oleracea and Dolycoris baccarum), which were frequent in the samples. Habitat generalists associated with drainage ditches have high colonization potential which is the result of the capability of those species to cross wide cropland areas and reach the most isolated ditches in the landscape (Seibold et al. 2019).
The presence of similar ruderal and invasive vegetation in agrarian ditches caused difficulties in distinguishing true bug communities of different substrates. The main reason for that could be the presence of similar environments in agrarian ditches that supported similar species composition of true bugs (Blowes et al. 2022; Gallé et al. 2023). Robust ruderal and invasive plants and woody vegetation increased the habitat complexity of ditches (Herzon and Helenius 2008; Marja and Herzon 2012), and consequently, contributed to the creation of new microhabitats and new food sources causing the enrichment in true bug communities (Zurbrügg and Frank 2006; Torma and Császár 2013; Stein et al. 2014; Simons et al. 2016; Gallé et al. 2023; Klimm et al. 2024). However, an augmented species number is not necessarily favourable, as a high prevalence of habitat generalists in the community and the replacement of specialists may cause functional and taxonomic homogenization and reduction in the conservation value of a habitat (Herzon and Helenius 2008; Clavel et al. 2011; Blowes et al. 2022; Gallé et al. 2023).
The only exception among ditches was saline grassland ditches with a distinctive true bug community of saline marshlands (Rabitsch 2012; Šeat and Nadaždin 2021; Torma et al. 2019). This community was represented by cluster A from saline habitats and the attributes describing this trait syndrome were small (< 4 mm), brachypterous species, which were oligophagous feeders on reed, sedge, bulrush or rush. These true bugs overwinter in the adult stage and prefer humid and open habitats. Low dispersal abilities (i.e. shortened wings and small size) of salt marsh true bugs in the intensively used agrarian landscape could reduce their success in reaching potentially suitable ditches (Birkhofer et al. 2015). Seibold et al. (2019) showed that the number of weak dispersers among grassland arthropods decreases if the cover of surrounding arable land increases. This could be an additional obstacle for true bugs of already endangered saline marshes (Janssen et al. 2016), which used to be a typical wetland type in the region but became scarce due to climate change and local drying effects, including drainage (Biró et al. 2007; Molnár et al. 2008).
Habitat stress affects community trait composition
Highly dispersive grass-feeding Mirids are resilient to seasonal droughts in saline and sandy semi-natural grasslands, making this group dominant in the driest habitats. Environmental filtering of species by habitat stress like drought may lead to convergence in trait values and lower functional diversity of the communities (Gallé et al. 2018). Similarly, land use intensity is also known to promote small highly dispersive species and reduce functional diversity in true bug communities (Birkhofer et al. 2015; Simons et al. 2016). Besides typical grass-feeders, dry Pannonian grasslands have a unique true bug fauna of habitat specialists. These harsh environments provide a peculiar and seasonally limited set of resources that only specialist species can utilize. Saline and sandy habitat specialists diversify the ecological functions of the true bug communities while being seed predators and trophic specialists of halophytes, but also add an extra value to the ditch habitats as very exclusive and stenotopic representatives of regional fauna (Achtziger et al. 2007; Rabitsch 2008).
In saline habitats, salinity in combination with water availability creates a gradient in habitat stress that causes the differentiation of trait syndromes in true bug communities. The same gradient can be observed in a single drainage ditch resulting in the coexistence of distinct true bug communities of saline marshes and saline grasslands. These ditches mimic the effect of naturally occurring microtopography unique to saline grassland-marshland mosaics (Molnár and Borhidi 2003; Šefferová-Stanová et al. 2008) by creating characteristic zonation of saline vegetation on ditch banks, from salt marsh vegetation on the bottom to Artemisia salt steppe on the top (Kelemen et al. 2012; Deák et al. 2014).
Conclusion
We argue that the drainage ditch system in central Hungary has the potential to support diverse communities of true bugs. Grassland ditches provide better secondary habitats for grassland true bugs than agrarian ones, however, saline grassland ditches provide safe havens for characteristic saline marshland communities. Agrarian and some grassland ditches which are greatly overgrown by invasive plants promote habitat generalists, which contribute to the conservation value of ditches only by increasing species richness without an increase in trait diversity. The main factors that determine the conservation value of drainage ditches in the region are habitat stress (caused by aridity and salinity) and landscape matrix. Increased habitat stress generates true bug communities richer in habitat specialists, whereas the increased distance between a ditch and the nearest cropland decreases the pressure that invasive and ruderal vegetation is putting on a ditch habitat. Keeping non-native vegetation in drainage ditches to a minimum and preserving natural seasonal migrations of salt and water in the soil would conserve species and trait composition in true bug communities which are similar to those in high-value grasslands and marshlands of the region.
Data availability
No datasets were generated or analysed during the current study.
References
Achtziger R, Frieß T, Rabitsch W (2007) True bugs (Insecta: Heteroptera) as suitable indicators for nature conservation. Insecta 10:93–127
Arnold GW (1983) The influence of ditch and hedgerow structure, length of hedgerows, and area of woodland and garden on bird numbers on farmland. J Appl Ecol 20(3):731–750. https://doi.org/10.2307/2403123
Balbuena JA, Monlleó-Borrull C, Llopis-Belenguer C, Blasco-Costa I, Sarabeev VL, Morand S (2021a) Fuzzy quantification of common and rare species in ecological communities (FuzzyQ). Methods Ecol Evol 12(6):1070–1079. https://doi.org/10.1111/2041-210X.13588
Balbuena JA, Monlleó-Borrull C, Llopis-Belenguer C, Blasco-Costa I, Sarabeev VL, Morand S (2021b) Code for fuzzy quantification of common and rare species in ecological communities (version v1.0). Zenodo. https://doi.org/10.5281/zenodo.4469291
Bignal EM, McCracken DI (1996) Low-intensity farming systems in the conservation of the countryside. Journal of Applied Ecology 33(3): 413–424. https://doi.org/10.2307/2404973
Birkhofer K, Smith HG, Weisser WW, Wolters V, Gossner MM (2015) Land-use effects on the functional distinctness of arthropod communities. Ecography 38(9):889–900. https://doi.org/10.1111/ecog.01141
Biró M, Révész A, Molnár Z, Horváth F (2007) Regional habitat pattern of the Danube-Tisza Interfluve in Hungary, I: the landscape structure and habitat pattern; the Fen and alkali vegetation. Acta Bot Hungarica 49(3–4):267–303. https://doi.org/10.1556/ABot.49.2007.3-4.4
Biró M, Révész A, Molnár Z, Horváth F, Czúcz B (2008) Regional habitat pattern of the Danube-Tisza Interfluve in Hungary II: the sand, the steppe and the riverine vegetation, degraded and regenerating habitats, regional habitat destruction. Acta Bot Hungarica 50(1–2):19–60. https://doi.org/10.1556/ABot.50.2008.1-2.2
Blowes SA, McGill B, Brambilla V, Chow CFY, Engel T, Fontrodona-Eslava A, Martins IS, McGlinn D, Moyes F, Sagouis A, Shimadzu H, van Klink R, Xu WB, Gotelli NJ, Magurran A, Dornelas M, Chase JM (2022) Synthesis reveals biotic homogenisation and differentiation are both common. bioRxiv 20220705498812. https://doi.org/10.1101/2022.07.05.498812
Chester ET, Robson BJ (2013) Anthropogenic refuges for freshwater biodiversity: Their ecological characteristics and management. Biological Conservation 166: 64–75. https://doi.org/10.1016/j.biocon.2013.06.016
Clavel J, Julliard R, Devictor V (2011) Worldwide decline of specialist species: toward a global functional homogenization? Front Ecol Environ 9(4):222–228. https://doi.org/10.1890/080216
Council Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora
Dániel-Ferreira J, Fourcade Y, Bommarco R, Wissman J, Öckinger E, (2023) https://doi.org/10.1111/1365-2664.14378
Deák B, Valkó O, Alexander C, Mücke W, Kania A, Tamás J, Heilmeier H (2014) Fine-scale vertical position as an indicator of vegetation in alkaligrasslands – case study based on remotely sensed data. Flora 209(12):693–697. https://doi.org/10.1016/j.flora.2014.09.005
Deák B, Tóthmérész B, Valkó O, Sudnik-Wójcikowska B, Moysiyenko II, Bragina TM, Apostolova I, Dembicz I, Bykov NI, Török P (2016) Cultural monuments and nature conservation: a review of the role of kurgans in the conservation and restoration of steppe vegetation. Biodivers Conserv 25:2473–2490. https://doi.org/10.1007/s10531-016-1081-2
DeClerck FAJ, Chazdon R, Holl KD, Milder JC, Finegan B, Martinez-Salinas A, Imbach P, Canet L, Ramos Z (2010) Biodiversity conservation in human-modified landscapes of Mesoamerica: Past, present and future. Biological Conservation 143(10): 2301–2313. https://doi.org/10.1016/j.biocon.2010.03.026
Decleer K, Maes D, Van Calster H, Jansen I, Pollet M, Dekoninck W, Baert L, Grootaert P, Van Diggelen R, Bonte D (2015) Importance of core and linear marsh elements for wetland arthropod diversity in an agricultural landscape. Insect Conserv Divers 8(4):289–301. https://doi.org/10.1111/icad.12110
Dollinger J, Dagès C, Bailly JS, Lagacherie P, Voltz M (2015) Managing ditches for agroecological engineering of landscape. A review. Agron Sustain Dev 35:999–1020. https://doi.org/10.1007/s13593-015-0301-6
Dollinger J, Vinatier F, Voltz M, Dagès C, Bailly JS (2017) Impact of maintenance operations on the seasonal evolution of ditch properties and functions. Agric Water Manage 193:191–204. https://doi.org/10.1016/j.agwat.2017.08.013
Dorotovičová Cs (2013) Man-made canals as a hotspot of aquatic macrophyte biodiversity in Slovakia. Limnologica 43(4): 277–287. https://doi.org/10.1016/j.limno.2012.12.002
Dray S, Dufour A (2007) The ade4 Package: implementing the duality Diagram for ecologists. J Stat Softw 22(4):1–20. https://doi.org/10.18637/jss.v022.i04
Dray S, Choler P, Dolédec S, Peres-Neto PR, Thuiller W, Pavoine S, ter Braak CJF (2014) Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95(1):14–21. https://doi.org/10.1890/13-0196.1
Duelli P, Obrist MK (1998) In search of the best correlates for local organismal biodiversity in cultivated areas. Biodivers Conserv 7:297–309. https://doi.org/10.1023/A:1008873510817
Fauvel G (1999) Diversity of Heteroptera in agroecosystems: role of sustainability and bioindication. Agriculture. Ecosyst Environ 74(1–3):275–303. https://doi.org/10.1016/S0167-8809(99)00039-0
Gallé R, Szabó Á, Császár P, Torma A (2018) Spider assemblage structure and functional diversity patterns of natural forest steppes and exotic forest plantations. For Ecol Manag 411:234–239. https://doi.org/10.1016/j.foreco.2018.01.040
Gallé R, Tölgyesi C, Szabó AR, Korányi D, Bátori Z, Hábenczyus A, Török E, Révész K, Torma A, Gallé-Szpisjak N, Lakatos T, Batáry P (2023) Plant invasion and fragmentation indirectly and contrastingly affect native plants and grassland arthropods. Sci Total Environ 903:166199. https://doi.org/10.1016/j.scitotenv.2023.166199
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850. https://doi.org/10.1007/s10841-013-9565-9
Gossner MM, Simons NK, Achtziger R, Blick Th, Dorow WHO, Dziock F, Köhler F, Rabitsch W, Weisser WW (2015a) A summary of eight traits of Coleoptera, Hemiptera, Orthoptera and Araneae, occurring in grasslands in Germany. Sci Data 2:150013. https://doi.org/10.1038/sdata.2015.13
Gossner MM, Simons NK, Höck L, Weisser WW (2015b) Morphometric measures of Heteroptera sampled in grasslands across three regions of Germany. Ecology 96(4):1154. https://doi.org/10.1890/14-2159.1
Herzon I, Helenius J (2008) Agricultural drainage ditches, their biological importance and functioning. Biol Conserv 141(5):1171–1183. https://doi.org/10.1016/j.biocon.200803.005
Jansen SHDR, Holmgren M, van Langevelde F, Wynhoff I (2012) Resource use of specialist butterflies in agricultural landscapes: conservation lessons from the butterfly Phengaris (Maculinea) nausithous. J Insect Conserv 16:921–930. https://doi.org/10.1007/s10841-012-9479-y
Janssen JAM, Rodwell JS, García Criado M, Gubbay S, Haynes T, Nieto A, Sanders N, Landucci F, Loidi J, Ssymank A, Tahvanainen T, Valderrabano M, Acosta A, Aronsson M, Arts G, Attorre F, Bergmeier E, Bijlsma RJ, Bioret F, Valachovič M (2016) European Red List of habitats, Part 2. Terrestrial and freshwater habitats. Publications Office of the European Union, Luxembourg
Kelemen A, Török P, Valkó O, Miglécz T, Tóthmérész B (2012) Mechanisms shaping plant biomass and species richness: plant strategies and litter effect in alkali and loess grasslands. J Veg Sci 24:1195–1203. https://doi.org/10.1111/jvs.12027
Kantsa A, Tscheulin T, Junker RR, Petanidou T, Kokkini S (2013) Urban biodiversity hotspots wait to get discovered: The example of the city of Ioannina, NW Greece. Landscape and Urban Planning 120: 129–137. https://doi.org/10.1016/j.landurbplan.2013.08.013
Kenyeres Z, Bauer N (2021) Conservation possibilities of Isophya costata (Orthoptera: Tettigoniidae: Phaneropterinae) based on frequency, population size, and habitats. J Orthoptera Res 30(1):35–41. https://doi.org/10.3897/jor.30.59262
Kleijn D, Sutherland WJ (2003) How effective are European agri-environment schemes in conserving and promoting biodiversity? Journal of Applied Ecology 40(6): 947–969. https://doi.org/10.1111/j.1365-2664.2003.00868.x
Kleyer M, Dray S, de Bello F, Lepš J, Pakeman RJ, Strauss B, Thuiller W, Lavorel S (2012) Assessing species and community functional responses to environmental gradients: which multivariate methods? J Veg Sci 23(5):805–821. https://doi.org/10.1111/j.1654-1103.2012.01402.x
Klimm FS, Bräu M, König S, Mandery K, Sommer C, Zhang J, Krauss J (2024) Importance of habitat area, quality and landscape context for heteropteran diversity in shrub ecotones. Landscape Ecol 39(3):1–17. https://doi.org/10.1007/s10980-024-01798-z
Kment P, Hradil K, Straka M, Sychra J (2017) Heteroptera (ploštice). In: Hejda, R., Farkač, J., Chobot, K. (Eds.): Red list of threatened species in the Czech Republic. Invertebrates. Příroda Praha 36: 137–147
Korányi D, Gallé R, Torma A, Gallé-Szpisjak N, Batáry P (2023) Small grassland fragments and connectivity support high arthropod functional diversity in highly modified landscapes. Insect Conserv Divers 16(5):701–711. https://doi.org/10.1111/icad.12668
Kőrösi Á, Batáry P, Orosz A, Rédei D, Báldi A (2012) Effects of grazing, vegetation structure and landscape complexity on grassland leafhoppers (Hemiptera: Auchenorrhyncha) and true bugs (Hemiptera: Heteroptera) in Hungary. Insect Conserv Divers 5(1):57–66. https://doi.org/10.1111/j.1752-4598.2011.00153.x
Kowarik I (2011) Novel urban ecosystems, biodiversity, and conservation. Environmental Pollution 159(8–9): 1974–1983. https://doi.org/10.1016/j.envpol.2011.02.022
Langheinrich U, Tischew S, Gersberg RM, Lüderitz V (2004) Ditches and canals in management of fens: opportunity or risk? A case study in the Drömling Natural Park, Germany. Wetlands Ecol Manage 12:429–445. https://doi.org/10.1007/s11273-004-0700-y
Marja R, Herzon I (2012) The importance of drainage ditches for farmland birds in agricultural landscapes in the Baltic countries: does field type matter? Ornis Fennica 89(3):170–181
Martinez Arbizu P (2020) pairwiseAdonis: pairwise multilevel comparison using adonis. R Package Version 0.4
Molnár Zs, Biró M, Bölöni J, Horváth F (2008) Distribution of the (semi-)natural habitats in Hungary I. marshes and grasslands. Acta Bot Hungarica 50(Suppl):59–105. https://doi.org/10.1556/ABot.50.2008.Suppl.5
Molnár Zs, Borhidi A (2003) Hungarian alkali vegetation: origins, landscape history, syntaxonomy, conservation. Phytocoenologia 33(2–3):377–408. https://doi.org/10.1127/0340-269X/2003/0033-0377
Natuhara Y (2022) Conservation of endangered species in Japan’s agroecosystems: focusing on specified class II nationally rare species of wild fauna/flora. Landscape Ecol Eng 18:309–320. https://doi.org/10.1007/s11355-021-00470-x
Oksanen FJ, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagne H (2017) Vegan: Community Ecology Package. R package Version 2.4-3
Pásztor L, Laborczi A, Bakacsi Zs, Szabó J, Illés G (2018) Compilation of a national soil-type map for Hungary by sequential classification methods. Geoderma 311:93–108. https://doi.org/10.1016/j.geoderma.2017.04.018
Perelman SB, Chaneton EJ, Batista WB, Burkart SE, León RJC (2007) Habitat stress, species pool size and biotic resistance influence exotic plant richness in the flooding pampa grasslands. J Ecol 95(4):662–673. https://doi.org/10.1111/j.1365-2745.2007.01255.x
Péricart J (1972) Hémiptères. Anthocoridae, Cimicidae at Microphysidae de l’Ouest-Paléarctique. Fauna de l’Europe et du Bassin Méditerranéen, Vol. 7, Paris
Péricart J (1983) Hémiptères. Tingidae euro-méditerranéens. Fauna de France 69. Fédération Française des Sociétés de Sciences Naturelles, Paris
Péricart J (1984) Hémiptères. Berytidae euro-méditerranéens. Fauna de France 70. Fédération Française des Sociétés de Sciences Naturelles, Paris
Péricart J (1987) Hémiptères. Nabidae d’Europe occidentale et du Maghreb. Fauna de France 71. Fédération Française des Sociétés de Sciences Naturelles, Paris
Péricart J (1998) Hémiptères. Lygaeidae euro-méditerranéens. Fauna de France 84 A, B, C. Fédération Française des Sociétés de Sciences Naturelles, Vol. 1–3, Paris
Phillips BB, Wallace C, Roberts BR, Whitehouse AT, Gaston KJ, Bullock JM, Dicks LV, Osborne JL (2020) Enhancing road verges to aid pollinator conservation: a review. Biol Conserv 250:108687. https://doi.org/10.1016/j.biocon.2020.108687
Pokluda P, Hauck D, Cizek L (2012) Importance of marginal habitats for grassland diversity: fallows and overgrown tall-grass steppe as key habitats of endangered ground-beetle Carabus Hungaricus. Insect Conserv Divers 5(1):27–36. https://doi.org/10.1111/j.1752-4598.2011.00146.x
Queiroz C, Beilin R, Folke C, Lindborg R (2014) Farmland abandonment: threat or opportunity for biodiversity conservation? A global review. Front Ecol Environ 12(5): 288–296. https://doi.org/10.1890/120348
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rabitsch W (2008) Notes on the true bugs (Insecta, Heteroptera) in the National Park Lake Neusiedl–Seewinkel and comments about their suitability as indicators for a habitat management (Austria). Abh Zool -Bot Ges Österreich 37:155–172
Rabitsch W (2012) Checklist and red list of true bugs of Burgenland (Insecta, Heteroptera). Wissenschaftliche Mitteilungen Niederösterreichisches Landesmuseum 23:161–306
Rolke D, Jaenicke B, Pfaender J, Rothe U (2018) Drainage ditches as important habitat for species diversity and rare species of aquatic beetles in agricultural landscapes (Insecta: Coleoptera). J Limnol 77(3):466–482. https://doi.org/10.4081/jlimnol.2018.1819
Šeat J, Nadaždin B (2021) True bugs (Heteroptera) of the Pannonic salt steppes and salt marshes in Serbia and their conservation status in the pannonian countries. Ann De La Société entomologique de France (N S) 57(2):107–138. https://doi.org/10.1080/00379271.2021.1888155
Šeat J, Nadaždin B, Milić N, Ćuk M, Torma A (2021) How steady is the nested pattern in saline grassland true bug communities? Effects of sampling effort and data completeness on nestedness. Acta Oecol 110:103670. https://doi.org/10.1016/j.actao.2020.103670
Šefferová-Stanová V, Janák M, Ripka J (2008) Management of Natura 2000 habitats. 1530 *Pannonic salt steppes and salt marshes. European Commission. ISBN 978-92-79-08316-7
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze ED, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Simons NK, Weisser WW, Gossner MM (2016) Multi-taxa approach shows consistent shifts in arthropod functional traits along grassland land-use intensity gradient. Ecology 97(3):754–764. https://doi.org/10.1890/15-0616.1
Simaika JP, Samways MJ, Frenzel PP (2016) Artificial ponds increase local dragonfly diversity in a global biodiversity hotspot. Biodivers Conserv 25:1921–1935. https://doi.org/10.1007/s10531-016-1168-9
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880. https://doi.org/10.1111/ele.12277
Štepanovičová O, Bianchi Z (2001) Red (Ecosozological) List of Heteroptera of Slovakia. In: Balaž D, Marhold K, Urban P (Eds.) Red List of plants and animals of Slovakia Nature Conservation. Ochrana Prírody 20 (Suppl.): 105–106
Szinetár C, Eichardt J, Horváth R (2005) Data on the Biology of Alopecosa psammophila Buchar 2001 (Araneae, Lycosidae). The Journal of Arachnology 33(2), 16th International Congress of Arachnology, pp. 384–389. https://doi.org/10.1636/05-1.1
Tölgyesi Cs, Torma A, Bátori Z, Šeat J, Popović M, Gallé R, Gallé-Szpisjak N, Erdős L, Vinkó T, Kelemen A, Török P (2022) Turning old foes into new allies—harnessing drainage canals for biodiversity conservation in a desiccated European lowland region. J Appl Ecol 59(1):89–102. https://doi.org/10.1111/1365-2664.14030
Tölgyesi Cs, Zalatnai M, Erdős L, Bátori Z, Hupp N, Körmöczi L (2016) Unexpected ecotone dynamics of a sand dune vegetation complex following water table decline. J Plant Ecol 9(1):40–50. https://doi.org/10.1093/jpe/rtv032
Torma A, Császár P (2013) Species richness and composition patterns across trophic levels of true bugs (Heteroptera) in the agricultural landscape of the lower reach of the Tisza River Basin. J Insect Conserv 17:35–51. https://doi.org/10.1007/s10841-012-9484-1
Torma A, Gallé R, Bozsó M (2014) Effects of habitat and landscape characteristics on the arthropod assemblages (Araneae, Orthoptera, Heteroptera) of sand grassland remnants in Southern Hungary. Agric Ecosyst Environ 196:42–50. https://doi.org/10.1016/j.agee.2014.06.021
Torma A, Bozsó M, Tölgyes C, Gallé R (2017) Relationship of different feeding groups of true bugs (Hemiptera: Heteroptera) with habitat and landscape features in Pannonic salt grasslands. J Insect Conserv 21(4):645–656. https://doi.org/10.1007/s10841-017-0007-y
Torma A, Bozsó M, Gallé R (2018) Secondary habitats are important in biodiversity conservation: a case study on orthopterans along ditch banks. Anim Biodivers Conserv 41(1):97–108. https://doi.org/10.32800/abc.2018.41.0097
Torma A, Császár P, Bozsó M, Deák B, Valkó O, Kiss O, Gallé R (2019) Species and functional diversity of arthropod assemblages (Araneae, Carabidae, Heteroptera and Orthoptera) in grazed and mown salt grasslands. Agric Ecosyst Environ 273:70–79. https://doi.org/10.1016/j.agee.2018.12.004
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity – ecosystem service management. Ecology Letters 8(8): 857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
Varga Z (1995) Geographical patterns of biological diversity in the Palaearctic Region and the Carpathian Basin. Acta Zool Academiae Scientiarum Hung 41(2):71–92
Verdonschot RCM, Keizer-vlek HE, Verdonschot PFM (2011) Biodiversity value of agricultural drainage ditches: a comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquat Conserv Mar Freshw Ecosyst 21(7):715–727. https://doi.org/10.1002/aqc.1220
Von Königslöw V, Mupepele AC, Klein AM (2021) Overlooked jewels: existing habitat patches complement sown flower strips to conserve pollinators. Biol Conserv 261:109263. https://doi.org/10.1016/j.biocon.2021.109263
Wachmann E, Melber A, Deckert J (2004) Wanzen, Vol. 2, Tierw 75, Deutschlds
Wachmann E, Melber A, Deckert J (2006) Wanzen, Vol. 1, Tierw 77, Deutschlds
Wachmann E, Melber A, Deckert J (2007) Wanzen, Vol. 3, Tierw 78, Deutschlds
Wachmann E, Melber A, Deckert J (2008) Wanzen, Vol. 4, Tierw 81, Deutschlds
Wagner E, Weber HH (1964) Hétéroptères. Miridae. Fauna De France 67. Fédération Française des Sociétés de Sciences Naturelles, Paris
Zurbrügg C, Frank T (2006) Factors influencing bug diversity (Insecta: Heteroptera) in semi-natural habitats. Biodivers Conserv 15(1):275–294. https://doi.org/10.1007/978-1-4020-5204-0_17
Acknowledgements
A.T., Z.B. and C.T. were supported by the grants of the National Research, Development and Innovation Office of Hungary (A.T.: NKFIH KKP 133839, Z.B.: NKFIH FK 142428, C.T.: NKFIH K 146137). Z.B. was additionally supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. M.P. was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (451-03-9/2023-14/200124).
Funding
Open access funding provided by University of Szeged. The preparation of this manuscript was funded by the National Research, Development and Innovation Office of Hungary (grant of Csaba Tölgyesi, NKFIH K 146137).
Open access funding provided by University of Szeged.
Author information
Authors and Affiliations
Contributions
J.Š. original draft writing, data collecting, data analysis; A.T. conceptualization, data collecting, manuscript writing; J.Š. and A.T. contributed equally to the preparation of the manuscript; C.T. experimental design, data collecting, manuscript editing, funding acquisition; Z.B. and M.P. data collecting, final reviewing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Andreas Schuldt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Šeat, J., Torma, A., Bátori, Z. et al. Landscape matrix and substrate jointly shape the trait composition of true bug (Heteroptera) communities in drainage ditches. Biodivers Conserv 33, 2363–2380 (2024). https://doi.org/10.1007/s10531-024-02860-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-024-02860-7